Abstract
Myasthenia gravis (MG) is an autoimmune neuromuscular disease characterized by the presence of antibodies interacting at the neuromuscular junction (NMJ), resulting in loss of strength and severe exhaustibility of striated muscles. The abnormal production of these antibodies is triggered mainly in the thymus, and hence thymectomy in MG is considered a universally recommended treatment in order to improve the symptomatologic condition of this pathology. Currently, minimally invasive thymectomy using the Da Vinci robot system is certainly one of the most innovative techniques, performed in Pisa since 2001. This approach provides a valuable alternative to the traditional thymectomy through median sternotomy. The contribution of a neurologist is fundamental for preoperative patient selection and for the peri-operative clinical assistance in both approaches. We believe that in the robotic approach, the multidisciplinary collaboration between the neurologist, thoracic surgeon and anesthetist is important in reducing perioperative complications and ensuring a higher rate of complete remission or stable clinical improvement of MG.
Keywords: Myasthenia gravis (MG), acetylcholine receptor antibodies (AchRAb), thymic hyperplasia, thymoma, robotic thymectomy
Introduction
Myasthenia gravis (MG) is a neuromuscular disease characterized in most of the cases by the presence of antibodies interacting at the neuromuscular junction (NMJ) that results in loss of strength and severe exhaustibility of striated muscles which can even reach a point of impairment of vital functions (1).
The abnormal antibody production is triggered mainly in the thymus. In these categories of patients, the thymus presents morphological abnormalities as: hyperplasia (65% cases), tumors (thymoma) in 10−15% or atrophy (1−2%).
Thymectomy in MG is a universally recommended procedure, especially in MG with positive anti-acetylcholine receptor antibodies (AchRAb) (2,3) with the intent to improve symptoms and, in many cases, to achieve the complete remission of MG (4).
Our centre has dedicated a particular interest in MG and thymic-related pathologies, and has performed 730 thymectomies in patients with MG since 1990.
This great case volume necessitated development of a dedicated multidisciplinary team with collaboration between neurologists, thoracic surgeons and anesthetists (5).
We believe that this multidisciplinary approach is paramount in reducing perioperative complications, as well as optimizing the possibility of complete remission or a stable clinical improvement of the patient’s condition.
Minimally-invasive thymectomy, using the da Vinci robot system, is one of the most innovative approaches for this type of operation. Despite of the fact that this technique was adopted since 2001 in Pisa for the treatment of lung tumors, its application for the treatment of thymic disease was only initiated in 2011, due to the excellent results achieved with thymectomy via sternotomy approach and also because an aesthetic skin access did not seem to justify the use of the da Vinci system.
Since 2011, 66 patients have undergone robotic thymectomy in our center. Within this group, our experience in 36 patients with MG suggests that thymectomy was a valid alternative for the treatment of their disease (6). The same team of the neurologist, thoracic surgeon and anesthetist who took care of thymectomy patients through the median sternotomy approach also managed patients through this minimally invasive approach (5) (Figures 1,2).
Figure 1.
Setting of the operating theatre for robotic surgery in myasthenia gravis patient with the multidisciplinary team in Pisa (in Figure 1A, from left to right neurologist, anesthetist, thoracic surgeon and collaborators).
Figure 2.
Operating theatre for robotic surgery during a thymectomy case in Pisa.
MG and thymic pathology: therapy
MG is an autoimmune neurological disease of multifactorial etiology in which genetic predisposition plays an important role (7,8). Furthermore, specific genetic polymorphisms has been linked to the incidence of thymoma in MG patients (9).
Knowledge of the pathogenesis of MG has enabled the development of different therapeutic approaches on a symptomatic and etiologic basis.
Symptomatic treatment through anticholinesterase drugs, mainly pyridostigmine bromide, is the most traditional therapy of the disease (10), and also the most commonly used as the initial approach (11). These are generally administered orally in chronic MG, but can be administered via intramuscular or intravenous, in cases of emergency or in the immediate postoperative phase. Other alternative ways of rapid administration, such as intranasally, have been studied (12).
The anticholinesterase drugs are efficacious in those MG forms with positive anti-acetylcholine receptors (AChR) antibodies, but are rarely efficacious and are responsible for the important cholinergic side effects in the seronegative forms and even more so in those MG patients with positive Anti-MuSK antibodies (13,14).
The use of anticholinesterases is also limited in that they are purely used for symptomatic relief and in order to act, need some residual function of the NMJ. With disease progression, and the reduction of the number of AChR, anticholinesterase therapy alone can become insufficient to control the myasthenic symptoms.
Once this occurs, it becomes necessary to use the etiologic treatments that act upon the pathologic autoimmune mechanisms responsible for the disease, particularly corticosteroids, which, according to our experience, often represent the immediate choice after the use of anticholinesterase drugs. Corticosteroids have been used since 1970 to treat MG (15), as they have the advantage to act rapidly and effectively in most forms of the disease. When steroid treatment is not sufficiently effective or when it is contraindicated, a number of cytotoxic immunosuppressive drugs, monoclonal antibodies, intravenous immunoglobulins (IVIG), plasma exchange and thymectomy are used. The use of IVIG in treating MG dates back to 1984 (16-19), and in our centre, this sort of treatment is considered a better option than the plasma exchange in all those cases that require rapid improvement of the patient’s clinical condition (17,19).
In the overarching management of these patients, thymectomy is the only strategic intervention capable of modifying the natural history of the disease. Thymectomy increases significantly the possibility of success in the treatment of MG: only 10% of the non-thymectomized patients achieve remission from the disease while the percentage increases significantly in those patients that undergo thymectomy (4,20), particularly young individuals with a recent onset of MG.
Thymectomy in MG has been universally used for many years (20-22), even though nowadays, based on our experience, we recommend this procedure exclusively for AChRAb-positive MG patients, which represent the majority of subjects affected by MG (2,3).
We also believe that patients with ocular AChRAb-positive MG and thymic hyperplasia can be good candidates for surgery, as in these cases, the ocular symptoms often represent the early phase of a form of generalized MG. The minimally invasive nature of robotic thymectomy is an additional reason to also recommend this surgical technique in patients younger than 50 years with ocular AChRAb positive-MG.
In conjunction with the thymectomy, it is important to assign specific pre- and postoperative neurological therapy, which, according to our experience, should primary be based on the use of steroids, combined with anticholinesterase drugs when useful. Corticosteroids seem to increase the therapeutic effect of the surgery, allowing a rapid improvement of the clinical condition that can even reach a complete remission from the disease (5).
Our experience and numerous scientific publications highlight the importance of the radical thymectomy, with the intent to excise not only the thymus but also the surrounding adipose and ectopic tissue that could be responsible for perseverance of MG (5,23-25).
A particularly attentive analysis of the chest computed tomography (CT) scan of the thymic region is essential in order to choose the most appropriate surgical approach; chest CT scan detection of a thymoma sized >5 cm with infiltration of the capsule or surrounding structures is the main exclusion criteria for the robotic approach.
Role of the neurologist
The neurologist manages the MG patient starting from arrival in the outpatient clinic, and is aware of all the clinical information for each individual. The role of the neurologist is to use all the most appropriate medical and surgical tools in order to achieve a stable clinical improvement or complete remission of the condition.
MG is a disease characterized by a huge clinical diversity, both from the symptoms as well as the pharmacological response point of view. For this reason, the constant presence of the neurologist is necessary throughout the surgical management of these patients.
Preoperatively, the aim of the neurologist is to optimize symptom control, using all the appropriate specific therapies for each individual patient. The primary treatment, in our experience, is generally the use of steroids in combination with anticholinesterases, in personalized doses based on disease severity in individual patient. In this situation, prednisone offers the possibility of a faster and better control of myasthenic symptoms than the other immunosuppressive drugs and with a minor risk of side effects. In our experience, we did not encounter any additional infectious complications or delay in the healing of the surgical wound.
Steroids should be associated to a hyposodic and hypocaloric diet, plus potassium supplements in order to avoid hypokalaemia. In addition, calcium supplements and calcitriol are given to prevent osteoporosis.
During the whole perioperative period, the anticholinesterase and steroid treatment are usually maintained at the same dosages as those given preoperatively. On the day of the operation, oral prednisone is replaced by an equivalent dosage of intramuscular dexamethasone.
We are not in favor of the use of immunosuppressants before the thymectomy, but if the patient has ongoing treatment with azathioprine or cyclosporine A, it is advisable to suspend these medications for approximately 10 days during the perioperative period with the intent to reduce the possibility of infectious complications.
Based on the collaboration between the neurologist and the anaesthetist in the operating theatre, general anesthesia is performed according to the patient’s characteristics and severity of MG. The use of low-dose latest generation curare, such as rocuronium, has significantly reduced the rate of postoperative respiratory complications.
Through this multidisciplinary approach, our patients do not need to stay in the intensive care unit following surgery. Instead, they are directly transferred to the ward or stay for 24 hours in the high dependency unit before being brought to the ward.
The collaboration between the neurologist and anesthetist is also valuable in determining an appropriate post-surgical pain therapy. In our experience, intra-venous non-steroidal anti-inflammatory drugs, paracetamol and tramadol therapy are favoured over other types of therapies. The use of low dosage morphine and opiates is reserved in those cases with the best clinical MG offset. Following robotic thymectomy the average hospital stay is of three to four days.
The use of steroids, based on our experience, seems to be beneficial not only for the induction and maintenance of clinical pre- and postoperative improvements, but also in achieving complete remission from the disease or on its long-term consistent improvement. These key clinical outcomes of MG can be obtained in a few months after the surgical procedure, but usually they can occur after an average period of three years or more (5,26).
Our methods
Multidisciplinary collaboration
From 1976 to 2015, our centre in Pisa has performed extended thymectomies through the median sternotomy approach, which have allowed almost half of the cases to reach complete remission of the disease (5,27).
Over 4,950 MG patients have been treated in our centre, and 730 have undergone thymectomy in the Hospital of Pisa Thoracic Surgery Department.
Since 1993, in conjunction with the surgical approach, a multidisciplinary system was activated (Figures 1,2). Based on a close collaboration with the neurologist, the patients with MG were inserted into a diagnostic therapeutic pathway, characterized also by an attentive pharmacological control of the MG during the perioperative period.
Robotic system
In 2001, the robotic surgery utilizing the “da Vinci” system was introduced in Pisa as well; this is considered a natural evolution of the video-assisted thoracoscopic surgery (VATS) approach. This method has the advantage of having a three-dimensional high definition vision of the operating field and better articulation of surgical instruments within the chest cavity. These features allow precise isolation of anatomical structures, safe manipulation of the tissues, and also guarantee a complete radical excision with the operation (6,28-31).
This innovative tool has been used also in thymectomy for MG patients in Pisa since 2011, showing that this approach can guarantee in selected cases a radical excision of the thymic gland and the entire perithymic adipose tissue with a comparable surgical outcome and clinical effects of the median sternotomy approach.
Robotic thymectomy offers a number of advantages compared to thymectomy through median sternotomy. The main advantage is reduced invasiveness of surgery, that according to our experience in Pisa, requires only one thoracoscopic camera access and two accesses for the robotic arms (29,30).
This method offers a better aesthetic result and is therefore preferred by young and mostly female patients, which are the predominant demographic affected by MG. In addition, in most cases, this surgical approach offers a rapid postoperative course making it a more appealing option than median sternotomy. The aesthetic result and the rapid functional recovery are also important elements to convince those patients concerned about the thymectomy through median sternotomy.
Selection criteria
The principal elements used by the neurologist to select patients to undergo a minimally invasive approach include: the type of thymic pathology, the patient body habitus, comorbidities, the ongoing medical therapy, and symptom severity according to Myasthenia Foundation of American Clinical Classification (MGFA).
The neurologist makes a diagnosis of MG through accurate anamnesis, a complete neurological examination and through the presence of the anti-AChRAb. Positivity for AChRAb represents a clear diagnostic indicator in these patients, making unnecessary the use of electromyography and pharmacological tests, which sometimes may also be confounding factors for diagnosis. The presence of anti-MuSK antibodies represents a contraindication for thymectomy; indeed, numerous publications confirm its therapeutic inefficacy in this form of myasthenia (32,33).
In patients seronegative for both AChRAb and anti-MuSK, we have noticed no improvement in the clinical conditions following thymectomy; therefore, we have stopped various years ago to operate these patients.
Patients with MG and positive dosage of AChRAb should always undergo a chest CT to evaluate the presence of a thymic pathology. Thymectomy is indicated in patients with radiologic evidence of thymoma and those with thymic hyperplasia associated with positivity for AChRAb and, in most cases, with an age less than 50 years. Robotic thymectomy can be an option in those patients older than 50 years with AChRAb positivity, associated with thymic hyperplasia or thymic residue, MG poorly responsive to medical therapy and without major comorbidities.
The full inclusion and exclusion criteria for robotic thymectomy in MG patients are as follows:
-
Inclusion criteria
Patients with AChRAb-positive, mild-moderate MG according to the MGFA classification (including ocular forms of MG) and generally aged less than 50 years;
Chest CT scan detection of thymic hyperplasia or thymic residue associated with AChRAb-positive MG in patients generally aged less than 50 years;
Chest CT scan detection of a thymoma sized <5 cm without capsular infiltration or of the surrounding structures (29,30).
-
Exclusion criteria
Severe MG based on the MGFA classification;
AchRAb-negative MG patients (including ocular forms);
Anti-MuSK positive MG patients;
Severe obesity;
Presence of important comorbidities, mainly cardiac and respiratory;
Chest CT scan detection of a thymoma >5 cm in size with capsular infiltration or surrounding structures;
Patients older than 50 years with MG responsive to medical therapy.
Possible neurological complications
In MG patients, it is always appropriate to consider the possible complications related to this disease, particularly those resulting from reduced muscular strength that may lead to altered respiratory function.
Thanks to our multidisciplinary approach, postoperative myasthenic crisis is considered a very rare complication in our centre. Despite the 10−20% incidence of post-thymectomy myasthenic crisis described in the literature, this complication only occurred in our centre after extended operations that required prolonged general anesthesia in patients with myasthenia not well controlled by specific medical therapy (34,35).
Our experience
A total of 728 MG patients have undergone thymectomy in our centre since 1993. We retrospectively studied 36 patients who underwent robotic thymectomy between 2011 and 2015 (9 males and 27 females, mean age 36±12 years, mean age of onset of MG 34±12 years). Eleven patients were included in class 1, 11 patients in class 2A, 18 patients in class 2B, and two patients in class 3B according to MGFA classification. The mean operating time was 166±64 minutes.
In all cases, thymus excision was complete with perithymic adipose tissue removal, and in a patient with associated hyperthyroidism, the thyroid gland was also removed. All patients were given steroids throughout the entire perioperative period, and one patient required additional treatment with a cycle of IVIG for five days prior to the thymectomy in order to optimise the patient preoperative conditions. One patient was on azathioprine, which was suspended during the perioperative period for 10 days. No significant intra- or postoperative complications occurred, aside from transient recurrent laryngeal nerve irritation (n=1) and pleural effusion (n=1). The mean postoperative hospital stay was three days (ranging between two to five days).
Histologically, four patients were affected by thymoma (two thymoma A, one thymoma AB, and one patient thymoma B1) while 32 patients were shown to have thymic hyperplasia (Figure 3).
Figure 3.
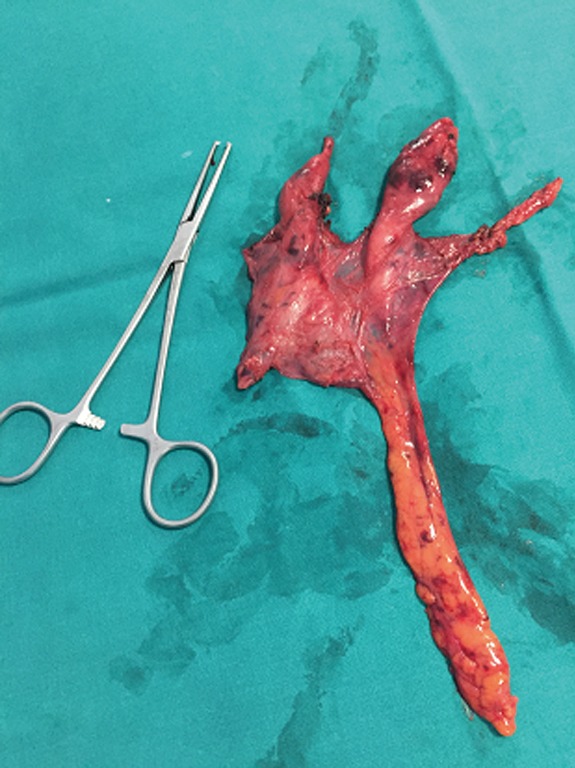
Thymic hyperplasia excised with robotic technique.
During this brief period of follow-up, complete stable remission (CSR) from the disease were observed in four patients who underwent thymectomy for hyperplasia and in two for thymoma. Eleven patients achieved pharmacologic remission after a mean period of seven months; five patients presented significant clinical improvement with minimal signs of the disease; and two patients showed marginal improvement. Three patients, however, did not show any significant clinical changes. None of the patients had worsened symptoms. There was no appropriate follow-up available for nine patients operated on in 2015; however, we can already point out that one patient has suspended medical therapy, two are controlled exclusively with anticholinesterases, and six are treated with a combination therapy of anticholinesterases and low-dose prednisone.
Conclusions
The minimally invasive robotic thymectomy is a safe alternative to the median sternotomy approach in those forms of MG associated with thymic hyperplasia or non-invasive thymoma. Our encouraging results also derive from a multidisciplinary approach, personalized preoperative therapy, meticulous patient selection, and the use of steroids in the entire perioperative phase.
These particular measures were not only used to reduce the postoperative complications but also to guarantee a better chance for improvement of the clinical condition. The robotic technique is a valid alternative to the invasive surgery, particularly for clinically well-compensated young patients in whom the mini-invasive approach and the rapid postoperative recovery could represent an important additional factor for the achievement of an optimal therapeutic result.
According to this analysis, an appropriate neurological treatment prior to thymectomy and a correct anesthetic approach represent a safety factor during the whole surgical pathway minimizing the risk of complications.
These results were also achieved thanks to an integrated approach between the neurologist, thoracic surgeon and anaesthetist, using experience acquired from numerous sternotomies performed in Pisa.
We would also like to highlight that the association between radical thymectomy and appropriate neurological treatment during the postoperative years may be an additional factor for the achievement of CSR or the clinical improvement of myasthenic patients.
Myasthenia gravis is a disease where emotional stress can lead to exacerbation of symptoms, and in which the diagnosis and the search for right therapy could be very difficult and be an additional cause of prolonged suffering. These negative experiences could worsen the severity of the disease. We believe that it is therefore paramount to achieve a compassionate approach between doctor and patient; this empathy can contribute to obtaining the best therapeutic result. We consider this element a further fundamental pillar of our model of cure of MG (26).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lindstrom JM, Seybold ME, Lennon VA, et al. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 1976;26:1054-9. [DOI] [PubMed] [Google Scholar]
- 2.Luo Y, Pan DJ, Chen FF, et al. Effectiveness of thymectomy in non-thymomatous myasthenia gravis: a systematic review. J Huazhong Univ Sci Technolog Med Sci 2014;34:942-9. [DOI] [PubMed] [Google Scholar]
- 3.Marx A, Pfister F, Schalke B, et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev 2013;12:875-84. [DOI] [PubMed] [Google Scholar]
- 4.Jaretzki A, 3rd. Thymectomy for myasthenia gravis: analysis of controversies-patient management. Neurologist 2003;9:77-92. [DOI] [PubMed] [Google Scholar]
- 5.Mussi A, Lucchi M, Murri L, et al. Extended thymectomy in myasthenia gravis: a team-work of neurologist, thoracic surgeon and anaesthesist may improve the outcome. Eur J Cardiothorac Surg 2001;19:570-5. [DOI] [PubMed] [Google Scholar]
- 6.Rückert JC, Ismail M, Swierzy M, et al. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann NY Acad Sci 2008;1132:329-35. [DOI] [PubMed] [Google Scholar]
- 7.Provenzano C, Ricciardi R, Scuderi F, et al. PTPN22 and myasthenia gravis: replication in an Italian population and meta-analysis of literature data. Neuromuscul Disord 2012;22:131-8. [DOI] [PubMed] [Google Scholar]
- 8.Renton AE, Pliner HA, Provenzano C, et al. A genome-wide association study of myasthenia gravis. JAMA Neurol 2015;72:396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppedè F, Ricciardi R, Denaro M, et al. Association of the DNMT3B -579G>T polymorphism with risk of thymomas in patients with myasthenia gravis. PLoS One 2013;8:e80846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker MB. Treatment of myasthenia gravis with physostigmine. Lancet 1934;223:1200-1. [Google Scholar]
- 11.Ricciardi R, Fontana GP. La terapia anticolinesterasica nella Miastenia Gravis. Elsevier Masson, Milano, 2004. [Google Scholar]
- 12.Ricciardi R, Rossi B, Nicora M, et al. Acute treatment of myasthenia gravis with intranasal neostigmine: clinical and electromyographic evaluation. J Neurol Neurosurg Psychiatry 1991;54:1061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakami Y, Ito M, Hirayama M, et al. Anti-MuSK autoantibodies block binding of collagen Q to MuSK. Neurology 2011;77:1819-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori S, Kubo S, Akiyoshi T, et al. Antibodies against muscle-specific kinase impair both presynaptic and postsynaptic functions in a murine model of myasthenia gravis. Am J Pathol 2012;180:798-810. [DOI] [PubMed] [Google Scholar]
- 15.Warmolts JR, Engel WK, Whitaker JN. Alterate day prednisone in a patient with myasthenia gravis. Lancet 1970;2:1198-9. [DOI] [PubMed] [Google Scholar]
- 16.Fateh-Moghadam A, Wick M, Besinger U, et al. High-dose intravenous gammaglobulin for myasthenia gravis. Lancet 1984;1:848-9. [DOI] [PubMed] [Google Scholar]
- 17.Gajdos P, Outin H, Elkharrat D, et al. High-dose intravenous gammaglobulin for myasthenia gravis. Lancet 1984;1:406-7. [DOI] [PubMed] [Google Scholar]
- 18.Ricciardi R, Fontana GP. La terapia con immunoglobuline, endovena e plasmaferesi nella miastenia gravis. Elsevier Masson, Milano, 2006. [Google Scholar]
- 19.Gajdos P, Tranchant C, Clair B, et al. Treatment of myasthenia gravis exacerbation with intravenous immunoglobulin: a randomized double-blind clinical trial. Arch Neurol 2005;62:1689-93. [DOI] [PubMed] [Google Scholar]
- 20.Jaretzki A., 3rd Thymectomy for myasthenia gravis: an analysis of the controversies regarding technique and results. Neurology 1997;48:S52-S63. [Google Scholar]
- 21.Blalock A, Mason MF, Morgan HJ, et al. Myasthenia gravis and tumors of the thymic region: report of a case in which the tumor was removed. Ann Surg 1939;110:544-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blalock A, McGehee Harvey A, Ford FR, et al. The treatment of myasthenia gravis by removal of the thymus gland preliminary report. JAMA 1941;117:1529-33. [Google Scholar]
- 23.Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Ann Thorac Surg 2000;70:327-34. [DOI] [PubMed] [Google Scholar]
- 24.Marulli G, Rea F. Myasthenia gravis and thymectomy: many doubts and few certainties. Eur J Cardiothorac Surg 2015;48:46-7. [DOI] [PubMed] [Google Scholar]
- 25.Masaoka A, Monden Y. Compaison of the results of transsternal simple, transcervical simple and extended thymectomy. Ann N Y Acad Sci 1981;377:755-65. [DOI] [PubMed] [Google Scholar]
- 26.Ricciardi R, Fontana GP. Vivere la Miastenia (Nuova edizione). Franco Angeli Editore, Milano, Italy, 2012. [Google Scholar]
- 27.Lucchi M, Ricciardi R, Melfi F, et al. Association of thymoma and myasthenia gravis: oncological and neurological results of the surgical treatment. Eur J Cardiothorac Surg 2009;35:812-6. [DOI] [PubMed] [Google Scholar]
- 28.Rea F, Marulli G, Bortolotti L, et al. Experience with the “da Vinci” robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg 2006;81:455-9. [DOI] [PubMed] [Google Scholar]
- 29.Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. [DOI] [PubMed] [Google Scholar]
- 30.Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6. [DOI] [PubMed] [Google Scholar]
- 31.Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [DOI] [PubMed] [Google Scholar]
- 32.Lauriola L, Ranelletti F, Maggiano N, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology 2005;64:536-8. [DOI] [PubMed] [Google Scholar]
- 33.Leite MI, Ströbel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol 2005;57:444-8. [DOI] [PubMed] [Google Scholar]
- 34.Ando T, Omasa M, Kondo T, et al. Predictive factors of myasthenic crisis after extended thymectomy for patients with myasthenia gravis†. Eur J Cardiothorac Surg 2015;48:705-9. [DOI] [PubMed] [Google Scholar]
- 35.Yu S, Lin J, Fu X, et al. Risk factors of myasthenic crisis after thymectomy in 178 generalized myasthenia gravis patients in a five-year follow-up study. Int J Neurosci 2014;124:792-8. [DOI] [PubMed] [Google Scholar]