Abstract
Background
Obstructive sleep apnea (OSA) is the most common form of sleep-disordered breathing frequently associated with obesity. Obese subjects undergoing elective surgical procedures with general anesthesia are potentially at risk if this condition is not identified. Our aim was to assess the prevalence of bariatric patients with undiagnosed OSA following pre-operative assessment and who could benefit from peri-procedural respiratory management.
Methods
Patients who were referred for prospective bariatric surgery were screened using the STOP-BANG questionnaire. If patients scored >4 points they underwent a home-based nocturnal pulse oximetry. Severity of OSA was defined by the 4% oxygen desaturation index (ODI) combined with a physician’s review. Data were compared using unpaired two-tailed t-test and Chi-square test. Linear regression models were used to assess associations between clinical parameters.
Results
Sleep-disordered breathing of any degree was evident in 103 of 141 patients (73%). Thirteen (9%) patients had severe, 19 (13%) moderate, and 34 (24%) mild OSA, 38 (27%) patients had no OSA. 34 (24%) patients were initiated on continuous positive airway pressure (CPAP) prior to the surgical procedure, 15 (11%) were admitted for further respiratory assessment and two of them were given CPAP following inpatient sleep study. Thirteen (9%) patients were advised to use a mandibular advancement device for mild but symptomatic OSA. Out of all patients, 76 (54%) were advised that no treatment was required.
Conclusions
OSA is highly prevalent in a cohort of bariatric surgery patients screened with STOP-BANG questionnaires. Almost 3/4 of this cohort have at least some degree of sleep-disordered breathing, and approximately half of them require a plan for the respiratory management perioperatively.
Keywords: Obstructive sleep apnea (OSA), perioperative risk, obesity
Introduction
The United Kingdom has one of the fastest growing rates of obesity in the developed world (1). In 2013, approximately a quarter of adults (26% of men and 24% of women) were obese and 2% were morbidly obese (2). Analysis by the UK government estimates that over half of the UK population could be obese by 2050, posing a significant challenge to the National Health Service (NHS). Obesity causes an increased load on the respiratory system (3,4) leading to breathlessness and is associated with a number of respiratory conditions including asthma, pulmonary embolism, pneumonia and obstructive sleep apnea (OSA) (5).
OSA is the most common sleep-related breathing disorder, characterized by recurrent episodes of upper airway occlusion leading to apneas and hypopneas whilst the patient is asleep (6). These episodes cause intermittent hypoxia, arousal from sleep and lead to increased sympathetic activity, increased respiratory effort (3,4) and sleep fragmentation (7). OSA causes daytime sleepiness and has been associated with co-morbidities (8) including type 2 diabetes mellitus (T2DM) (9), hypertension (10), and increased cardiovascular risks, including stroke (11).
The prevalence of OSA is increasing, it affected 4% of middle aged men and 2% of middle aged women in the United States in the early 1990’s (12), but with the obesity epidemic the prevalence is now at 10% for 30–49-year-old men and at 3% for 30–49-year-old women in the US (13). In obese subjects, prevalence can be as high as 60-70% (14) and most patients remain undiagnosed (15) and untreated (8).
Continuous positive airway pressure (CPAP) is the best available treatment for OSA (16), the National Institute of Health and Clinical Excellence (NICE) recommends the use of CPAP as a treatment option for adults with moderate to severe OSA and mandibular advancement devices for mild conditions (17). In obese patients, weight loss can improve sleep-disordered breathing (18). In severe obesity which is refractory to diet, physical exercise and bariatric surgery effectively support weight loss (19). However, weight loss due to bariatric surgery has, so far, failed to prove its superiority compared to conventional weight loss strategies with respect to its impact on the severity of OSA, as measured by the apnea-hypopnea-index (20).
Patients who are eligible for bariatric surgery may suffer from undiagnosed OSA (21) potentially putting them at risk when undergoing general anesthesia (22-27). A meta-analysis investigating the association between OSA and post-operative complications of any intervention found that the incidence of post-operative desaturations, respiratory failure, cardiac events and transfers to the intensive care unit was higher in patients with untreated OSA (23).
The standard to diagnose OSA is overnight polysomnography (PSG), but this is an expensive and time-intense procedure and not widely available (8,28). In contrast, a case-finding screening tool often used to classify patients at risk of OSA is the STOP-BANG questionnaire (29,30). Combining the STOP-BANG questionnaire with the recording of a physiological signal, nocturnal pulse oximetry, can help to improve diagnostic accuracy (31).
We hypothesized that screening for sleep-disordered breathing following STOP-BANG assessment in the pre-bariatric population, using nocturnal pulse oximetry as part of the assessment, will assist in the diagnosis of patients with previously unknown OSA.
Patients and methods
Screening of bariatric patients
Patients undergoing bariatric surgery at Guy’s & St Thomas’ NHS Foundation Trust, London, UK between 12/2013 and 12/2014 were screened as part of the pre-operative assessment using the STOP-BANG questionnaire. STOP-BANG is the acronym for snoring [‘does the patient snore loudly (loud enough to be heard through closed doors or your bed-partner elbows you for snoring at night?)’], tiredness (‘does the patient often feel tired, fatigued, or sleepy during the daytime?’), observed apnea (‘has anyone observed the patient stop breathing or choking/gasping during sleep?’), blood pressure (BP) (‘does the patient have or is the patient being treated for high BP?’), body mass index (BMI >35 kg/m2), age (>50 years), neck circumference (>40 cm), and gender (male). Each item scores either ‘0’ (if the answer is no) or ‘1’ (if the answer is yes) point. Those who scored >4 points are likely to be at risk of OSA and were referred to the Sleep Disorder Centre for a comprehensive evaluation. A STOP-BANG score of >4 points has been found to have a sensitivity of 88% for the diagnosis of OSA (32).
As part of the evaluation expert sleep physicians assessed the patients’ history of the sleep complaint, brief examination, recorded weight, height, BP and other co-morbidities and measured the epworth sleepiness scale (ESS) (33).
Patient data
Patient demographics included age, sex, BMI, ESS score, comorbidities including hypertension, T2DM, depression and ischemic heart disease (IHD).
Nocturnal pulse oximetry
Patients underwent nocturnal pulse oximetry (Pulsox 300i, Konica Minolta sensing Inc., Hachioji, Tokyo, Japan) for two consecutive nights at home. The following data were recorded and calculated:
Mean peripheral capillary oxygen saturation (SpO2)
4% and 3% oxygen desaturation index (ODI, h-1)
Total time with oxygen saturation <90% (percent of the total night)
Mean pulse rate (h-1)
Pulse rises [>6 beats per minute (bpm); h-1]
The recorded traces were reviewed and interpreted by an experienced sleep physician. Based on the results of the tests the sleep physician provided an interpretation. OSA was defined as a 4% ODI of >5 h-1. Severity of OSA was classified as mild (4% ODI 5-15 h-1), moderate (4% ODI 15-30 h-1) and severe (4% ODI >30 h-1) (34).
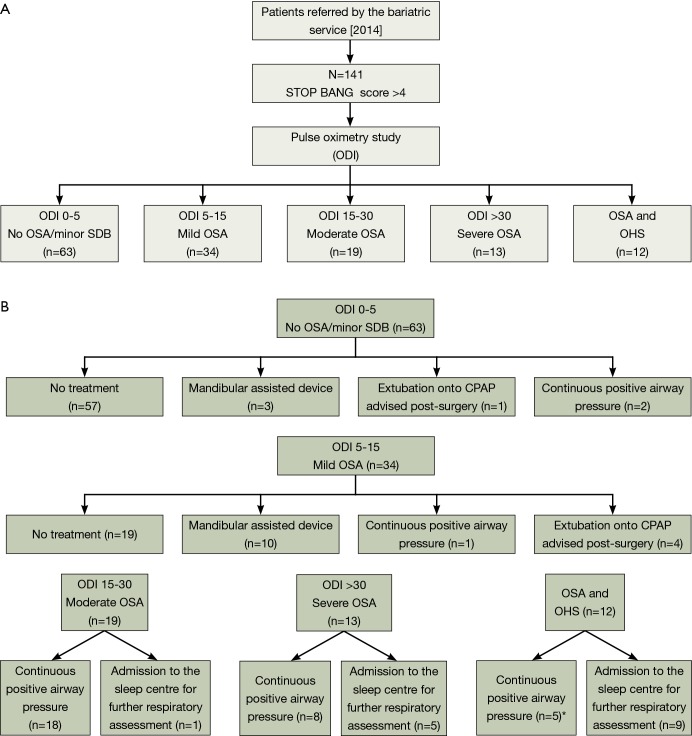
Treatment decisions for the patient, based on the results of the above assessment, included (Figure 1A,B):
Figure 1.
Flow diagram of decisional management of bariatric cohort. *, two patients given CPAP and admitted. ODI, oxygen desaturation index; SDB, sleep disordered breathing; OSA, obstructive sleep apnea; OHS, obesity hypoventilation syndrome; CPAP, continuous positive airway pressure.
No significant sleep-disordered breathing, no action required;
Mild OSA, if the patients were symptomatic it was recommended to consider a mandibular advancement device for symptom management independent of the surgical intervention;
Moderate-severe OSA, trial of CPAP;
Extubation onto CPAP advised post-surgery due to significant desaturations;
Suggested admission to the sleep center for further respiratory assessment, if the results of the pulse oximetry were inconclusive.
Statistical analysis
Continuous variables are presented as mean (standard deviation) and categorical variables as percentages. For unadjusted comparisons between OSA and non-OSA patients, continuous variables were compared using an unpaired two-tailed t-test. All other categorical variables were compared using the Chi-square test. For unadjusted comparisons between mild, moderate and severe OSA, continuous variables were compared using the Kruskal-Wallis one-way ANOVA with Dunnparisons between multiple comparisons. All other categorical variables (class II or III obesity, hypertension, T2DM, depression and IHD) were compared using Chi-square test. In the univariate analysis we compared the dependent variables 4% ODI h-1 (and 3% ODI h-1) with other independent variables (gender, age, BMI, ESS, class II/III obesity, hypertension, diabetes, IHD, SpO2, Time with oxygen saturation <90% (%), pulse rate (h-1) and pulse rise (h-1) using a Spearman correlation test. We finally performed a multiple linear regression analysis to identify the variables that significantly correlated with the dependent variables; 3% ODI h-1 and 4% ODI h-1. A P value <0.05 was assumed to represent statistical significance.
Results
Demographics
The studied cohort included a total of 141 patients awaiting bariatric surgery who scored >4 points on STOP-BANG in their pre-operative assessment [70% female, age 49 (±12) years, BMI 48.7 (±7.3) kg/m2; Table 1]. Systemic hypertension was prevalent in 40%, T2DM was present in 35%, and 25% of the patients had depression. The majority of the patients were not excessively sleepy [ESS 7.3 (±4.9) points].
Table 1. Baseline data of the cohort of 141 patients studied.
| Variables | Participants (n=141) |
|---|---|
| Sex (M:F) | 42:99 |
| Age (years) | 48.8±(12.2) |
| BMI (kg/m2) | 48.7±(7.3) |
| ESS (/15) | 7.3±(4.9) |
Data are presented as mean ± (SD). BMI, body mass index; ESS, epworth sleepiness scale.
Sleep apnea prevalence and treatment recommendations
Sleep-disordered breathing of any degree was evident in 103 of 141 patients (73%). 13 of 141 patients (9%) were found to have severe OSA, 19 of 141 (13%) had moderate OSA and 34 of 141 (24%) had mild OSA. 12 of 141 (9%) suffered with moderate to severe OSA with significant periods of the night below SpO2 of 90%, potentially indicating co-existing obesity hypoventilation syndrome (OHS) (35).
Based on these findings, 34 of 141 (24%) patients who were excessively sleepy were given CPAP prior to surgery and in 5 of 141 (4%) patients’ extubation to CPAP post-surgery was recommended. Fifteen of 141 patients (11%) were admitted for further respiratory assessment to the sleep laboratory, and in 13 of 141 patients (9%) the use of a mandibular advancement device was recommended (Figure 1A,B).
Nocturnal pulse oximetry
There were no significant differences in parameters between the nocturnal pulse oximetry data on night 1 vs. night 2 (Table 2). Bariatric patients who had OSA were older (P<0.004), more obese (P<0.008), and more sleepy (P<0.05). They had more co-morbidities with hypertension (P<0.05) and T2DM (P<0.05) (Table 3). A multiple linear regression analysis revealed that time with SpO2 saturation <90% (P<0.001), pulse rise index (P<0.001) and ESS (P<0.001) were independently associated with the oxygen desaturation indices (Table 4).
Table 2. Nocturnal pulse oximetry data of the patients studied, comparison of night 1 vs. night 2.
| Nocturnal oximetry | Night 1 | Night 2 | P value |
|---|---|---|---|
| SpO2 (%) | 94 (2.8) | 93.9 (2.9) | 0.79 |
| 4% ODI (h-1) | 17.1 (25.3) | 19 (27.5) | 0.09 |
| 3% ODI (h-1) | 21.6 (27.2) | 21.9 (28.1) | 0.54 |
| Time with O2 sats <90% (%) | 8.9 (17.1) | 8.3 (16.3) | 0.84 |
| Pulse rate (h-1) | 35.3 (12) | 35.5 (11.4) | 0.91 |
| Pulse rise (h-1; >6 bpm) | 75.4 (21.1) | 74.9 (20.4) | 0.37 |
There were no significant differences between the nights. The results indicated moderate sleep-disordered breathing with almost 1/10 of the night below oxygen saturations of 90%, and an increased pulse rise index caused by arousal from sleep. All data are presented as mean (SD). SpO2, peripheral capillary oxygen saturation; ODI, oxygen desaturation index; bpm, beats per minute.
Table 3. Comparison of patients with OSA and those without.
| Characteristics | OSA | No OSA | P value |
|---|---|---|---|
| Age (years) | 48.5 (12.3) | 42.0 (10.6) | 0.004 |
| BMI (kg/m2) | 49.6 (7.8) | 46.0 (5.7) | 0.008 |
| BMI score 35-39 | 97.0 (68.7) | 35.0 (92.1) | 0.65 |
| ESS | 7.6 (5.1) | 6.0 (4.3) | 0.05 |
| Nocturnal oximetry | |||
| SpO2 (%) | 93.4 (2.9) | 96.0 (1.1) | <0.001 |
| 4% ODI (h-1) | 22.3 (27.8) | 3.0(1.2) | <0.001 |
| 3% ODI (h-1) | 27.9 (29.5) | 4.0 (1.8) | <0.001 |
| Time with O2 sats <90% (%) | 12.1 (19.0) | 0 (0.6) | <0.001 |
| Pulse rate (h-1) | 75.4 (11.7) | 74.0 (8.1) | 0.38 |
| Pulse rise (h-1) (>6 bpm) | 37.5 (23.0) | 31.0 (15.2) | 0.06 |
| Co-morbidities | |||
| Hypertension | 46.0 (44.6) | 10.0 (26.3) | 0.05 |
| T2DM | 40.0 (38.8) | 8.0 (21.1) | 0.05 |
| Depression | 24.0 (23.3) | 11.0 (28.9) | 0.49 |
| IHD | 2.0 (1.9) | 1.0 (2.6) | 0.73 |
Hypertension ≥140/90 mmHg. Patients with OSA were older, more obese, sleepier and had more comorbidities with hypertension and T2DM. BMI, body mass index; ESS, epworth sleepiness scale; SpO2, peripheral capillary oxygen saturation; ODI, oxygen desaturation index; T2DM, type 2 diabetes mellitus; IHD, ischemic heart disease; OSA, obstructive sleep apnea.
Table 4. Spearman correlation between the dependent variables (4% ODI and 3% ODI) and other independent variables.
| Variables | 4% ODI | 3% ODI |
|---|---|---|
| Gender | 0.26‡ | 0.29† |
| Age | 0.17‡ | 0.18‡ |
| BMI (kg/m2) | 0.30† | 0.28† |
| ESS (score) | 0.28† | 0.28‡ |
| Class II obesity | 0.06‡ | 0.07‡ |
| Class III obesity | 0.06‡ | 0.07‡ |
| Hypertension | 0.18‡ | 0.19‡ |
| T2DM | 0.16‡ | 0.16‡ |
| Depression | 0.05‡ | 0.04‡ |
| IHD | 0.01‡ | 0.01‡ |
| SpO2 | 0.64† | 0.65† |
| Time with O2 saturation <90% (%) | 0.89† | 0.87† |
| Pulse rate (h-1) | 0.28† | 0.29† |
| Pulse rise (h-1) | 0.35† | 0.36† |
Hypertension ≥140/90 mmHg. ‡, P<0.05, †, P<0.001. BMI, body mass index; ESS, epworth sleepiness scale; SpO2, peripheral capillary oxygen saturation; ODI, oxygen desaturation index; T2DM, type 2 diabetes mellitus; IHD, ischemic heart disease.
Discussion
Patients undergoing bariatric surgery who score >4 on the STOP-BANG questionnaire have a high prevalence of OSA with almost three quarters of patients having some abnormal nocturnal breathing pattern. Approximately one quarter of the patients had moderate-severe OSA, while 1/10 had a recording that was consistent with combined OSA/OHS (35). In more than one quarter of all patients CPAP was employed for peri-operative management. 11% of the patients were invited for further respiratory assessment for an inpatient sleep study in the sleep laboratory (pre-operatively); 9% were recommended to use a mandibular advancement device in future for mild but symptomatic OSA. Bariatric patients who were more obese, older, more sleepy and who had more comorbidities (hypertension, diabetes) were more likely to have significant sleep-disordered breathing.
Clinical significance of findings
Almost half of the patients were given some recommendation for further respiratory management, no further action was required for 54% of patients. CPAP is a common and effective treatment for OSA (36). It reduces symptoms like daytime sleepiness, but it has also been found to improve cognitive function (37) and cardiovascular risks (38). As our understanding about prevalence in bariatric surgery cohorts improves, it is important that we diagnose and treat OSA in these patients to reduce peri-operative complications. Our study also found that patients with OSA compared to those without OSA were older, heavier and sleepier, they were also more likely to have hypertension and T2DM. Although this is not causal evidence in this small cohort, it supports the suggestion that OSA is a risk factor for cardiovascular and metabolic conditions (13).
Review of literature
Our results are consistent with previous studies describing the prevalence of OSA to be 78.3% in patients who have bariatric surgery (39), while another study found that 77% of patients for bariatric surgery had OSA (40). It has been reported that patients with OSA who received CPAP therapy before surgery have less peri-operative complications compared to patients with undiagnosed and untreated OSA (25). This underlines the importance to diagnose and treat OSA in these patients to reduce the risk of complications during and following bariatric surgery.
Data from the national bariatric surgery registry in the UK show that in 2006 there were fewer than 500 bariatric surgery procedures and that this increased to over 6,000 bariatric surgery procedures in 2013 (41), a trend that is mirrored the world over. Therefore the number of patients at risk of peri-operative complications is increasing and diagnosis and treatment of patients with OSA using CPAP could optimize peri-operative management.
Limitations of the study
Patients were from a selected cohort, screened using the STOP-BANG score, and final outcomes and complications after surgery was not recorded, peri-operative risks were not assessed. In order to investigate the effectiveness of CPAP treatment future studies would need to randomize identified cases of patients with OSA undergoing bariatric surgery to a trial of CPAP or sham-CPAP peri-operatively. It would also be of interest to extend this research to other surgical non-bariatric cohorts. Further, the absolute prevalence of OSA in all bariatric surgery patients may differ due to the exclusion of patients who scored less than four points on the STOP-BANG questionnaire.
Conclusions
There is a high prevalence of sleep-disordered breathing in patients undergoing bariatric surgery who score >4 points on STOP-BANG. Screening these patients for OSA prior to bariatric surgery using nocturnal pulse oximetry and offering treatment with CPAP could reduce peri-operative risks.
Acknowledgements
This work was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.McPherson K, Marsh T, Brown M. Tackling obesities: future choices—modelling future trends in obesity and the impact on health. 2nd Edition. Foresight 2007. [Google Scholar]
- 2.HSCIS. Health Survey for England 2013. Health, social care and lifestyles. Summary of key findings. Available online: http://www.hscic.gov.uk/catalogue/PUB16076/HSE2013-Sum-bklet.pdf
- 3.Steier J, Jolley CJ, Seymour J, et al. Neural respiratory drive in obesity. Thorax 2009;64:719-25. [DOI] [PubMed] [Google Scholar]
- 4.Steier J, Jolley CJ, Seymour J, et al. Increased load on the respiratory muscles in obstructive sleep apnea. Respir Physiol Neurobiol 2010;171:54-60. [DOI] [PubMed] [Google Scholar]
- 5.Zammit C, Liddicoat H, Moonsie I, et al. Obesity and respiratory diseases. Int J Gen Med 2010;3:335-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remmers JE, deGroot WJ, Sauerland EK, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol 1978;44:931-8. [DOI] [PubMed] [Google Scholar]
- 7.Sharma SK, Agrawal S, Damodaran D, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med 2011;365:2277-86. [DOI] [PubMed] [Google Scholar]
- 8.Steier J, Martin A, Harris J, et al. Predicted relative prevalence estimates for obstructive sleep apnoea and the associated healthcare provision across the UK. Thorax 2014;69:390-2. [DOI] [PubMed] [Google Scholar]
- 9.West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 2006;61:945-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjöström C, Lindberg E, Elmasry A, et al. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax 2002;57:602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034-41. [DOI] [PubMed] [Google Scholar]
- 12.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [DOI] [PubMed] [Google Scholar]
- 13.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguiar IC, Freitas WR, Santos IR, et al. Obstructive sleep apnea and pulmonary function in patients with severe obesity before and after bariatric surgery: a randomized clinical trial. Multidiscip Respir Med 2014;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh M, Liao P, Kobah S, et al. Proportion of surgical patients with undiagnosed obstructive sleep apnoea. Br J Anaesth 2013;110:629-36. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1:862-5. [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Clinical Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome, 2008:26. Available online: https://www.nice.org.uk/guidance/ta139
- 18.Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery 2007;141:354-8. [DOI] [PubMed] [Google Scholar]
- 19.Fritscher LG, Mottin CC, Canani S, et al. Obesity and obstructive sleep apnea-hypopnea syndrome: the impact of bariatric surgery. Obes Surg 2007;17:95-9. [DOI] [PubMed] [Google Scholar]
- 20.Dixon JB, Schachter LM, O'Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA 2012;308:1142-9. [DOI] [PubMed] [Google Scholar]
- 21.Frey WC, Pilcher J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg 2003;13:676-83. [DOI] [PubMed] [Google Scholar]
- 22.Proczko MA, Stepaniak PS, de Quelerij M, et al. STOP-Bang and the effect on patient outcome and length of hospital stay when patients are not using continuous positive airway pressure. J Anesth 2014;28:891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaw R, Chung F, Pasupuleti V, et al. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth 2012;109:897-906. [DOI] [PubMed] [Google Scholar]
- 24.Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005;352:1206-14. [DOI] [PubMed] [Google Scholar]
- 25.Gupta RM, Parvizi J, Hanssen AD, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc 2001;76:897-905. [DOI] [PubMed] [Google Scholar]
- 26.Mokhlesi B, Hovda MD, Vekhter B, et al. Sleep-disordered breathing and postoperative outcomes after bariatric surgery: analysis of the nationwide inpatient sample. Obes Surg 2013;23:1842-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schumann R, Shikora SA, Sigl JC, et al. Association of metabolic syndrome and surgical factors with pulmonary adverse events, and longitudinal mortality in bariatric surgery. Br J Anaesth 2015;114:83-90. [DOI] [PubMed] [Google Scholar]
- 28.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth 2010;57:423-38. [DOI] [PubMed] [Google Scholar]
- 29.Chung F, Subramanyam R, Liao P, et al. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth 2012;108:768-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toshniwal G, McKelvey GM, Wang H. STOP-Bang and prediction of difficult airway in obese patients. J Clin Anesth 2014;26:360-7. [DOI] [PubMed] [Google Scholar]
- 31.Whitelaw WA, Brant RF, Flemons WW. Clinical usefulness of home oximetry compared with polysomnography for assessment of sleep apnea. Am J Respir Crit Care Med 2005;171:188-93. [DOI] [PubMed] [Google Scholar]
- 32.Chung F, Yang Y, Liao P. Predictive performance of the STOP-Bang score for identifying obstructive sleep apnea in obese patients. Obes Surg 2013;23:2050-7. [DOI] [PubMed] [Google Scholar]
- 33.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992;15:376-81. [DOI] [PubMed] [Google Scholar]
- 34.National Institute for Health and Clinical Excellence. Final appraisal determination – Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. 2007:1-25. Available online: http://www.nice.org.uk/guidance/ta139/documents/sleep-apnoea-final-appraisal-determination-22
- 35.Mandal S, Suh ES, Boleat E, et al. A cohort study to identify simple clinical tests for chronic respiratory failure in obese patients with sleep-disordered breathing. BMJ Open Respir Res 2014;1:e000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006;(1):CD001106. [DOI] [PubMed] [Google Scholar]
- 37.Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain Res Bull 2003;61:87-92. [DOI] [PubMed] [Google Scholar]
- 38.Doherty LS, Kiely JL, Swan V, et al. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 2005;127:2076-84. [DOI] [PubMed] [Google Scholar]
- 39.Lopez PP, Stefan B, Schulman CI, et al. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg 2008;74:834-8. [PubMed] [Google Scholar]
- 40.O'Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg 2004;14:23-6. [DOI] [PubMed] [Google Scholar]
- 41.Welbourn R, Small P, Finlay I, et al. The United Kingdom National Bariatric Surgery Registry. Second Registry Report 2014. Nbsr 2014:1-220. Available online: http://nbsr.co.uk/wp-content/uploads/2014/11/Extract_from_the_NBSR_2014_Report.pdf