Abstract
Castleman disease (CD) is a chronic lymphoproliferative disorder characterized by unexplained enlarged lymph nodes. According to lymph nodes distribution it contains two types of single-centric and multicentric (more than one site) disease. Multicentric Castleman disease (MCD) is rare, and shows unspecific manifestation with high misdiagnosis rate. Here we reported a case of MCD in a 43-year-old male. 18F-FDG PET/CT imaging demonstrated higher FDG uptake in multiple lymph nodes and slightly FDG uptake in spleen and bone marrow. Right inguinal Lymph node biopsy was taken and the results confirmed CD.
Keywords: Multicentric Castleman disease (MCD), 18F-FDG PET/CT, treatment evaluation, differential diagnosis
Introduction
Castleman disease (CD) is a rare chronic lymphoproliferative disorder characterized by unexplained enlarged lymph nodes that was first described by Benjamin Castleman in 1956 (1). It is a non-caseous lymphoid proliferation and also known as angiofollicular lymphoid hyperplasia, giant lymph node hyperplasia or angiomatous lymphoid hamartoma. According to the distribution of lymph nodes two types were defined as unicentric CD and multicentric Castleman disease (MCD) (more than one site) which was reported first in 1978 by Gaba et al. (2). Pathologically there are four subtypes of CD including hyaline-vascular CD, plasma cell CD, HHV-8-associated (plasmablastic) MCD and MCD, not otherwise specified (NOS) (3). The diagnosis of MCD is very difficult on clinical due to lower incidence and unspecific clinical manifestations. Until now few studies investigated the role of 18F-FDG PET/CT in CD. In this study we analyzed MCD metabolic characteristics of one patient in our hospital and reviewed some relevant literature in order to strengthen the understanding of MCD and improve the diagnosis of it. The institutional review board of our hospital approved this study and waived the requirement for obtaining informed consent.
Case presentation
A 43-year-old male was seen in clinic for two years history of recurrent fever (37.6–38.5 centigrade, irregular), aggravated gradually anemia and decreased appetite and loss of weight for 10 kg in 2 years. Hormone and immunosuppressant treatments and empirical antituberculosis therapy (10 days) were all ineffective. Initial physical examination revealed anemia appearance and a coin size brown ecchymosis in chest as well as in the middle of back with no surface bulging and tender. A bean size, hard, movable and nontender lymph node was also noted in left neck. The other regional lymph nodes were non-palpable. Initial laboratory values included rheumatoid factor: 38 IU/mL (95% CI, 0–15.9), antistreptolysin O: 283 IU/mL (95% CI, 0–200), C-reactive protein (CRP): 111 mg/L (95% CI, 0–200), ferritin: 725 µg/L (95% CI, 20–200), IgG: 48.1 g/L (95% CI, 7.0–16.0), IgM: 2.87 g/L (95% CI, 0.4–2.3), IgA: 5.49 g/L (95% CI, 0.7–4.0), blood beta 2 microglobulin: 6.42 mg/L (95% CI, 1.09–2.53), Coombs test (+), and T-SPORT (+). TB, viral infection, thyroid function and tumor marker were normal.
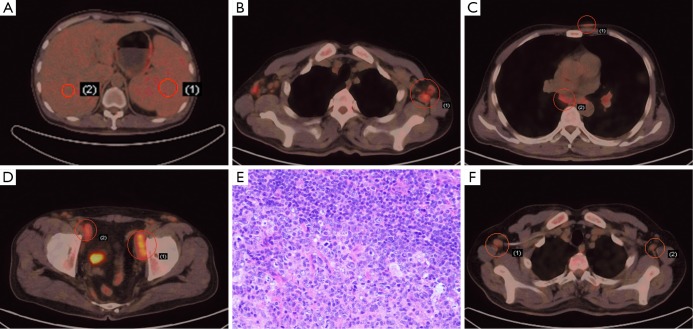
18F-FDG PET/CT imaging demonstrated an enlarged spleen with little higher FDG uptake (SUVmax =2.6) compared to liver (SUVmax =1.8), and this slight uptake was homogeneous which should be caused by anemia rather than tumors such as lymphoma. Some enlarged lymph nodes were noted in bilateral neck, axilla, mediastinum, hila of lung, pelvic walls and inguinal area, while others were near abdominal aorta and bilateral iliac artery. The diameter of most lymph nodes was less than 2 cm and the SUVmax was less than 5.0; the SUVmax of one largest nodule measuring 4.6 cm × 1.5 cm was 5.2. 18F-FDG PET/CT also showed multiple subcutaneous nodules in anterior chest wall, chest and back (Figure 1A-D). Multiple scattered plaque shadows and nodules were found in inferior lobes of lung and the FDG uptake was mildly higher (SUVmax =1.2) than the normal section (SUVmax =0.7). Moreover, the bone marrow showed slight FDG metabolism (SUVmax =3.1) which was considered to be normal.
Figure 1.
18F-FDG PET/CT and histology findings of MCD in a 43-year-old male. (A) 18F-FDG PET/CT demonstrated slight FDG metabolism of spleen and liver. Glucose uptake is little higher in spleen (SUVmax =2.3, right) than that of liver (SUVmax =2.0, left); (B) 18F-FDG PET/CT showed increased FDG metabolism of lymph nodes in axilla (SUVmax =3.9); (C) 18F-FDG PET/CT revealed subcutaneous nodules and lymph nodes in mediastinum with higher glucose uptake (SUVmax =1.8, upper; SUVmax =3.0, lower); (D) enlarged lymph nodes on both sides of the pelvic wall on 18F-FDG PET/CT (SUVmax =5.2, right; SUVmax =3.7, left); (E) the histological results of the right inguinal lymph nodule biopsy (HE, ×200); (F) following 3 cycles of R-CHOP regimen, 18F-FDG PET/CT showed shrunken lymph nodes in both axillas and decreased FDG metabolism (SUVmax =2.5).
Further bone marrow biopsy revealed active proliferation of granulocyte, especially for erythrocyte and megakaryocyte, and cluster platelet was found. No specific evidence was confirmed. Biopsies of right inguinal lymph nodes were collected. The histological results demonstrated that these proliferated lymph tissues were stained CD20 (++), PaX-5 (+), CD3 (++) and CD43 (++), and the germinal centers were Ki-67 (+) and CD21 (+), both of which indicated the diagnosis of MCD (plasma cell type) combining autoimmune hemolytic anemia (Figure 1E).
R-CHOP regimen (combined chemotherapy with Rituxan 700 mg d0, epirubicin 150 mg d1, cyclophosphamide 1.4 g d1, vindesine 5 mg d1 and hydroprednisone 60 mg bid d1-5) was administered for 3 cycles. 18F-FDG PET/CT was then reperformed and showed remarkably decline in lymph nodes diameter and FDG metabolism (SUVmax of one axillary lymph node was from 3.9 to 2.5). Although the sizes of some small lymph nodes remained unchanged but their FDG uptake disappeared, which indicated optimistic therapeutic effects (Figure 1F).
Discussion
MCD patients often suffer from systemic symptoms such as fever, fatigue, weight loss, anemia, elevated erythrocyte sedimentation rate (ESR), hypoalbuminemia and so on. Increased serum polyclonal immunoglobulin may be found in Laboratory test, and in some cases IgM appears. Some studies reported that patients who underwent bone marrow examination had increased plasma cells number from 2–20% with normal morphology. When these nonspecific physical symptoms and indications appear on one patient it’s very difficult to make an accurate diagnosis timely.
Compared to conventional CT, PET/CT examination can get two types of information: morphological image and metabolic function. Lee et al. (4) reported that the FDG uptake was well correlated with disease multicentricity and clinical manifestation, suggesting that it would be a significant imaging marker for severity or prognosis of CD. By analyzing PET/CT data from before and after treatment, we found not only the size changes of the lesions but also their glucose uptake status was crucial for treatment evaluation and clinical decision-making. The 2012 National Comprehensive Cancer Network (NCCN) guidelines (5) suggest PET/CT result as an evaluation criterion of lymphoma. In this case, 18F-FDG PET/CT reexamination after 3-cycles treatment demonstrated diminished glucose uptake and good outcome in spite of unchanged lesion size in part, which suggest PET/CT examination might be better than CT during MCD treatment.
A differential diagnosis should be taken into account between MCD and lymphoma, lymph node tuberculosis, Wegener’s granulomatosis, sarcoidosis or multiple myeloma. MCD shares some imaging features with lymphoma, including multisite enlarged lymph nodes and a swollen spleen. But the average SUVmax of lymphoma is often much higher, indicating higher degree of malignancy. Moreover, in the spleen of this case, both slightly increased SUVmax before treatment and no change after chemotherapy represented reactive hyperplasia due to anemia, unlike increasing uptake caused by lymphoma infiltration (6). There are, however, some difficulties in distinguishing MCD from tuberculosis by PET/CT, especially lymph node tuberculosis during proliferative phase without caseous necrosis (7). On the other hand, laboratory test results always provide valuable information. For example, cytoplasmic antineutrophil cytoplasmic antibodies (cANCA) are usually seen in Wegener’s granulomatosis which is the specific antibody of the disease. PET/CT image analysis combining with laboratory tests maybe improve diagnostic efficiency and accuracy of MCD.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Castleman B, Iversonl L, Menendez VP, et al. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer 1956;9:822-30. [DOI] [PubMed] [Google Scholar]
- 2.Gaba AR, Stein RS, Sweet DL, et al. Multicentric giant lymph node hyperplasia. Am J Clin Pathol 1978;69:86-90. [DOI] [PubMed] [Google Scholar]
- 3.Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol 2009;16:236-46. [DOI] [PubMed] [Google Scholar]
- 4.Lee ES, Paeng JC, Park CM, et al. Metabolic characteristics of Castleman disease on 18F-FDG PET in relation to clinical implication. Clin Nucl Med 2013;38:339-42. [DOI] [PubMed] [Google Scholar]
- 5.Zelenetz AD, Wierda WG, Abramson JS, et al. Non-Hodgkin's Lymphomas, version 3.2012. J Natl Compr Canc Netw 2012;10:1487-98. [DOI] [PubMed] [Google Scholar]
- 6.Hill AJ, Tirumani SH, Rosenthal MH, et al. Multimodality imaging and clinical features in Castleman disease: single institute experience in 30 patients. Br J Radiol 2015;88:20140670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alan Selçuk N, Fenercioğlu A, Selçuk HH, et al. Multifoci bone tuberculosis and lymphadenitis in mediastinum mimics malignancy on FDG-PET/CT: a case report. Mol Imaging Radionucl Ther 2014;23:39-42. [DOI] [PMC free article] [PubMed] [Google Scholar]