Abstract
Transcatheter valve-in-valve (VIV) implantation for degenerated aortic bioprostheses has emerged as a promising alternative to redo conventional aortic valve replacement (cAVR). However there are concerns surrounding the efficacy and safety of VIV. This systematic review aims to compare the outcomes and safety of transcatheter VIV implantation with redoes cAVR. Six databases were systematically searched. A total of 18 relevant studies (823 patients) were included. Pooled analysis demonstrated VIV achieved significant improvements in mean gradient (38 mmHg preoperatively to 15.2 mmHg postoperatively, P<0.001) and peak gradient (59.2 to 23.2 mmHg, P=0.0003). These improvements were similar to the outcomes achieved by cAVR. The incidence of moderate paravalvular leaks (PVL) were significantly higher for VIV compared to cAVR (3.3% vs. 0.4%, P=0.022). In terms of morbidity, VIV had a significantly lower incidence of stroke and bleeding compared to redo cAVR (1.9% vs. 8.8%, P=0.002 & 6.9% vs. 9.1%, P=0.014, respectively). Perioperative mortality rates were similar for VIV (7.9%) and redo cAVR (6.1%, P=0.35). In conclusion, transcatheter VIV implantation achieves similar haemodynamic outcomes, with lower risk of strokes and bleeding but higher PVL rates compared to redo cAVR. Future randomized studies and prospective registries are essential to compare the effectiveness of transcatheter VIV with cAVR, and clarify the rates of PVLs.
Keywords: Transcatheter, valve-in-valve, reoperative, outcomes, aortic valve replacement
Introduction
The rapidly ageing population, lower thromboembolic and hemorrhagic risks, and proven increased durability of tissue valves have generated enthusiasm and extended indications of bioprostheses (1). However, bioprostheses have limited durability, with studies reporting expected valve degeneration within 10 to 20 years follow-up. In these cases, patients are indicated for a reoperative valve replacement, with higher risk compared to the first operation (2,3).
Transcatheter valve-in-valve (VIV) implantation for failing aortic bioprostheses has recently emerged as a promising alternative to surgical aortic valve replacement due to degenerated bioprostheses (2,4). However, there are still concerns surrounding short the long-term safety and efficacy of this approach, as well as durability of the VIV implant (2,5-9).
In order to assess the relative benefits and risks of transcatheter VIV implantation compared reoperative conventional aortic valve replacement (cAVR), a systematic review and meta-analysis was performed.
Methods
Literature search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses PRISMA guidelines were followed for the present systematic review (10,11). Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, and Database of Abstracts of Review of Effectiveness (DARE), from their dates of inception to February 2015. To achieve maximum sensitivity of the search strategy, we combined the terms “transcatheter valve replacement” or “transcatheter valve in valve implantation” or “transcatheter heart valves” or “transcatheter aortic valve in valve implantation” which were searched as text words and exploded as MeSH headings where possible. Two authors performed the search independently, and any discrepancies were resolved by discussion. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies, assessed using the inclusion and exclusion criteria. Expert academic cardiothoracic surgeons were consulted as to whether they knew of any unpublished data. For redo cAVR outcomes, a recent review (12) was included in the present comparison between VIV and redo cAVR.
Selection criteria
Eligible studies for the present systematic review and meta-analysis included those in which patient cohorts underwent transcatheter aortic VIV implantation. Studies that did not include mortality or complications as endpoints were excluded. Studies with fewer than 10 patients in their cohort were also excluded. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials, reviews and expert opinions were excluded.
Data extraction and critical appraisal
All data including baseline characteristics, operational parameters, and safety and efficacy outcomes were extracted from article texts, tables and figures. The primary outcome was perioperative or 30-day mortality. Other outcomes extracted included: myocardial infarctions (MI), strokes, bleeding, permanent pacemaker implantations, vascular complications, acute kidney injury, postoperative mean gradients, mild paravalvular leak (PVL), and moderate PVL. Two investigators independently reviewed each retrieved article. Discrepancies between the two reviewers were resolved by discussion and consensus. The quality of studies was assessed using criteria recommended by the National Health Service Centre for Reviews and Dissemination case series quality assessment criteria (University of York, Heslington, United Kingdom) (13). The final results were reviewed by the senior investigators.
Statistical analysis
Transcatheter VIV data are presented as mean ± standard deviation. For weighted pooled means, a meta-analysis of proportions was conducted. Firstly, to establish variance of raw proportions, a Freeman-Tukey transformation was applied (14). To incorporate heterogeneity (anticipated among the included studies), transformed proportions were combined using DerSimonian-Laird random effects models (15). Finally the pooled estimates were back-transformed. Heterogeneity was evaluated using Cochran Q and I2 test. Weighted means were calculated by determining the total number of events divided by total sample size.
For comparison between valve-in-valve and conventional reoperative AVR, data from prior published reviews on conventional reoperative AVR (12) outcomes were used. A formal comparison was performed between VIV and conventional reoperative aortic valve replacement using mixed-effects meta-regression with a fixed-effect moderator variable for interventional technique. Outcomes for analysis included 30-day mortality rates, cardiovascular death, MI, strokes, permanent pacemaker implantations, vascular complications, acute kidney disease, postoperative mean gradient and postoperative peak gradient, mild and moderate PVL.
All analyses were performed using the meta for package for R version 3.02. P values <0.05 were considered statistically significant.
Results
Quality of studies
A total of 366 studies were identified through six electronic database searches for VIV studies and from other sources including reference lists (Figure 1). After exclusion of duplicate or irrelevant references, 360 potentially relevant articles were retrieved. A total of 18 relevant VIV studies were identified and included in the present systematic review and meta-analysis (4-9,16-27) (Table 1). Of these, there were 8 prospective studies (5-7,19,21,22,26,27) and 10 retrospective studies (4,8,9,16-18,20,23-25). A total of 823 patients were included for analysis (Table 1). Eight out of 18 included studies reported mean follow-up of 12 months or longer (5,7,16,17,20-22,24). All studies reported 30-day mortality rates. Postoperative mean gradients were reported in all included studies except three studies (9,24,26). Patient-prosthesis mismatch was reported in only three studies (16,17,21). Rates of PVL was reported in eight studies (5,9,16,18,20-22,27). Quality assessment performed according to the Moose Checklist of the Dutch Cochrane review group is shown in Supplementary Table S1. From a prior review on reoperative cAVR (12), 6 studies were included for the present analysis.
Figure 1.
Search strategy for the present systematic review on valve-in-valve implantation.
Table 1. Study characteristics.
| First author | Year | Study period | Institution | Country | Study type | N | Degenerated valve type (%) | VIV type mm (%) | Mean follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Camboni | 2015 | 2009-2014 | University Medical Center Regensburg | Germany | R, OS | 31 | Mitroflow (42.8), Perimount (42.8), CLOB (14.3) | CV 26 (20.0), Engager 23 (20), Engager 26 (6.7), ES 23 (6.7), ES XT 23 (20.0), ES XT 26 (20.0), Symetis S (6.7) | 1.0 |
| Subban | 2014 | 2009-2014 | Prince Charles Hospital | Australia | R, OS | 12 | CLOB (8.3), Perimount (50.0), Biocor (8.3), Toronto (16.7), Mosaic (16.7) | CV 29 (16.7), CV 26 (25), ES 26 (33.3), ES 23 (16.7), CV 23 (8.3) | 26.0 |
| Stähli | 2014 | 2008-2012 | Heart Hospital London; University Hospital Zurich | England; Switzerland | R, OS | 14 | Mitroflow (14.3), Medical Epic (14.3), Perimount (14.3), Freestyle (7.1), Mosaic (14.3), Lifesciences (7.1), Aortic Valve Conduit (7.1), Biomedica (7.1), Mosaic Handcock II (7.1), Homograft 1994 (7.1) | ES 23 (57.1), ES 26 (21.4), CV 31 (7.1), CV 26 (7.1), CV 29 (7.1) | 1.0 |
| Dvir | 2014 | 2007-2013 | VIVID Registry | International | R, registry | 459 | ≤ 21 mm (29.0), >21 mm and <25 mm (38.3), ≥2 mm (30.3) | ES XT 20, 23, 26, 29 (58.9), self-expandable 23, 26, 29, 31 | 25.1 |
| Diemert | 2014 | 2013 | University Heart Center Hamburg | Germany | R, OS | 16 | Pericarbon Freedom (25.0), Mosaic (11.1), Mitroflow (11.1), Edwards (25.0), Epic (6.3), Intact (11.1), Hancock II (6.3) | CoreValve Evolut THV 23 (100.0) | 1.0 |
| Bapat | 2014 | 2010-2014 | St. Thomas Hospital, London | United Kingdom | P, case series | 10 | Homograft (60.0), Cryolife (10.0), Pericarbon stentless (10.0), Toronto SPV root (10.0), Freestyle root (10.0) | ES 26 (20.0), 23 (10.0), XT 23 (40.0), XT 26 (20.0), 3 26 (10.0) | 8.1 |
| Ihlberg | 2013 | 2008-2012 | The Nordic Valve-in-Valve Registry | International | R, registry | 45 | Biocor/Epic (7.0), Carpentier Edwards SAV (22.0), Mosaic (4.0), Mosaic Ultra (2.0), Shelhigh (2), Mitroflow (33.0), Magna (2.0), Perimount (9.0), Soprano (2.0), Freestyle (7.0), Toronto SPV (2.0), Homograft (7.0) | ES 23 (55.6), 26 (15.6), 29 (6.7), CV 26 (26.7) | 14.4 |
| Toggweiler | 2012 | 2005-2011 | St. Paul’s Hospital, Vancouver; the Quebec Heart and Lung Institute, Quebec City; and the Cleveland Clinic, Cleveland | Canada, USA | P, OS | 21 | Cribier-Edwards (9.5), ES (81.0), Sapien XT (9.5) | CE, ES, Sapien XT 23 (43.0), 26 (57.0) | 12.0 |
| Seiffert | 2012 | 2008-2011 | University Heart Center Hamburg | Germany | P, OS | 11 | Hancock (54.5), Carpentier Edwards (9.1), Biocor (18.2), Mosaic (9.1), Freestyle (9.1) | ES 23 (90.9), 26 (9.1) | 12.0 |
| Linke | 2012 | NR | University of Leipzig Heart Centre | Germany | P, OS | 27 | Mitroflow (7.4), Epic (40.74), Mosaic (3.7), Perimount (18.5), Shelhigh (3.7), ATS 3f (3.7), Baxter (3.7), Hancock II (3.7), Toronto (7.4), Aspire (7.4) | CV 26 (77.8), 29 (22.2) | 14.0 |
| Latib | 2012 | NR | San Raffaele Scientific Institute | Italy | R, OS | 18 | Carpentier-Edwards (38.9), St. Jude (11.1), Mosaic (16.7), Freestyle (5.6), Xenomedica (5.6), Pericarbon (5.6), Bravo (5.6), Hancock (5.6), Mitroflow (5.6) | ES 23 (44.4), 26 (11.1), ES XT 23 (27.8), 26 (16.7) | 11.0 |
| Gaia | 2012 | 2008-2011 | Federal University of São Paulo | Brazil | R, OS | 14 | Biomedica (21.4), Hancock (21.4), Biocor (14.3), unknown (42.9) | Biomedica 20 (14.3), 22 (50.0), 24 (35.7) | 1.0-33.0 |
| Ussia | 2011 | NR | Italian CoreValve Registry | Italy | R, registry | 24 | 18-F CoreValve (100.0) | CRS 26 (62.5), CRS 29 (37.5) | 10.5 |
| Pasic | 2011 | 2008-2010 | Deutsches Herzzentrum Berlin | Germany | P, OS | 14 | Homograft (21.4), Hancock (14.3), Sapien (7.1), Freestyle (7.1), Carpentier-Edwards (14.3), Mosaic (7.1), Hancock Ultra (14.3), | ES 26 (28.6), 23 (71.4) | 12.1 |
| Eggebrecht | 2011 | 2005-2010 | viv-TAVI registry | Germany & Switzerland | R, registry | 47 | Perimount, Perimount Magna, Porcine, Hancock III, Mosaic, Mosaic Ultra, Biocor, Epic, Mitroflow | ES 23 (61.7), 26 (12.7), CV 26 (23.4), CV 29 (2.1) | 1.0 |
| Bedogni | 2011 | NR | 8 Italian Centres | Italy | P, registry | 25 | Pericarbon (8.0), Mitroflow (12.0), Carpentier-Edwards (20.0), Toronto (16.0), Biocor (16.0), Mosaic (12.0), Hancock (4.0), Cyrolife (4.0), Cribier (4.0), Elan (4.0) | CRS 26 (76.0), 29 (24.0) | 6.0 |
| Webb | 2010 | NR | Multiple | Canada, England | P, OS | 24 | Mitrial: Edwards SAV, Baxter Edwards, Mosaic, Intact; Aortic: Carpentier-Edwards, Shiley, Superstentless, Freestyle, Mosaic; Pulmonary: Xenograft, Homograft | Cribier-Edwards, SAPIEN, or SAPIEN XT | 4.5 |
| Kempfert | 2010 | 2007-2009 | Universität Leipzig | Germany | P, OS | 11 | Perimount (27.3), Carpentier-Edwards (18.2), Hancock (18.2), Mosaic (9.1), Medtronic (9.1), Mitroflow (9.1), Epic (9.1) | Cribier-Edwards (27.3), ES (72.7) | 330.0±293.0 |
R, retrospective; OS, observation study; n, number of patients, VIV, valve-in-valve transcatheter aortic valve implantation; CV, CoreValve; ES, Edwards Sapien.
Patient characteristics
Baseline characteristics of included studies on transcatheter VIV implantations and reoperative cAVR are summarized in Table 2. Overall in the VIV group, 58.0% of patients were male, with a pooled weighted mean age of 77.5 years (range, 68–82.4 years). The mean logistic EuroSCORE was 31 whilst the mean LVEF was 51.0%. Weighted average eGFR was 53.7 mL/min (range, 43–62.6 mL/min). The prevalence of hypertension, diabetes mellitus, chronic kidney disease, and peripheral vascular disease was 74.8%, 27.3%, 44.6%, and 26.3%, respectively. Aortic stenosis was indicated in 39.8% (range, 22–72.7%) of patients, whilst aortic regurgitation was indicated in 33.7% (range, 0–100%) of patients. Atrial fibrillation affected 23.5% (range, 12.5–43%) of all VIV patients, and 16.3% (range, 4.2–36%) had pacemakers. Pooled weighted mean body mass index (BMI) was 26.5 kg/m2. Coronary artery disease affected 50% of all VIV patients, whilst 25.8% of patients had history of a MI and 12.4% had a history of a stroke. Of the included VIV patients, 31.8% (range, 12.5–57.1%) had a previous coronary bypass graft surgery (CABG), whilst 17.8% (range, 0-33.3%) had prior percutaneous coronary intervention (PCI). The preoperative mean gradient was 36.9 mmHg (range, 16–45.4 mmHg). The mean implanted valve size was 24.6 mm (range, 22.1–27.1 mm).
Table 2. Patients’ clinical characteristics of all included studies for transcatheter VIV implantation and redo cAVR.
| First author | Males (%) | Average age (years) | Logistic EuroSCORE | STS-PROM (%) | LVEF (%) | HT (%) | DM (%) | CKD (%) | PVD (%) | AF (%) | Pacemaker (%) | CAD (%) | Prior MI (%) | Prior stroke (5) | Prior CABG (%) | Prior PCI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transcatheter VIV implantation | ||||||||||||||||
| Camboni | NR | 77.8±6.3 | NR | 20.9±8.6 | 55.6±8.0 | NR | NR | NR | NR | NR | NR | 64.0 | NR | 15.0 | NR | NR |
| Subban | 75.0 | 78.0±7.0 | NR | 3.7±3.7 | 58.0±11.0 | 58.3 | 16.7 | 58.3 | NR | 41.7 | NR | 50.0 | NR | NR | 33.0 | 25.0 |
| Stähli | 43.0 | 75.0±4.0 | NR | NR | 56.0±4.0 | 79.0 | 14.0 | NR | 7.0 | 14.0 | 36.0 | 43.0 | NR | NR | 14.0 | 0 |
| Dvir | 56.0 | 77.6±9.8 | 29.0 | 10.0 | 50.3±13.1 | NR | 28.7 | 48.8 | 26.1 | NR | NR | NR | NR | 11.7 | NR | NR |
| Diemert | 50.0 | 78.9±6.7 | 42.8±7.8 | 9.5±5.6 | NR | 18.8 | 12.5 | 12.5 | 31.3 | 18.8 | 18.8 | 31.3 | NR | 18.8 | 12.5 | 6.3 |
| Bapat | 70.0 | 73.3±14.0 | 31.2±19.0 | 7.0±5.2 | 45.4±14.2 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Ihlberg | 57.8 | 80.6 | 35.4±16.1 | 15.0±10.8 | 46.3±12.8 | NR | 18.0 | 20 | 20 | NR | NR | NR | 13.0 | NR | NR | NR |
| Toggweiler | 52.0 | 81.0±7.0 | NR | 8.6±3.7 | 51.0±13.0 | NR | 24.0 | NR | NR | 43.0 | NR | 91.0 | 48.0 | 24.0 | 52.0 | NR |
| Seiffert | 81.8 | 79.3±6.1 | 31.8±23.4 | 12.5±11.1 | 50.0±17.4 | NR | NR | NR | 36.4 | NR | NR | 45.5 | 27.3 | NR | NR | NR |
| Linke | 70.4 | 74.8±8.0 | 31.3±16.5 | 10.0±7.9 | 52.0±15.0 | 100.0 | 59.0 | 11.0 | 26.0 | NR | NR | 44.0 | 33.0 | 0 | 33.0 | 18.5 |
| Latib | 66.7 | 75.0±12.6 | 37.4±20.8 | 8.2±5.2 | 52.9±10.8 | NR | 16.7 | 61.1 | NR | 22.2 | NR | NR | NR | NR | NR | NR |
| Gaia | 86.0 | 69.8 | 42.9 | 38.6 | 51.0±15.9 | 100.0 | 57.1 | NR | 42.8 | 14.2 | NR | 78.5 | NR | 0 | NR | NR |
| Ussia | 45.9 | 80.3±6.2 | 23.6±14.3 | NR | 49.3±15.1 | NR | 16.6 | 20.8 | 16.7 | 12.5 | 4.2 | 58.3 | 20.9 | 12.5 | 16.7 | 33.3 |
| Pasic | 64.3 | 73.3±13.1 | 45.3±22.2 | 21.9±10.9 | 45.0±13.0 | NR | NR | NR | 57.1 | NR | 14.3 | 85.7 | NR | 42.9 | 57.1 | NR |
| Eggebrecht | 60.0 | 79.8±7.1 | 35.0±18.5 | 11.6±8.5 | 52.0±12.0 | NR | 28.0 | 60.0 | NR | NR | NR | 60.0 | 26.0 | NR | 34.0 | 17.0 |
| Bedogni | NR | 82.4±3.2 | 31.5±14.8 | 8.2±4.2 | 56.5±12.5 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Webb | NR | 68.0±21.8 | 30.9±7.7 | 12.4±4.4 | 58.3±11.2 | NR | 8.3 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Kempfert | 63.6 | 78.0±6.0 | 31.7±14.5 | 7.2±2.6 | 52.8±7.7 | NR | NR | NR | 27.3 | NR | NR | NR | NR | NR | NR | NR |
| Weighted average | 58.0 | 77.5 | 31.0 | 11.3 | 51.0 | 74.8 | 27.3 | 44.6 | 26.3 | 23.5 | 16.3 | 59.8 | 25.8 | 12.4 | 31.8 | 17.8 |
| Redo cAVR | ||||||||||||||||
| Pechlivanidis | 67.2 | 65.6±12.9 | NR | NR | 51.8±10.5 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Papadopoulos | 18.6 | 82.0±5.0 | 25.0±5.0 | 11.0±4.0 | 39.0±18.0 | 13.2 | 12.0 | 18.0 | 14.0 | NR | NR | 27.0 | NR | 12.0 | NR | NR |
| Wilbring | 66.0 | 77.6±2.7 | 26.4±12.9 | NR | NR | NR | 43.0 | 60.0 | 32.0 | 36.0 | NR | 100.0 | NR | 15.0 | 93.0 | NR |
| Leontyev | 76.8 | 58.1±16.3 | 27.0±23.0 | NR | NR | NR | 17.0 | 4.5 | 3.0 | NR | NR | NR | NR | 16.0 | 10.0 | NR |
| Eitz | 39.4 | NR | NR | NR | NR | 62.0 | 32.0 | 44.0 | 13.0 | 27.0 | NR | 63.0 | NR | 11.0 | NR | NR |
| Davierwala | 72.2 | 59.0±14.0 | NR | NR | NR | 41.0 | 9.0 | 22.0 | 6.0 | NR | NR | NR | NR | 18.0 | 31.0 | NR |
| Weighted average | 57.6 | 66.7 | 26.0 | NA | NA | 34.1 | 16.8 | 22.3 | 10.1 | NA | NA | 49.0 | NA | 15.0 | 31.0 | NA |
VIV, valve-in-valve transcatheter aortic valve implantation; cAVR, redo conventional aortic valve replacement; NR, not reported; LVEF, left ventricular ejection fraction; HT, hypertension; DM, diabetes mellitus; CKD, chronic kidney disease; PVD, peripheral vascular disease; AF, atrial fibrillation; CAD, coronary artery disease; MI, myocardial infarction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
For the redo cAVR group, the average age was 66.7 years, 57.6% were males and the mean logistic EuroSCORE of 26. The prevalence of hypertension, diabetes mellitus, chronic kidney disease, and peripheral vascular disease was 34.1%, 16.8%, 22.4%, and 10.1%, respectively. Of the all patients undergoing cAVR, 49% had CAD, 15% had a history of strokes, and 31% had previous CABG.
Operative outcomes
For the VIV population, the pooled mean procedural time was 87.8 min (95% CI, 70.7–104.9 min; I2=92%). Average fluoroscopy time was 16.8 min (95% CI, 6.9–30.8 min; I2=99.5%). The weighted average hospital stay was 9.7 days (95% CI, 7.6–11.8 days; I2=70%).
Assessment of safety
Pooled overall 30-day all-cause mortality rate was similar for transcatheter VIV implantation (6.4%; 95% CI, 4.5–8.2%) and cAVR (6.5%; 95% CI, 5.3–7.7%) (P=0.353). At the latest follow-up, overall VIV all-cause mortality was 12.6% (95% CI, 5.6–21.4%; I2=77.5%) (Table 3).
Table 3. Comparison of outcomes for transcatheter valve-in-valve (VIV) versus reoperative conventional aortic valve replacement (cAVR).
| Outcome | n/N (%) | Pooled weighted estimate (95% CI) | I2 | P value for heterogeneity | P value |
|---|---|---|---|---|---|
| Perioperative mortality (%) | 0.353 | ||||
| VIV | 65/823 (7.9) | 6.4 (4.5–8.2) | 4.87 | 0.397 | |
| cAVR | 38/626 (6.1) | 6.5 (5.3–7.7) | 51.45 | <0.001 | |
| Myocardial infarctions (%) | NA | ||||
| VIV | 6/271 (2.2) | 3.0 (1.0–5.0) | 0.00 | 0.997 | |
| cAVR | NA | NA | NA | NA | |
| Strokes (%) | 0.002** | ||||
| VIV | 15/802 (1.9) | 2.0 (1.0–3.0) | 0.00 | 0.998 | |
| cAVR | 40/793 (8.8) | 4.7 (3.2–6.2) | 0.00 | 0.713 | |
| Bleeding (%) | 0.014* | ||||
| VIV | 47/681 (6.9) | 4.6 (1.7–7.4) | 51.68 | 0.029 | |
| cAVR | 53/585 (9.1) | 9.0 (6.7–11.3) | 0.00 | 0.911 | |
| Permanent pacemaker implantation (%) | 0.257 | ||||
| VIV | 66/802 (8.2) | 6.5 (4.3–8.7) | 17.12 | 0.258 | |
| cAVR | 61/662 (9.2) | 8.2 (2.9–13.5) | 86.51 | <0.001 | |
| Vascular complications (%) | |||||
| VIV | 49/634 (7.7) | 5.4 (2.6–8.1) | 32.73 | 0.156 | |
| cAVR | NA | NA | NA | NA | NA |
| Acute kidney injury (%) | 0.927 | ||||
| VIV | 52/697 (7.5) | 7.0 (5.1–8.9) | 0.00 | 0.936 | |
| cAVR | 62/740 (8.4) | 8.6 (4.4–12.8) | 79.24 | 0.001 | |
| Postoperative mean peak gradient (mmHg) | 0.545 | ||||
| VIV | – | 15.2 (13.4–17.1) | 89.29 | <0.001 | |
| cAVR | – | 13.5 (6.8–20.3) | 99.32 | <0.001 | |
| Mild paravalvular leak (%) | 0.175 | ||||
| VIV | 26/199 (13.1) | 9.7 (3.1–16.3) | 76.00 | <0.001 | |
| cAVR | 0/220 (0) | 0.4 (0–1.1) | 0.00 | 0.646 | |
| Moderate paravalvular leak (%) | 0.022* | ||||
| VIV | 7/199 (3.5) | 3.3 (0.9–5.8) | 0.00 | 0.936 | |
| cAVR | 0/220 (0) | 0.4 (0–1.1) | 0.00 | 0.646 | P value |
*, P<0.05; **, P<0.01. n, no of events; N, total number of patients; CI, confidence interval; NA, not available.
The incidence of perioperative strokes was significantly less in the VIV group compared to cAVR (2.0% vs. 4.7%, P=0.002). Bleeding rates were also lower in the VIV group (4.6%) compared to cAVR group (9.0%, P=0.014).
In the VIV group, the rate of cardiovascular-related 30-day mortality was 4.9% (3.4–6.5%; I2=0%) and incidence of MI in the VIV group was 3.0% (0.9–2.3%, I2=0%). A comparison with cAVR could not be undertaken as cAVR studies poorly reported the rate of cardiovascular-related deaths or the incidence of perioperative MI. Permanent pacemaker implantations were similar in the VIV group compared to cAVR (6.5% vs. 8.2%, P=0.257). The incidence of acute kidney disease was similar between VIV vs. cAVR (7.0% vs. 8.6%, P=0.927).
Assessment of hemodynamic outcomes
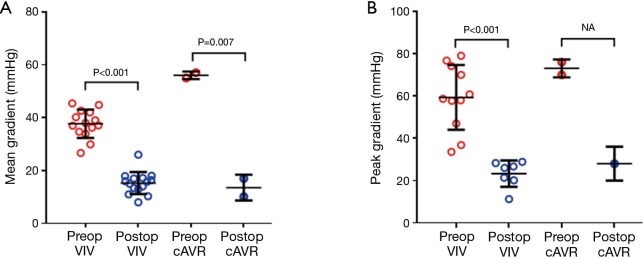
In the VIV group, the pooled postoperative mean gradient of 15.2 mmHg was significantly lower compared to preoperative mean gradient (36.9 mmHg, P<0.0001). Similarly, the postoperative peak gradient of 23.2 mmHg was significantly lower than preoperative peak gradient (59 mmHg, P=0.0003). Pooled postoperative aortic valve area was 1.435 cm2, which was a significant improvement compared to preoperative aortic valve area (P<0.0001). Significant increases in indexed effective orifice area was also noted (P=0.004). There were significant improves across all hemodynamic outcomes following the VIV implantation (Figure 2). Similar postoperative mean gradients were seen between VIV and cAVR groups (15.2 vs. 13.5 mmHg; P=0.545).
Figure 2.
Preoperative versus postoperative transcatheter valve-in-valve hemodynamics. VIV, valve-in-valve; cAVR, reoperative conventional aortic valve replacement.
The incidence of mild PVL were similar between VIV and cAVR (9.7% vs. 0.4%, P=0.175). However, only 2 cAVR studies had data available for analysis. For moderate PVL, VIV had higher rates compared to cAVR (3.3% vs. 0.4%, P=0.022). Severe PVL was not reported in VIV or cAVR groups.
Discussion
Transcatheter VIV implantation in patients with failing aortic bioprostheses has been recently proposed as an alternative to surgical re-operative aortic valve replacement, particularly in high-risk patients (6,27,28). In order to assess the relative benefits and risks of transcatheter VIV implantation compared with reoperative cAVR, a systematic review and meta-analysis was performed.
This review highlights that transcatheter VIV implantation is currently being prescribed for higher risk patients who are older, have higher rates of comorbidities and cardiac operative risk compared to patients offered reoperative AVR via the conventional open approach. The mean age of the VIV group was more than a decade older than those in the cAVR group (77.5 and 66.7 years, respectively) and more than double the prevalence of hypertension (74.8% vs. 34.1%), peripheral vascular disease (26.3% vs. 10.1%) and chronic kidney disease (44.6% vs. 22.3%). Unsurprisingly, the Logistic EuroSCORE was substantially higher in the VIV group compared to cAVR.
Interestingly, despite the increased prevalence of comorbidities in the VIV cohort, the meta-regression revealed that transcatheter aortic VIV implantation had similar hemodynamic outcomes and perioperative mortality, whilst having a lower incidence of strokes and bleeding compared to cAVR. These findings are somewhat surprising as transcatheter aortic VIV implantation holds equivocal outcomes to the gold standard treatment of cAVR. A likely explanation for this finding is the use of smaller valves in secondary cAVR than those used in primary cAVR that leads to the hemodynamic outcomes that are suboptimal compared to primary cAVR (29), but comparable with VIV. As VIV has a lower incidence of stroke and bleeding compared to secondary cAVR, it suggests that VIV may be the safer option in patients with failing aortic bioprostheses. The surgical approach is associated with more surgical trauma and anaesthetic risks compared to the minimally invasive transcatheter alternative. This improved safety with VIV is likely to be even greater as the patients were of higher risk in the VIV cohort compared to the cAVR cohort. Future studies with more comparable patient groups may be of interest.
It is important to note that some studies have suggested that mortality and complication rates are associated with smaller first implant valve diameter size and baseline stenosis. In 2014, Dvir and colleagues (17) studied 459 transcatheter aortic VIV procedures in an international registry. Patients with smaller valves (≤21 mm) had worse one-year actuarial survival compared to intermediate and large diameter size valves. The Global VIV Registry demonstrated that lower gradients could be achieved in patients with small internal diameter bioprosthesis when a supra-annular TAVI prosthesis was chosen, such as the CoreValve design. To further investigate this, Diemert and colleagues (18) studied 16 high-risk patients with <21 mm first bioprosthesis diameter who underwent VIV with a 23-mm CoreValve Evolut prosthesis, a later generation valve with a lower profile design. Implantation was successful in all but two patients (87.5%), with no postoperative signs of aortic regurgitation, stenosis or major adverse event within 30-day follow-up.
While suturing a conventional prosthesis to the aortic valve annulus undoubtedly remains the most effective technique to minimize the post-operative occurrence of PVL, for transcatheter valve anchoring, the perfectly circular and rigid prosthetic valve stent is more reliable than the native calcified and irregular aortic valve annulus. Moreover, the valvular prosthetic ring prevents post-operative intra-ventricular conduction disturbances by protecting the His bundle from tension and injuries during percutaneous valve deployment.
Limitations
This study has limitations that need to be considered when interpreting the results. Firstly, the indications for a transcatheter VIV procedure were inherently heterogeneous across studies, which may undermine the validity of the presented data. The VIV procedure can be performed in a wider variety of settings, including degeneration by stenosis subsequent to calcification, pannus, thrombosis, aortic regurgitation, structural degeneration, or a combination of these factors. Given the limited number of studies published to date, subgroup analysis was not feasible in order to determine whether there were any differences in outcomes between these indications. Secondly, there is lack of long-term durability and hemodynamic outcomes for VIV interventions. As such, cAVR should remain the gold standard therapy for reoperative aortic valve surgery, especially for low and intermediate risk patients, until long-term VIV data is available for assessment. Finally, the majority of included studies had a small-sample size with short-term follow-up. Therefore, comparative meta-analysis was performed based on meta-regression analysis, since there were few studies which directly compared outcomes between transcatheter VIV and minimally invasive reoperative AVR cohorts. Large randomised controlled trials to examine long-term outcomes and allow for subgroup analysis according to bioprosthesis valve size and degeneration mechanisms are warranted.
Conclusions
In conclusion, current lower quality evidence suggests that transcatheter VIV implantation may achieve similar haemodynamic outcomes whilst significantly minimising the risk of strokes and bleeding compared to redo cAVR. However VIV was associated with an increased rate of moderate PVL s compared to cAVR. Future randomized studies and prospective registries are essential to examine the long-term effectiveness of transcatheter VIV.
Acknowledgements
None.
Table S1. Assessment of quality of included studies.
| First author | Clear definition of study population | Clear definition of outcomes and outcome assessment | Independent assessment of outcome parameters | Sufficient duration of follow-up | No selective loss during follow-up | Important confounders and prognostic factors identified |
|---|---|---|---|---|---|---|
| Camboni | Yes | Yes | Yes | No | Yes | Yes |
| Subban | Yes | No | Yes | Yes | Yes | Yes |
| Stähli | Yes | Yes | Yes | No | Yes | Yes |
| Dvir | Yes | Yes | Yes | Yes | Yes | Yes |
| Diemert | Yes | Yes | Yes | No | Yes | No |
| Bapat | Yes | Yes | Yes | No | Yes | Yes |
| Ihlberg | Yes | Yes | Yes | Yes | Yes | Yes |
| Toggweiler | Yes | Yes | Yes | Yes | Yes | Yes |
| Seiffert | Yes | Yes | Yes | Yes | Yes | Yes |
| Linke | Yes | Yes | Yes | Yes | Yes | Yes |
| Latib | Yes | Yes | Yes | No | Yes | Yes |
| Gaia | Yes | Yes | Yes | Unclear | Unclear | Yes |
| Ussia | Yes | Yes | Yes | No | Yes | Yes |
| Pasic | Yes | Yes | Yes | Yes | Yes | Yes |
| Eggebrecht | Yes | Yes | Yes | No | Yes | Yes |
| Bedogni | Yes | Yes | Yes | No | Yes | Yes |
| Webb | Yes | No | Yes | No | Yes | Yes |
| Kempfert | Yes | Yes | Yes | No | Yes | Yes |
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Brown JM, O'Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg 2009;137:82-90. [DOI] [PubMed] [Google Scholar]
- 2.Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126:2335-44. [DOI] [PubMed] [Google Scholar]
- 3.Jones JM, O'kane H, Gladstone DJ, et al. Repeat heart valve surgery: risk factors for operative mortality. J Thorac Cardiovasc Surg 2001;122:913-8. [DOI] [PubMed] [Google Scholar]
- 4.Camboni D, Holzamer A, Flörchinger B, et al. Single institution experience with transcatheter valve-in-valve implantation emphasizing strategies for coronary protection. Ann Thorac Surg 2015;99:1532-8. [DOI] [PubMed] [Google Scholar]
- 5.Toggweiler S, Wood DA, Rodés-Cabau J, et al. Transcatheter valve-in-valve implantation for failed balloon-expandable transcatheter aortic valves. JACC Cardiovasc Interv 2012;5:571-7. [DOI] [PubMed] [Google Scholar]
- 6.Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010;121:1848-57. [DOI] [PubMed] [Google Scholar]
- 7.Pasic M, Unbehaun A, Dreysse S, et al. Transapical aortic valve implantation after previous aortic valve replacement: clinical proof of the "valve-in-valve" concept. J Thorac Cardiovasc Surg 2011;142:270-7. [DOI] [PubMed] [Google Scholar]
- 8.Stähli BE, Reinthaler M, Nguyen-Kim TD, et al. Transcatheter aortic valve-in-valve implantation: clinical outcome as defined by VARC-2 and postprocedural valve dysfunction according to the primary mode of bioprosthesis failure. J Invasive Cardiol 2014;26:542-7. [PubMed] [Google Scholar]
- 9.Ussia GP, Barbanti M, Ramondo A, et al. The valve-in-valve technique for treatment of aortic bioprosthesis malposition an analysis of incidence and 1-year clinical outcomes from the italian CoreValve registry. J Am Coll Cardiol 2011;57:1062-8. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phan K, Tian DH, Cao C, et al. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015;4:112-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tourmousoglou C, Rao V, Lalos S, et al. What is the best approach in a patient with a failed aortic bioprosthetic valve: transcatheter aortic valve replacement or redo aortic valve replacement? Interact Cardiovasc Thorac Surg 2015;20:837-43. [DOI] [PubMed] [Google Scholar]
- 13.NHS Centre for Reviews and Dissemination. Undertaking systematic reviews of research on effectiveness: CRD's guidance for those carrying out or commissioning reviews. York: University of York. CRD Report 4 (2nd edition). 2001. [Google Scholar]
- 14.Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann Math Statist 1950;21:607-11. [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 16.Subban V, Savage M, Crowhurst J, et al. Transcatheter valve-in-valve replacement of degenerated bioprosthetic aortic valves: a single Australian Centre experience. Cardiovasc Revasc Med 2014;15:388-92. [DOI] [PubMed] [Google Scholar]
- 17.Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014;312:162-70. [DOI] [PubMed] [Google Scholar]
- 18.Diemert P, Seiffert M, Frerker C, et al. Valve-in-valve implantation of a novel and small self-expandable transcatheter heart valve in degenerated small surgical bioprostheses: the Hamburg experience. Catheter Cardiovasc Interv 2014;84:486-93. [DOI] [PubMed] [Google Scholar]
- 19.Bapat V, Davies W, Attia R, et al. Use of balloon expandable transcatheter valves for valve-in-valve implantation in patients with degenerative stentless aortic bioprostheses: technical considerations and results. J Thorac Cardiovasc Surg 2014;148:917-22; discussion 922-4. [DOI] [PubMed] [Google Scholar]
- 20.Ihlberg L, Nissen H, Nielsen NE, et al. Early clinical outcome of aortic transcatheter valve-in-valve implantation in the Nordic countries. J Thorac Cardiovasc Surg 2013;146:1047-54; discussion 1054. [DOI] [PubMed] [Google Scholar]
- 21.Seiffert M, Conradi L, Baldus S, et al. Impact of patient-prosthesis mismatch after transcatheter aortic valve-in-valve implantation in degenerated bioprostheses. J Thorac Cardiovasc Surg 2012;143:617-24. [DOI] [PubMed] [Google Scholar]
- 22.Linke A, Woitek F, Merx MW, et al. Valve-in-valve implantation of Medtronic CoreValve prosthesis in patients with failing bioprosthetic aortic valves. Circ Cardiovasc Interv 2012;5:689-97. [DOI] [PubMed] [Google Scholar]
- 23.Latib A, Ielasi A, Montorfano M, et al. Transcatheter valve-in-valve implantation with the Edwards SAPIEN in patients with bioprosthetic heart valve failure: the Milan experience. EuroIntervention 2012;7:1275-84. [DOI] [PubMed] [Google Scholar]
- 24.Gaia DF, Couto A, Breda JR, et al. Transcatheter aortic valve-in-valve implantation: a selection change? Rev Bras Cir Cardiovasc 2012;27:355-61. [DOI] [PubMed] [Google Scholar]
- 25.Eggebrecht H, Schäfer U, Treede H, et al. Valve-in-valve transcatheter aortic valve implantation for degenerated bioprosthetic heart valves. JACC Cardiovasc Interv 2011;4:1218-27. [DOI] [PubMed] [Google Scholar]
- 26.Bedogni F, Laudisa ML, Pizzocri S, et al. Transcatheter valve-in-valve implantation using Corevalve Revalving System for failed surgical aortic bioprostheses. JACC Cardiovasc Interv 2011;4:1228-34. [DOI] [PubMed] [Google Scholar]
- 27.Kempfert J, Van Linden A, Linke A, et al. Transapical off-pump valve-in-valve implantation in patients with degenerated aortic xenografts. Ann Thorac Surg 2010;89:1934-41. [DOI] [PubMed] [Google Scholar]
- 28.Di Eusanio M, Saia F, Pellicciari G, et al. In the era of the valve-in-valve: is transcatheter aortic valve implantation (TAVI) in sutureless valves feasible? Ann Cardiothorac Surg 2015;4:214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phan K, Zhou JJ, Niranjan N, et al. Minimally invasive reoperative aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]