Abstract
The population of patients with congenital genitourinary disorders has unique healthcare demands that require an additional interpersonal and medical skillset. Adults with congenital neurogenic bladder may have complex urinary anatomy, abnormal bladder function and atypical voiding mechanisms. While initial surgery and care of these patients is typically managed by a pediatric urologist, growth and development into adulthood necessitates transition of care to an adult care team. Failure of transition to adult care has been demonstrated to result in lower quality healthcare and increased risk of developing preventable complications.
Keywords: Congenitalism, spina bifida (SB), myelodysplasia, neuropathic bladder, transition of care
Introduction
Urologic congenitalism encompasses all genitourinary disorders diagnosed at or shortly after birth. Among the organs affected by congenital diseases, the bladder is commonly involved. Bladder problems include congenital uropathies related to obstruction, neurologic dysfunction and primary anatomic disorders outlined in Table 1. Of these, myelomeningocele, otherwise referred to as spina bifida (SB), is the most common and makes up the largest group of adult congenital urological patients in the United States (1).
Table 1. The most common congenital urologic diseases by primary pathology.
| Classification | Underlying etiologies |
|---|---|
| Obstructive | Posterior urethral valves |
| Urethral atresia | |
| Obstructing ureteroceles | |
| Ectopic ureters | |
| Primary bladder | Epispadias |
| Bladder exstrophy | |
| Cloacal exstrophy | |
| Eagle-Barrett disease (prune belly) | |
| Neurological | Neural tube defects including myelomeningocele |
| Cerebral palsy | |
| Degenerative neuromuscular diseases |
These diseases range in severity, effect on bladder function and impact on the patient’s life. They are often managed in early life by a pediatric urologist and later in life care is transferred to adult specialists. Because urologic congenital disease is a wide spectrum of disorders that can lead to neurogenic bladder, it can be challenging to evaluate and treat these patients. Diseases can be isolated to the urinary system (hypospadias) or can affect multiple organ systems (SB). As patients age, they may develop many comorbidities, such as upper and lower gastrointestinal motility dysfunction, cardiopulmonary disease, and orthopedic and metabolic issues related to their primary condition. These comorbidities can complicate urological and surgical care and may be challenging to navigate in an adult care environment where care can be more disjointed.
Aside from progression of disease, patients with congenital urological diseases often encounter urological issues that are unique to adult life, such as sexuality and fertility. In addition, age-related urological problems like prostatic hyperplasia and pelvic organ prolapse may be encountered. All of these issues necessitate an adult-urological care skillset. On the other hand, knowledge of congenital conditions and historical procedures to address those conditions often requires a pediatric skillset. Thus, the urologist treating these patients must have an excellent working knowledge in both areas.
Transitioning care
The primary goal of transition is to ensure developmentally appropriate, uninterrupted care for patients as they transfer their medical care to the adult setting (2). Transitioning will often include a change in physical location from the pediatric to adult hospital or clinic. The patient must also build new relationships with adult providers after being comfortable with years of care from pediatric providers. Though transition may occur at any time, success can be maximized when the patient is physically and mentally prepared and has the emotional maturity to assume independence in making healthcare decisions.
Transitioning care from a pediatric to an adult specialist can be a challenging time for patients and their families. Successful transition depends on a patient’s readiness to assume responsibility for their own care. Most transition readiness tools require that patients demonstrate the ability to schedule appointments, understand and communicate their health needs with their providers, and have a solid appreciation of their medical problems and treatments prior to formal transition to the adult care team. Most programs endorse beginning this process as early as 12 years of age, with a goal of complete transition between 18–24 years of age (3).
Children with congenital urological deficits traditionally have had access to comprehensive, multidisciplinary care in many areas of the United States. However, until recently, access to even basic care was often quite limited for adults with the same conditions (4-6). The Affordable Care Act (ACA) mandated the provision of dependent coverage up to the age of 26 and prevented denial of care due to previously diagnosed conditions—two regulatory changes which benefitted patients with congenital conditions. However, despite increased access to care, and increased awareness of the need for coordination of transition, remarkably high rates of adults often fail to transition in urology. Even in centers of excellence in pediatric and adult urological care, the rate of transition failure has been reported to be as high as 40% (7,8). Risk factors for transition failure include black or Hispanic race/ethnicity, low income level, and not having English as a primary language. The negative effects can be quite pronounced. As recently as 2005, nearly three-quarters of adults with urologic congenital disorders could not identify or did not have a primary care physician (9).
Data are beginning to show that the costs of non-transition are considerable. Patients who do not transition successfully often have interruptions in their care and develop potentially preventable complications (10). One study showed that 34% of hospital admissions for adult patients with SB were for conditions that were avoidable (11). These complications can be extremely costly and have been estimated around $364 million over a 2-year period (11,12).
There is some evidence to suggest that young adult patients are at risk for declining health around the time of transition. In a study of patients with congenital urologic disorders that transitioned to a dedicated adult care clinic, most of the patients (85%) presented with an untreated or worsened urologic complaint (5). The most common urologic problems were urinary incontinence (52%), recurrent urinary tract infections (UTIs) (34%), difficulty with catheterization (12%) and urinary calculi (9%). In this same study, 97% required a medical intervention, including 34% who underwent an operative procedure. While the reason for these observations is unknown, one can assume that both patient factors and health care infrastructure factors may be responsible. Certainly, adolescents and young adults are well known to delay seeking medical care even when they do not have a chronic condition, and a life-long history of experience with health care providers may further exacerbate a young person’s reluctance to seek out care. Moreover, as adolescents become young adults, pediatric care providers may feel increasingly out of their comfort zone in identifying and treating new problems, particularly if these problems are “adult” in nature.
Evaluating the transitioning patient
For patients with congenital conditions involving multiple systems, multidisciplinary care is often needed. Many young adults are accustomed to obtaining care in multidisciplinary pediatric clinics and are ill-equipped to navigate the complexities of the adult-care world and synthesizing information across many providers. Patient’s often come to the urology office with both a need and an expectation that the urology team will assist in coordination of care. The family’s ability to help in this process may be an important factor in the patient’s success or failure in adulthood and a wise urologic provider will welcome their assistance. We utilize a stepwise method of approaching all transition patients at their initial intake visit (13). The steps are as follows:
Define the patient’s baseline urologic function;
Elicit the patient’s goals, resource constraints, executive/cognitive deficits, and social constraints;
Characterize the patient’s new or worsening complaint;
Determine the appropriate diagnostic tests that will aid in characterizing problems;
Present the patient with all available treatment options and describe the associated drawbacks or risks of each.
Evaluating new problems
After a congenital pediatric patient has undergone procedures to correct anatomic urinary dysfunction and a mode of voiding has been established, bladder or diversion function typically stabilizes. New problems, most often typified by recurrent/escalating infections, deteriorating renal function, or new incontinence, often indicate a change in the urinary system and should be judiciously and meticulously scrutinized. For example, a patient on intermittent self-catheterization (ISC) presenting with back-to-back urinary infections over a 3-month time period could have physiologic, anatomic, social or behavioral reasons for this presentation. Noncompliance with catheterization comes to mind as an obvious potential cause, but an astute provider who inquires exactly how the patient is catheterizing may find that the reason for noncompliance could be the development of a stricture or false passage (anatomic), a changing home environment related to his providers (social), a change in his insurance coverage which would alter catheter availability (paramedical), or shunt malfunction altering gross hand dexterity (physiologic).
While the original problem at birth may be important, by adulthood it is often more important to classify and assess patients based on their storage and voiding mechanisms. A patient with posterior urethral valves (PUV) with a continent diversion is more similar to an exstrophy patient with the same diversion than to another valve patient who voids per urethra with Valsalva maneuvers. Broad groups include: (I) voiding normally per urethra; (II) Valsava voiding per urethra; (III) ISC per bladder; (IV) ISC per augmentation cystoplasty; (V) ISC per pouch; (VI) incontinent to diaper; (VII) suprapubic tube; (VIII) vesicostomy or ileal conduit. It can be helpful to scrutinize new or worsening urinary problems in this context. For instance, continent diversions should always be scrutinized for stones in the setting of recurring protease-producing bacterial infections.
Another important factor to assess is the patient’s renal function. Oftentimes adults who are transitioning have experienced gaps in healthcare and may have had changes in bladder or kidney function since their last visit with a physician. While serum creatinine (SCr) is known to be a suboptimal indicator of renal function in many of these patients, trends in SCr may be instructive. We make great efforts to obtain prior SCr measurements for this purpose. All patients obtain some baseline assessment of renal function beyond laboratory work and select patients require renal functional testing with 24-hour urine for creatinine clearance, iothalamate renal scan, or cystatin C testing.
For patients with deteriorating renal function, escalating UTIs or changing continence, identification of the cause is of paramount importance. If a patient has changing or escalating urinary problems, video urodynamic study (UDS) and upper tract imaging is often warranted (14). To workup problems or for routine surveillance, ultrasound is a safe and sensitive test for assessing upper tract deterioration. To evaluate hematuria, flank pain and suspected renal or bladder calculi, computed tomography (CT) may be necessary. Patients with a neurologic cause of neurogenic bladder may also be complicated by neurogenic bowel. To assess for fecal impaction, abdominal radiography may be indicated.
In developing a differential, it can be helpful to classify the possible diagnoses based on upper or lower tract etiologies. Upper tract disease can be divided into nephrologic and urologic etiologies while lower tract disease can be divided into primary or secondary urologic conditions. Though the causes of urologic dysfunction are wide, examples of urologic problems and possible etiologies are listed in Table 2.
Table 2. Problems of the upper and lower urinary tracts and their potential causes.
| Problem | Cause |
|---|---|
| Deterioration | For bladder, tethered cord |
| For diversion/augment, contraction pouch | |
| Overflow | Noncompliance |
| Retention | Bladder diverticula |
| Reservoir dilation | |
| Foreign object | Stones, stents, tumors |
| Extrinsic | Constipation, pelvic mass |
| Bladder outlet obstruction | Pelvic organ prolapse |
| Benign prostatic hyperplasia | |
| Prostate cancer | |
| Urethral stricture | |
| Neurologic | Stroke, spinal stenosis |
Guidelines
For patients with stable renal function and no new symptoms, yearly screening with ultrasound, urinalysis and basic metabolic panel can be sufficient. If there has been diversion or augmentation, vitamin B12 levels should be ordered and followed. If patients present with recurrent UTIs or hematuria and have a history of augmentation cystoplasty or continent reservoir, our practice is to perform a baseline cystoscopy.
European Association of Urology (EAU) has established guidelines for management of patients with SB (15). They suggest urinalysis every other month and renal and bladder ultrasound every 6 months. This complements yearly physical exams with blood tests to assess SCr and urine tests. They recommend that specialized testing including UDS may be used every 1–2 years or sooner if the patient develops symptoms of a changing urinary system. Hallmarks of a changing urinary system include worsening incontinence, rising SCr or new hydronephrosis.
At our institution, we schedule patients for annual serum studies, renal ultrasound and urinalysis. Patients are seen more frequently if they have high-risk bladders or severe renal dysfunction. UDS and cystoscopy are performed based on symptoms suggestive of stones or bladder deterioration.
Renal function and assessment
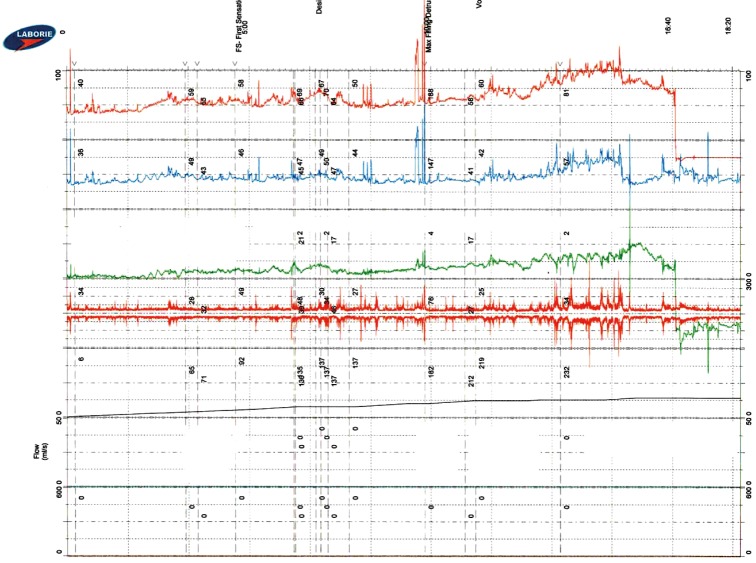
Patients with congenital neurogenic bladder have a higher risk for developing renal failure compared to the general population so life-long surveillance of renal function is vital (16). McGuire and colleagues demonstrated that the risk for upper tract deterioration substantially increases when detrusor leak point pressures exceed 40 cmH2O (17). Many SB patients with detrusor leak point pressures >40 mmHg who remain untreated will develop chronic kidney disease (CKD) during their life time (18). While some patients may “declare” a hostile bladder in childhood and necessitate augmentation cystoplasty early in life, young people with “safe” bladders may deteriorate in adulthood due to either primary or secondary causes (e.g., development of prostatic hyperplasia that reduces abdominal leak point pressures and causes secondary increased storage pressures) (Figure 1).
Figure 1.
Urodynamic testing demonstrating classic neuropathic bladder phenotype with poor compliance and elevated detrusor leak point pressure.
Even among patients “definitively” managed for a hostile bladder during childhood with augmentation or diversion, renal deterioration remains a great risk. Though most patients are able to maintain low bladder filling pressures after augmentation cystoplasty, some patients can develop decreased compliance (19). This population of patients may have severely scarred kidneys, anatomically or functionally solitary kidneys, and/or vesicoureteral reflux (VUR), all of which have been demonstrated to hasten the progression of renal disease (20,21). While it can be difficult to identify why a patient continues to lose renal function as they age into middle and late adulthood, if recurrent infections are noted, they should be aggressively addressed, as UTIs have been shown to be a risk factor for progressive renal dysfunction (22).
A special note should be made of patients with a history of PUV, as they demonstrate exceptionally high rates of renal demise in adult life (23). It is estimated that approximately one third of these patients may develop end stage renal disease (ESRD) by the time they reach early adulthood and even more progress to ESRD in adult life (24,25). As a result of this, it is estimated that approximately 17% of PUV patients undergo renal transplantation at some point in their lives (26). Patients with PUV may develop detrusor dysfunction which may not always be symptomatic and boys may present after they reach puberty with enlarged hypotonic bladders (27).
In bladder exstrophy spectrum patients, UTIs and renal scarring represent an important risk for renal deterioration (Figure 2). More than one fourth of patients may develop renal insufficiency (28,29). It is important to note that many exstrophy patients who void per urethra do so with elevated voiding pressures after bladder neck reconstruction, which may predispose to renal deterioration and urinary stasis with secondary recurrent UTIs. Thus, maintained renal surveillance is critical in this population (30).
Figure 2.
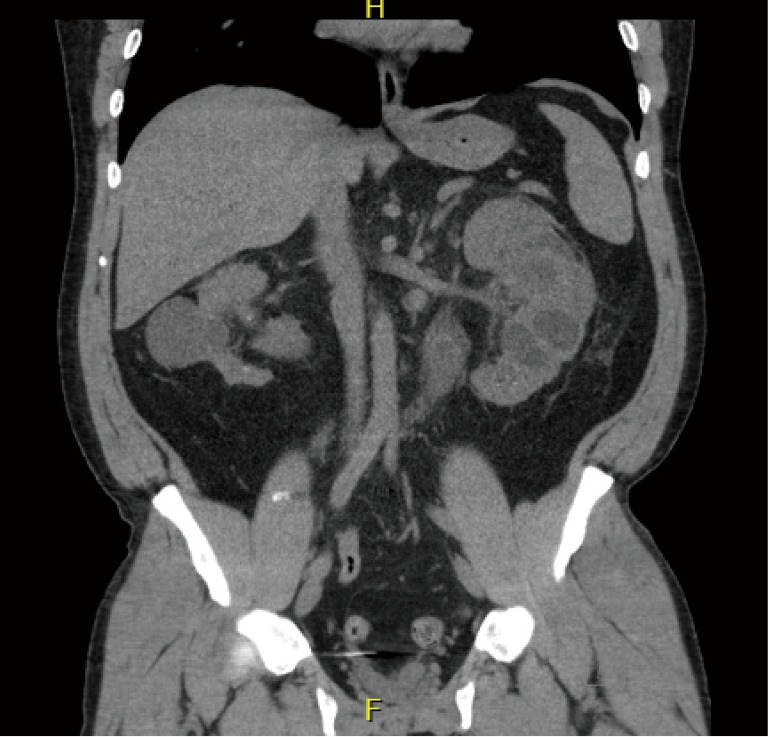
Forty-seven year old with bladder exstrophy and estimated glomerular filtration rate around 25. Kidneys are heavily scarred and atrophic appearing.
It may be challenging to monitor renal function as patients advance through adolescence and enter adulthood. Disproportionate increases in SCr are well described as patients progress through adolescence, which can make interpretation of data quite difficult during this time period (31). An especially difficult scenario is in estimating glomerular filtration rate (eGFR) in non-weight bearing adults with congenital disease as is often seen in SB patients. The lack of motility causes a disproportionate relationship between weight and muscle, rendering standard methods of calculating GFR (MDRD, Cockcroft-Gault) inaccurate (32). A “normal” SCr for an adult SB patient’s body mass index (BMI) may actually represent a patient with stage II or III CKD (33). In this sense, measuring SCr often overestimates eGFR in this population (34). Non-creatinine-based methods of estimating GFR have been suggested to be superior in this select population. Cystatin C has been shown to be more accurate than creatinine alone when evaluating renal insufficiency (35,36). However, in comparison to chromium 51 edetic acid clearance, cystatin C does not perform well when evaluating patients with severe renal impairment when accurate eGRF is most imperative. Importantly, none of these tests directly measures the extent of renal scarring. Though the exact effects of renal scarring on long-term patient outcomes are unclear, when present with proteinuria, renal scarring is a risk factor for development of ESRD (37).
Renal transplantation
Patients with congenital uropathies who require renal replacement therapy are an especially challenging subpopulation. Often, these patients have large, saccular kidneys that drain poorly and represent a liability for infection after initiation of immunosuppression, necessitating native nephrectomy. They may have limited options for dialysis related to presence of ventriculoperitoneal (VP) shunts or obesity and likewise present with surgical risk factors that impair placement of a graft kidney (38) (Figure 2). Other patients who have undergone transplant may experience recurrent ascending infection to the graft as the pouch or Mitrofanoff channel changes through time (Figure 3).
Figure 3.
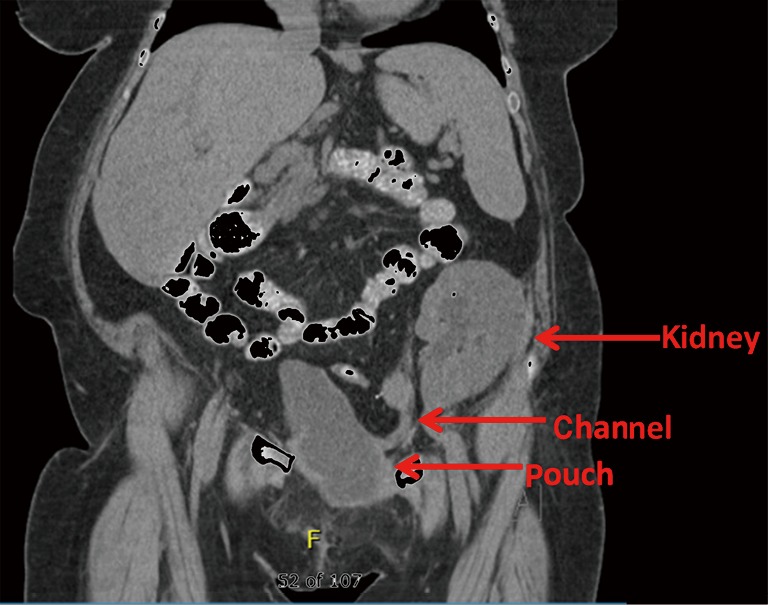
Fifty-two year old with congenital neuropathic bladder, congenital solitary kidney and Turner syndrome status post native nephrectomy and living related renal transplant and Indiana pouch reconstruction.
It is common for patients with PUV or bladder exstrophy to have had prior surgeries which place them at higher surgical risk. In fact, several studies have shown that PUV patients have poorer outcomes after renal transplantation surgery (39,40). Determination about whether a patient is a suitable candidate for transplant poses challenges if the lower urinary tract is dysfunctional. Lower urinary tract problems at the time of transplantation put the patient at higher risk for graft deterioration and possible graft loss. Patients with augmented bladders have demonstrated an increased risk for the development of UTIs after transplantation in comparison to patients with normal bladders (41).
Urinary continence
Incontinence is a common and potentially substantially limiting factor in the lives of patients with congenital neuropathic bladder. Poor continence affects self-esteem, the ability to have a social and sexual life and overall quality of life (42). Even in cases where patients are minimally bothered by leakage, incontinence can cause great disability in the form of skin breakdown and other sequelae. In addition to detrusor overactivity, urinary infections, bladder stones, an incompetent external sphincter and constipation may all promote incontinence in this population. Furthermore, overflow incontinence may be a problem in patients who cannot perform ISC or maintain a care environment where someone can provide this service for them. Among SB patients, continence affects over 50% and is a large source of psychosocial stress and morbidity (5,43).
Depending on a patient’s functional and physical status, he/she may be a candidate for ISC (44). If patients can perform clean intermittent catheterization (CIC) themselves, this mechanism can provide autonomy in maintaining social continence. Anticholinergic medications and botulinum toxin (Botox A®) may provide an adjunct in continence management either alone or in combination with CIC (45,46).
Surgical management of incontinence may be offered when medical therapy fails. Surgical therapy should be targeted to either increase bladder capacity (augmentation cystoplasty or supravesical diversion) or increase outlet resistance (urethral sling, artificial urinary sphincter, bladder neck reconstruction, or urethral suspension). Supravesical diversion with suprapubic tube or ileal conduit are typically reserved for patients with lower hand dexterity or cognitive/self-care abilities, while continent options are more appropriate for higher functioning individuals with adequate hand dexterity and independence in self-care. When a surgical therapy is being considered, urodynamics with or without video assistance may help classify if the leakage is due to detrusor overactivity, outlet incompetence or of mixed etiology (47,48). It is our practice to use video UDS routinely prior to planning operations that increase outlet resistance in this population and repeat studies after surgery to ensure that bladder storage pressures remain acceptable. Studies show that patients having placement of bladder neck sling may be at risk for high pressure storage post-operatively, suggesting that surveillance with UDS is warranted (47).
Urinary tract infections (UTIs)
While incomplete emptying remains the major risk factor for UTI in neuropathic bladder, retained foreign objects (stones) and introduction of pathogens via ISC can also be inciting factors. Urinary infections in these populations can be difficult to diagnose and manage, as patients with limited sensation may report atypical symptoms, including myalgias, headaches, and gastrointestinal upset. Furthermore, there remains no standardized definition of “UTI” in the congenital neuropathic bladder population. Patients with urinary reservoirs or augmentations constructed from gastrointestinal tissue typically experience chronic bacteruria and will have positive urine cultures even when asymptomatic (49). Patients should be coached to identify what physiologic changes typify infection and processes should be developed for prompt management to avoid unnecessary hospitalization and morbidity. Diagnosis and treatment of UTI should be based on both symptoms and presence of bacteria or leukocytes in the urine, along with positive culture (50). In our practice, the advent of electronic messaging through the medical record system has substantially hastened treatment for UTI in this population. Prompt management of infections is essential as pyelonephritis and its sequelae remain the most common cause of hospitalization for patients with neurogenic bladder (11,51).
Urolithiasis
Urinary tract calculi remain a large source of morbidity for patients with congenital neuropathic bladder (52,53). Patients with neurogenic bladder have a 50% incidence of urinary calculi over a 10-year period (52,54,55). Bladder stones can present as recurring UTIs, urinary obstruction, hematuria or pelvic/penile pain. Similarly to UTIs, patients may not experience the typical symptoms of stones if they lack the sensory abilities. Headache, nausea and abdominal pain may be the only complaints a patient might have (56). For these patients, imaging studies can help determine if a stone is the causative agent. It is also important to manage urinary infections in these patients as infection is associated with higher risk of stone recurrence (54). Infection-related stones often manifest as struvite or calcium-struvite stones (53,54). Recurrent infections from urease-producing bacteria (particularly Proteus species) should prompt consideration of bladder stones in this population (Figure 4).
Figure 4.
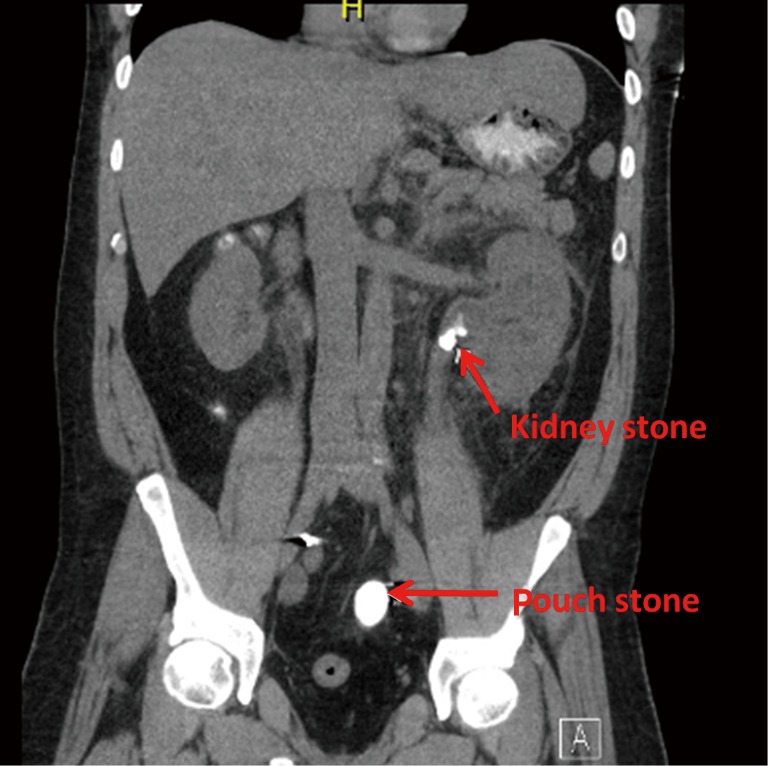
Forty-four year old with bladder exstrophy status post right Indiana pouch urinary diversion who was admitted for urosepsis and noted to have a large stone in the pouch as well as the left kidney. The patient underwent cystolithalopaxy and percutaneous nephrolithotomy.
As with patients without neurogenic bladder, the main strategies to prevent stones typically involve increased fluid intake. When stones are related to infections, the infections should be treated appropriately and focus should be placed on emptying the bladder often and to completion with ISC and bladder irrigation (52,57). If the stone is too large for irrigation, surgical therapy is indicated for bladder calculi. Most often, this can be accomplished endoscopically or percutaneously. A patient’s altered anatomy and body habitus as well as prior surgeries increase the risk of perioperative complications and necessitate altering the surgical approach. When endoscopy is used in a patient with continent channels or prior anti-incontinence therapy, an urologist is well-served to minimize scope caliber and trauma to prevent secondary incontinence post-operatively. Use of extracorporeal shockwave lithotripsy is limited by body habitus and has been shown to be less effective in this patient population, though it may be an option for a minority of patients with other surgical limitations that prevent more conventional approaches (58-60). When stones are large, percutaneous or open lithotomy may be necessary. However, congenital patients undergoing percutaneous lithotomy can have longer hospital admissions and may require additional procedures (61,62).
Bladder cancer
While prospective population based studies do not exist, a case control study by Higuchi et al. suggests that patients with neuropathic bladder with or without augmentation cystoplasty demonstrate elevated risk for bladder cancer approximately 2-fold over the general population (63). Moreover, these patients develop cancer about 25 years prior to the general population and manifest with more lethal disease as compared with patients without neuropathic bladder (64) (Figure 5). Estimates of patients with congenital anomalies of the bladder developing bladder cancer are between 1–2% per decade (63,65). However, screening for cancer in these patients is not sensitive or specific in diagnosis and routine screening has not been shown to reduce mortality (66,67). Even when annual cystoscopy is used, patients have been demonstrated to develop lethal bladder cancer. Thus, annual screening is not currently recommended for these patients who are asymptomatic. Select indications for screening are included in Table 3.
Figure 5.
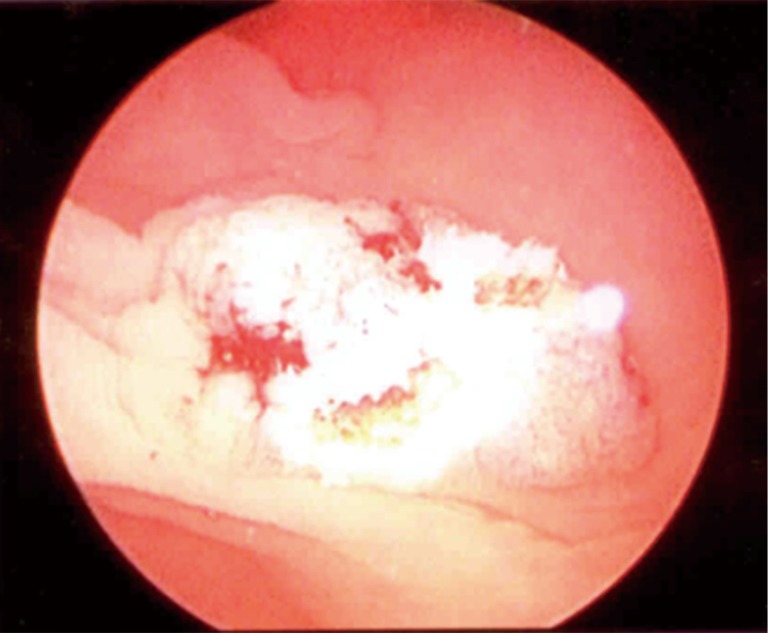
Microscopic view of colonic dysplasia in a colonic urostomy 20 years after cystectomy and neobladder reconstruction.
Table 3. Cancer screening recommendations for patients with neurogenic bladders.
| Diversion | Recommendation |
|---|---|
| All neurogenic bladder | Cystoscopy or upper tract investigation for episodes of hematuria |
| Colon-containing reservoir | Cystoscopy after age 50 and then every 2–3 years |
| Renal transplant with a history of BK virus | Cystoscopy at 10 years, then yearly |
| Exstrophy with prior ureterosigmoidostomy | Annual colonoscopy |
| Gastric augmentation | Biopsy of augment at 10 years at the gastric-bladder junction |
Abdominal herniae
Abdominal herniae, particularly in wheelchair-bound patients with SB, are common. However, there remains a dearth of literature on the topic. Risk factors for herniae in this population include prior abdominal explorations, obesity, suboptimal nutrition, chronic constipation, and weak abdominal musculature. Herniae are typically incisional or parastomal. Incisional herniae may secondarily fistulize, whereas the latter may present with poor fit of the stoma appliance or hydronephrosis and renal failure (Figure 6). We routinely employ consultation with a herniologist in these repairs, as complications and recurrence are high even in the best of hands.
Figure 6.
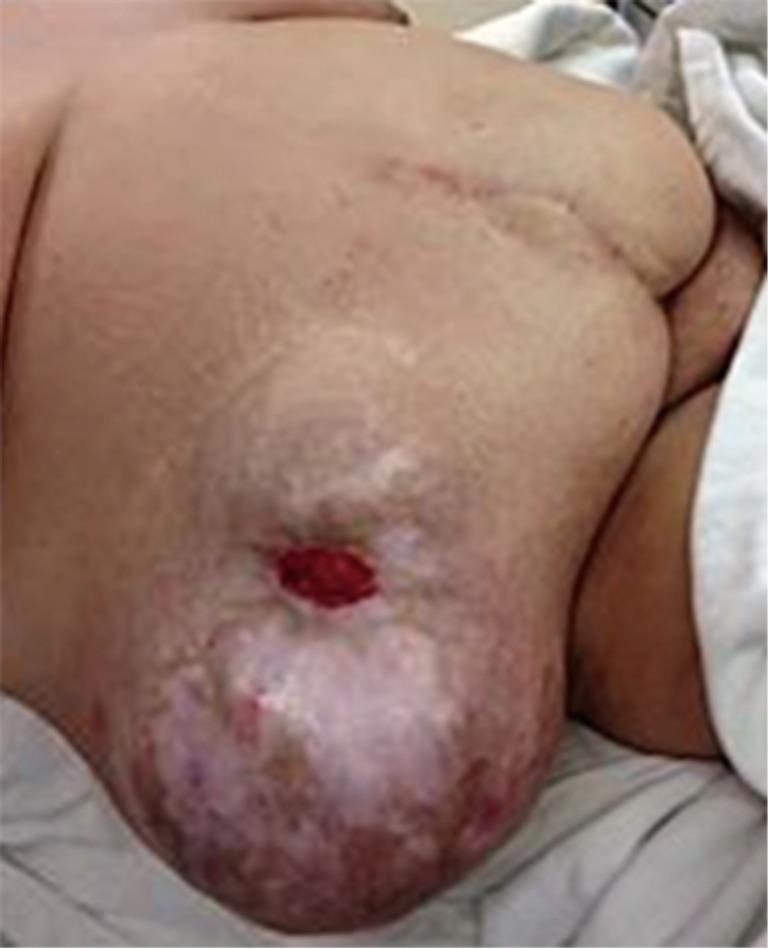
Massive parastomal hernia in a patient with inability to pouch her urostomy.
Stomal complications
Stomal retraction (in conduits) and stenosis (in Mitrofanoff or conduits) can also be problematic and may impair application of an appliance and/or compliance with irrigation and drainage of reservoirs, leading to secondary stone formation. The cause of stomal stenosis in ileal conduits is usually due to necrosis from tension or compression of the mesentery to the segment, which is more likely in obese patients. Many patients present with parastomal skin ulceration and trouble with securing an appliance, which can lead to frequent leaks and issues with skin breakdown and ulcers (Figure 7). Hydronephrosis, recurrent UTI, stones, and/or renal failure also may occur with stomal stenosis. Loopogram may help sort out the location of obstruction in a case of hydronephrosis, as the uretero-ileal anastomoses may also contribute to obstruction. Operative repair includes dissection of the conduit and release from the abdominal wall with amputation of the distal stenotic segment and advancement of the conduit. If there is not enough length to get a tension-free anastomosis with advancement, an alternative stoma site can be identified or an “add on” segment of small bowel with its own mesentery can be selected and attached. The final option would be complete revision with a new limb of bowel.
Figure 7.
Stomal stenosis. (A) Stomal stenosis with poor appliance fit and skin excoriation after ileal conduit; (B) stomal stenosis after appendicovesicostomy leading to stones in the pouch.
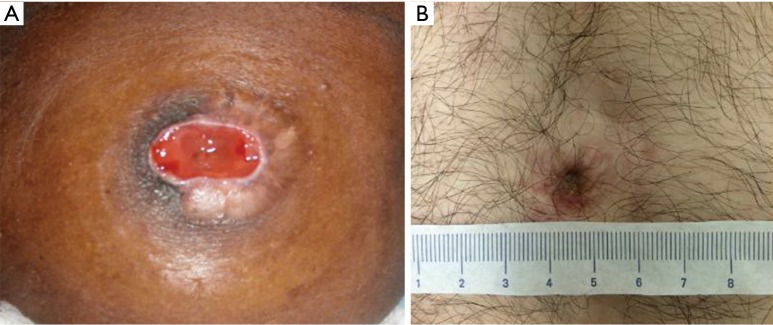
Mitrofanoff complications
The Mitrofanoff principle was initially described in 1980 as an alternative method of draining the bladder via intermittent catheterization (68). Many substrates, including ureter, fallopian tube, appendix, bladder tube, stomach tube, and plicated large and small bowel have been described. Among adult reconstructionists, plicated terminal ileum (in the fashion of Indiana) is most common, while reconfigured ileum (Yang-Monti) or appendix is favored in adult patients with congenital disorders. Surgical revision is estimated to be required in at least 40% of patients undergoing Mitrofanoff (6-year follow-up) (69). Long-term (decades or more) studies are lacking, but our experience is that patients can encounter stenosis or leakage many years after initial surgery. Anecdotally, stenosis is more common than de-novo incontinence in this group. A thorough review of the topic is provided by Farrugia and Malone (70).
Options to treat stomal stenosis of a Mitrofanoff include topical application of steroid ointment, use of an “L” stent for nighttime dilation, local excision of the scarred area and stomal advancement, or distal reconstruction with buccal graft (71,72). All of these require an outpatient operation at worst. However, long-segment stenosis that involves the intrafascial component or nipple valve may require exploratory laparotomy and replacement or relocation of the stoma.
Long-term rates of incontinence from Mitrofanoff channels are unknown. One report stated a 90% continence rate at 10.5 years after surgery (73). New incontinence in a patient previously dry for many years after treatment should prompt a thorough investigation of the bladder, including assessment for foreign object or tumor that may incite instability, pouch/bladder contraction or decreased compliance related to progression of neuropathic insult. If a thorough history does not corroborate a clear reason for the incontinence, urodynamics demonstrating visual leakage with stress from the stoma suggests that the stoma itself is the culprit. For new leakage where the urinary reservoir is proven to be responsible, treatment should obviously be targeted towards revising the pouch (tubularizing, enlarging or reducing). If the evaluation supports stress incontinence related to the channel itself, one can consider use of dextranomer/hyaluronic acid injection as a first step. While reports in the literature about the efficacy of this approach are quite limited, our experience has not been durable and it may burn bridges for salvage of the Mitrofanoff (Figure 8). Guidelines about how much and where the bulking agent should be injected are lacking. After failure, revision with re-tunneling the channel or replacement is the best option.
Figure 8.
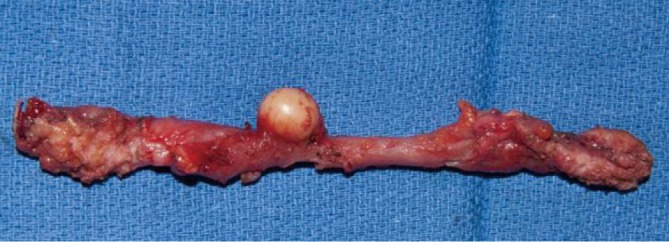
Mitrofanoff after injection with Deflux® that had several peri-stomal granulomas (only one shown) and necessitated removal and replacement of the channel).
Conclusions
Congenital urologic disease encompasses a heterogeneous population of patients who require special urological expertise. They are often lost to follow-up in the medical system after discontinuing care with their pediatric care provider(s). Appropriate transition of these individuals to adult-centered care is critical as these patients mature into adulthood to prevent unnecessary interruption of care and morbidity. In adulthood, the urologic care team should focus on setting realistic patient goals that are sustainable within the patient’s physiologic and anatomic limitations. Active investigation of new problems and monitoring of bladder and urinary changes are the hallmarks of long-term care for these patients.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Home—Spina Bifida Association. Cited Oct 9, 2015. Available online: http://spinabifidaassociation.org/
- 2.American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine . A consensus statement on health care transitions for young adults with special health care needs. Pediatrics 2002;110:1304-6. [PubMed] [Google Scholar]
- 3.GotTransition.org. Cited Jul 27, 2015. Available online: http://www.gottransition.org/
- 4.Cox A, Breau L, Connor L, et al. Transition of care to an adult spina bifida clinic: patient perspectives and medical outcomes. J Urol 2011;186:1590-4. [DOI] [PubMed] [Google Scholar]
- 5.Summers SJ, Elliott S, McAdams S, et al. Urologic problems in spina bifida patients transitioning to adult care. Urology 2014;84:440-4. [DOI] [PubMed] [Google Scholar]
- 6.Binks JA, Barden WS, Burke TA, et al. What do we really know about the transition to adult-centered health care? A focus on cerebral palsy and spina bifida. Arch Phys Med Rehabil 2007;88:1064-73. [DOI] [PubMed] [Google Scholar]
- 7.McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics 2013;131:1090-7. [DOI] [PubMed] [Google Scholar]
- 8.Lotstein DS, Ghandour R, Cash A, et al. Planning for health care transitions: results from the 2005-2006 National Survey of Children With Special Health Care Needs. Pediatrics 2009;123:e145-52. [DOI] [PubMed] [Google Scholar]
- 9.Young NL, Steele C, Fehlings D, et al. Use of health care among adults with chronic and complex physical disabilities of childhood. Disabil Rehabil 2005;27:1455-60. [DOI] [PubMed] [Google Scholar]
- 10.Exner G, Burgdörfer H, Bohatyrewicz A, et al. The lack of comprehensive care causing complications in patients with myelodysplasia. Paraplegia 1993;31:102-4. [DOI] [PubMed] [Google Scholar]
- 11.Dicianno BE, Wilson R. Hospitalizations of adults with spina bifida and congenital spinal cord anomalies. Arch Phys Med Rehabil 2010;91:529-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinsman SL, Doehring MC. The cost of preventable conditions in adults with spina bifida. Eur J Pediatr Surg 1996;6 Suppl 1:17-20. [DOI] [PubMed] [Google Scholar]
- 13.Wood HM, Yerkes EB. The Transition Process: Initial Assessment and Development of a Treatment Plan. In: Wood HM, Wood D, editors. Transition and Lifelong Care in Congenital Urology. Springer International Publishing, 2015:3-10. [Google Scholar]
- 14.Sawyer SM, Macnee S. Transition to adult health care for adolescents with spina bifida: research issues. Dev Disabil Res Rev 2010;16:60-5. [DOI] [PubMed] [Google Scholar]
- 15.Stöhrer M, Blok B, Castro-Diaz D, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol 2009;56:81-8. [DOI] [PubMed] [Google Scholar]
- 16.Lawrenson R, Wyndaele JJ, Vlachonikolis I, et al. Renal failure in patients with neurogenic lower urinary tract dysfunction. Neuroepidemiology 2001;20:138-43. [DOI] [PubMed] [Google Scholar]
- 17.McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol 1981;126:205-9. [DOI] [PubMed] [Google Scholar]
- 18.Woodhouse CR, Neild GH, Yu RN, et al. Adult care of children from pediatric urology. J Urol 2012;187:1164-71. [DOI] [PubMed] [Google Scholar]
- 19.Vainrib M, Reyblat P, Ginsberg DA. Differences in urodynamic study variables in adult patients with neurogenic bladder and myelomeningocele before and after augmentation enterocystoplasty. Neurourol Urodyn 2013;32:250-3. [DOI] [PubMed] [Google Scholar]
- 20.Chevalier RL, Thornhill BA, Forbes MS, et al. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol 2010;25:687-97. [DOI] [PubMed] [Google Scholar]
- 21.Lemelle JL, Guillemin F, Aubert D, et al. A multicenter evaluation of urinary incontinence management and outcome in spina bifida. J Urol 2006;175:208-12. [DOI] [PubMed] [Google Scholar]
- 22.Veenboer PW, de Kort LM, Chrzan RJ, et al. Urinary considerations for adult patients with spinal dysraphism. Nat Rev Urol 2015;12:331-9. [DOI] [PubMed] [Google Scholar]
- 23.Close CE, Carr MC, Burns MW, et al. Lower urinary tract changes after early valve ablation in neonates and infants: is early diversion warranted? J Urol 1997;157:984-8. [PubMed] [Google Scholar]
- 24.Parkhouse HF, Barratt TM, Dillon MJ, et al. Long-term outcome of boys with posterior urethral valves. Br J Urol 1988;62:59-62. [DOI] [PubMed] [Google Scholar]
- 25.King T, Coleman R, Parashar K. Mitrofanoff for valve bladder syndrome: effect on urinary tract and renal function. J Urol 2014;191:1517-22. [DOI] [PubMed] [Google Scholar]
- 26.Kousidis G, Thomas DF, Morgan H, et al. The long-term outcome of prenatally detected posterior urethral valves: a 10 to 23-year follow-up study. BJU Int 2008;102:1020-4. [DOI] [PubMed] [Google Scholar]
- 27.Lal R, Bhatnagar V, Agarwala S, et al. Urodynamic evaluation in boys treated for posterior urethral valves. Pediatr Surg Int 1999;15:358-62. [DOI] [PubMed] [Google Scholar]
- 28.Woodhouse CR, Ransley PG, Williams DI. The patient with exstrophy in adult life. Br J Urol 1983;55:632-5. [DOI] [PubMed] [Google Scholar]
- 29.Mesrobian HG, Kelalis PP, Kramer SA. Long-term followup of 103 patients with bladder exstrophy. J Urol 1988;139:719-22. [DOI] [PubMed] [Google Scholar]
- 30.Ebert AK, Schott G, Bals-Pratsch M, et al. Long-term follow-up of male patients after reconstruction of the bladder-exstrophy-epispadias complex: psychosocial status, continence, renal and genital function. J Pediatr Urol 2010;6:6-10. [DOI] [PubMed] [Google Scholar]
- 31.Hogg RJ, Furth S, Lemley KV, et al. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics 2003;111:1416-21. [DOI] [PubMed] [Google Scholar]
- 32.Dosa NP, Foley JT, Eckrich M, et al. Obesity across the lifespan among persons with spina bifida. Disabil Rehabil 2009;31:914-20. [DOI] [PubMed] [Google Scholar]
- 33.Quan A, Adams R, Ekmark E, et al. Serum creatinine is a poor marker of glomerular filtration rate in patients with spina bifida. Dev Med Child Neurol 1997;39:808-10. [DOI] [PubMed] [Google Scholar]
- 34.Seikaly MG, Browne R, Bajaj G, et al. Limitations to body length/serum creatinine ratio as an estimate of glomerular filtration in children. Pediatr Nephrol 1996;10:709-11. [DOI] [PubMed] [Google Scholar]
- 35.Fox JA, Dudley AG, Bates C, et al. Cystatin C as a marker of early renal insufficiency in children with congenital neuropathic bladder. J Urol 2014;191:1602-7. [DOI] [PubMed] [Google Scholar]
- 36.Shlipak MG, Matsushita K, Ärnlöv J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013;369:932-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jungers P, Houillier P, Chauveau D, et al. Pregnancy in women with reflux nephropathy. Kidney Int 1996;50:593-9. [DOI] [PubMed] [Google Scholar]
- 38.Power RE, O'Malley KJ, Little DM, et al. Long-term followup of cadaveric renal transplantation in patients with spina bifida. J Urol 2002;167:477-9. [DOI] [PubMed] [Google Scholar]
- 39.Reinberg Y, Gonzalez R, Fryd D, et al. The outcome of renal transplantation in children with posterior urethral valves. J Urol 1988;140:1491-3. [DOI] [PubMed] [Google Scholar]
- 40.Churchill BM, Sheldon CA, McLorie GA, et al. Factors influencing patient and graft survival in 300 cadaveric pediatric renal transplants. J Urol 1988;140:1129-33. [DOI] [PubMed] [Google Scholar]
- 41.Rigamonti W, Capizzi A, Zacchello G, et al. Kidney transplantation into bladder augmentation or urinary diversion: long-term results. Transplantation 2005;80:1435-40. [DOI] [PubMed] [Google Scholar]
- 42.Thorup J, Biering-Sorensen F, Cortes D. Urological outcome after myelomeningocele: 20 years of follow-up. BJU Int 2011;107:994-9. [DOI] [PubMed] [Google Scholar]
- 43.Verhoef M, Lurvink M, Barf HA, et al. High prevalence of incontinence among young adults with spina bifida: description, prediction and problem perception. Spinal Cord 2005;43:331-40. [DOI] [PubMed] [Google Scholar]
- 44.Bowman RM, McLone DG, Grant JA, et al. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg 2001;34:114-20. [DOI] [PubMed] [Google Scholar]
- 45.Verpoorten C, Buyse GM. The neurogenic bladder: medical treatment. Pediatr Nephrol 2008;23:717-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan R, Scovell J, Jeng Z, et al. The fate of transitional urology patients referred to a tertiary transitional care center. Urology 2014;84:1544-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snodgrass W, Barber T, Cost N. Detrusor compliance changes after bladder neck sling without augmentation in children with neurogenic urinary incontinence. J Urol 2010;183:2361-6. [DOI] [PubMed] [Google Scholar]
- 48.López Pereira P, Somoza Ariba I, Martínez Urrutia MJ, et al. Artificial urinary sphincter: 11-year experience in adolescents with congenital neuropathic bladder. Eur Urol 2006;50:1096-101; discussion 1101. [DOI] [PubMed] [Google Scholar]
- 49.Casey JT, Patel R, Wallner LP, et al. Infectious complications in patients with chronic bacteriuria undergoing major urologic surgery. Urology 2010;75:77-82. [DOI] [PubMed] [Google Scholar]
- 50.Sauerwein D. Urinary tract infection in patients with neurogenic bladder dysfunction. Int J Antimicrob Agents 2002;19:592-7. [DOI] [PubMed] [Google Scholar]
- 51.Manack A, Motsko SP, Haag-Molkenteller C, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urody 2011;30:395-401. [DOI] [PubMed] [Google Scholar]
- 52.Veenboer PW, Ruud Bosch JL, van Asbeck FW, et al. Urolithiasis in adult spina bifida patients: study in 260 patients and discussion of the literature. Int Urol Nephrol 2013;45:695-702. [DOI] [PubMed] [Google Scholar]
- 53.Raj GV, Bennett RT, Preminger GM, et al. The incidence of nephrolithiasis in patients with spinal neural tube defects. J Urol 1999;162:1238-42. [DOI] [PubMed] [Google Scholar]
- 54.Silver RI, Gros DA, Jeffs RD, et al. Urolithiasis in the exstrophy-epispadias complex. J Urol 1997;158:1322-6. [DOI] [PubMed] [Google Scholar]
- 55.Palmer LS, Franco I, Kogan SJ, et al. Urolithiasis in children following augmentation cystoplasty. J Urol 1993;150:726-9. [DOI] [PubMed] [Google Scholar]
- 56.Worley G, Wiener JS, George TM, et al. Acute abdominal symptoms and signs in children and young adults with spina bifida: ten years' experience. J Pediatr Surg 2001;36:1381-6. [DOI] [PubMed] [Google Scholar]
- 57.Clayman RV. Preventing reservoir calculi after augmentation cystoplasty and continent urinary diversion: the influence of an irrigation protocol. J Urol 2005;173:866-7. [DOI] [PubMed] [Google Scholar]
- 58.Deliveliotis C, Picramenos D, Kostakopoulos A, et al. Extracorporeal shock wave lithotripsy in paraplegic and quadriplegic patients. Int Urol Nephrol 1994;26:151-4. [DOI] [PubMed] [Google Scholar]
- 59.Niedrach WL, Davis RS, Tonetti FW, et al. Extracorporeal shock-wave lithotripsy in patients with spinal cord dysfunction. Urology 1991;38:152-6. [DOI] [PubMed] [Google Scholar]
- 60.Ramsey S, McIlhenny C. Evidence-based management of upper tract urolithiasis in the spinal cord-injured patient. Spinal Cord 2011;49:948-54. [DOI] [PubMed] [Google Scholar]
- 61.Alsinnawi M, Torreggiani WC, Flynn R, et al. Percutaneous nephrolithotomy in adult patients with spina bifida, severe spinal deformity and large renal stones. Ir J Med Sci 2013;182:357-61. [DOI] [PubMed] [Google Scholar]
- 62.Nabbout P, Slobodov G, Mellis AM, et al. Percutaneous nephrolithotomy in spinal cord neuropathy patients: a single institution experience. J Endourol 2012;26:1610-3. [DOI] [PubMed] [Google Scholar]
- 63.Higuchi TT, Granberg CF, Fox JA, et al. Augmentation cystoplasty and risk of neoplasia: fact, fiction and controversy. J Urol 2010;184:2492-6. [DOI] [PubMed] [Google Scholar]
- 64.Sung MT, Zhang S, Lopez-Beltran A, et al. Urothelial carcinoma following augmentation cystoplasty: an aggressive variant with distinct clinicopathological characteristics and molecular genetic alterations. Histopathology 2009;55:161-73. [DOI] [PubMed] [Google Scholar]
- 65.Austin JC, Elliott S, Cooper CS. Patients with spina bifida and bladder cancer: atypical presentation, advanced stage and poor survival. J Urol 2007;178:798-801. [DOI] [PubMed] [Google Scholar]
- 66.Higuchi TT, Fox JA, Husmann DA. Annual endoscopy and urine cytology for the surveillance of bladder tumors after enterocystoplasty for congenital bladder anomalies. J Urol 2011;186:1791-5. [DOI] [PubMed] [Google Scholar]
- 67.Castellan M, Gosalbez R, Perez-Brayfield M, et al. Tumor in bladder reservoir after gastrocystoplasty. J Urol 2007;178:1771-4; discussion 1774. [DOI] [PubMed]
- 68.Mitrofanoff P. Trans-appendicular continent cystostomy in the management of the neurogenic bladder. Chir Pediatr 1980;21:297-305. [PubMed] [Google Scholar]
- 69.Leslie B, Lorenzo AJ, Moore K, et al. Long-term followup and time to event outcome analysis of continent catheterizable channels. J Urol 2011;185:2298-302. [DOI] [PubMed] [Google Scholar]
- 70.Farrugia MK, Malone PS. Educational article: The Mitrofanoff procedure. J Pediatr Urol 2010;6:330-7. [DOI] [PubMed] [Google Scholar]
- 71.Radojicic ZI, Perovic SV, Stojanoski KD. Calibration and dilatation with topical corticosteroid in the treatment of stenosis of neourethral meatus after hypospadias repair. BJU Int 2006;97:166-8. [DOI] [PubMed] [Google Scholar]
- 72.Radojicic ZI, Perovic SV, Rados DP, et al. Buccal mucosa grafts for repair of stenotic catheterizable continent stoma. J Urol 2008;180:1767-9. [DOI] [PubMed] [Google Scholar]
- 73.Sahadevan K, Pickard RS, Neal DE, et al. Is continent diversion using the Mitrofanoff principle a viable long-term option for adults requiring bladder replacement? BJU Int 2008;102:236-40. [DOI] [PubMed] [Google Scholar]