Abstract
Background
Refractory gastroesophageal reflux-induced chronic cough (GERC) is difficult to manage. The purpose of the study is to evaluate the efficacy of a novel stepwise protocol for treating this condition.
Methods
A total of 103 consecutive patients with suspected refractory reflux-induced chronic cough failing to a standard anti-reflux therapy were treated with a stepwise therapy. Treatment commences with high-dose omeprazole and, if necessary, is escalated to subsequent sequential treatment with ranitidine and finally baclofen. The primary end-point was overall cough resolution, and the secondary end-point was cough resolution after each treatment step.
Results
High-dose omeprazole eliminated or improved cough in 28.1% of patients (n=29). Further stepwise of treatment with the addition of ranitide yielded a favorable response in an additional 12.6% (n=13) of patients, and subsequent escalation to baclofen provoked response in another 36.9% (n=38) of patients. Overall, this stepwise protocol was successful in 77.6% (n=80) of patients. The diurnal cough symptom score fell from 3 [1] to 1 [0] (Z=6.316, P=0.000), and the nocturnal cough symptom score decreased from 1 [1] to 0 [1] (Z=–4.511, P=0.000), with a corresponding reduction in the Gastroesophageal Reflux Diagnostic Questionnaire score from 8.6±1.7 to 6.8±0.7 (t=3.612, P=0.000). Conversely, the cough threshold C2 to capsaicin was increased from 0.49 (0.49) µmol/L to 1.95 (2.92) µmol/L (Z=–5.892, P=0.000), and the cough threshold C5 was increased from 1.95 (2.92) µmol/L to 7.8 (5.85) µmol/L (Z=–5.171, P=0.000).
Conclusions
Sequential stepwise anti-reflux therapy is a useful therapeutic strategy for refractory reflux-induced chronic cough.
Keywords: Baclofen, cough, gastroesophageal reflux, histamine H2 receptor antagonists, proton pump inhibitors (PPIs)
Introduction
Gastroesophageal reflux-induced chronic cough (GERC) is a special form of gastroesophageal reflux disease (GERD), and one of the three most common causes of chronic cough (1,2). GERC can be divided into acid and nonacid according to the nature of reflux inducing the cough (3). Proton pump inhibitors (PPIs) alone or in combination with prokinetic drugs are the standard therapy for GERC and can resolve cough in most patients (1), although there is controversy regarding its therapeutic effects (4,5). However, the failure to standard anti-reflux therapy, which we define as refractory GERC (6), is not rare and remains an unresolved challenge.
Currently, pharmacological anti-reflux therapy is still the mainstay in the management of refractory GERC (7). However, little is known regarding the appropriate therapeutic strategies. We have previously reported that baclofen, a selective gamma-aminobutyric acid B receptor agonist and a transient lower esophageal sphincter relaxation inhibitor, is helpful in the management of refractory GERC and that failure of response to baclofen might be resolved through subsequent intensified acid suppression using a high-dose PPI or the addition of a histamine H2 receptor antagonist to the pharmacological therapy (8). Based on this observation, we decided to investigate a stepwise protocol involving anti-reflux therapy for refractory GERC in a prospective study.
Materials and methods
Patients
Consecutive patients with suspected refractory GERC visiting our respiratory clinic were recruited into this study between February 2010 and August 2014. The cause of chronic cough was established according to a previously described algorithm (9). Refractory GERC was suspected when the patients met all of the following criteria: (I) the presence of chronic cough, with or without typical reflux symptoms; (II) multi-channel intraluminal impedance-pH monitoring (MII-pH) revealed abnormal acid (for acid GERC) or nonacid reflux (for nonacid GERC), as defined by a DeMeester score of ≥14.72 and/or a syndrome association probability (SAP) for acid or nonacid reflux of ≥95%; (III) cough failed to improve with an 8-week course of standard anti-reflux treatment consisting of omeprazole (20 mg, twice daily) and domperidone (10 mg, three times per day) (6,8). Current smokers and those who had ceased smoking for less than 2 years or patients who presented with concomitant alternative explanations for chronic cough, like the other pulmonary or extra-pulmonary etiologies such as asthma and rhinitis or postnasal drip, were excluded.
This study was a single-center, open-label interventional study. The protocol was approved by the Ethics Committee of Tongji Hospital and registered with the Chinese Clinical Trials Register (http://www.chictr.org/; number ChiCTR-ONC-13003066). Written informed consent was obtained from all of the participants. The preliminary results of this study was orally presented at 2012 Forth American Cough Conference held in New York (10).
Sequential stepwise of anti-reflux therapy
Following the identification and recruitment of suspected refractory GERC patients, the first step in the protocol was commencing high-dose PPI treatment, i.e., doubling the current dose of omeprazole to 40 mg, twice daily, plus domperidone (10 mg, three times daily) for 8 weeks. The patients who responded to the first-step of the protocol were maintained on this treatment until their cough resolved. If the cough was not resolved, ranitidine (150 mg, twice daily), a histamine H2 receptor antagonist, was added to the medication regimen for another 8 weeks, constituting the second step of the protocol. When this regimen improved the cough, the patients stayed on the augmented acid suppression until their symptoms disappeared. The remaining nonresponders were escalated to the third step of the protocol, which consisted of omeprazole (20 mg, twice daily) and baclofen (20 mg, three times daily), whereas domperidone and ranitidine were discontinued (6,8). If a favorable response occurred, the treatment was maintained until cough resolution was achieved. Otherwise, the therapy was ended 8 weeks later.
Outcome measures
The primary end-point was the overall rate of cough resolution. Cough severity was evaluated using the cough symptom score described by Hsu et al. (11), which grades daytime and nighttime cough on a six-point incremental scale, zero being no cough and five being a very severe cough occurring at the most of time. The cough was considered as controlled when the cough disappeared, improved when the combined daytime and nighttime cough symptom score decreased by two or more, and failed when the cough worsened or was not attenuated to a discernible degree (3,8).
The secondary end-points included the rate of cough resolution achieved with each step of therapy, the treatment duration required for responders, and changes in the cough symptom score, reflux symptom score, and cough sensitivity to capsaicin. The reflux symptom score was graded by a validated Chinese version of the Gastroesophageal Reflux Disease Questionnaire (GerdQ) (12), originally developed by Jones et al. (13), which consists of four positive reflux-related symptom items scored from zero to three and two negative reflux-related symptom items scored from three to zero, yielding a cumulative score ranging from 0 to 18. A higher GerdQ score represents more severe reflux-related symptoms (13). Cough sensitivity to capsaicin was established according to Fujimura et al. (14), with the modifications adapted to the ERS guidelines (15). The cough thresholds C2 and C5 were defined as the minimum concentrations of capsaicin for the induction of ≥2 or ≥5 coughs, respectively, and were used as indicators of cough sensitivity.
Follow-up
After the start of the stepwise protocol, patients were followed up during biweekly visits to the respiratory clinic, and changes in the cough symptom score and response to therapy were recorded. If the treatment was effective, the follow-up was prolonged to at least 12 weeks. When the therapy was ended, the GerdQ score and cough sensitivity to capsaicin were again established.
Statistical analysis
Data with normal distribution were expressed as mean ± standard deviation (SD), whereas data with skewed distribution were expressed as medians (interquartile). One-way analysis of variance followed by the Newman-Keuls test, the Kruskal-Wallis test followed by the Mann-Whitney U-test, and the unpaired Student’s t-test were used for comparison of data, where applicable. Software (SPSS 17.0, Chicago, IL, USA) was used for statistical calculations.
Results
Of the 286 patients diagnosed with suspected GERC during the study, standard anti-reflux therapy resolved the cough in 183 patients (64.0%), and the remaining 103 patients (36.0%) were recruited into this study. Their characteristics and responses to therapy are shown in Table 1 and Figure 1.
Table 1. Demographic characteristics of 103 patients with suspected refractory GERC.
| Item | Value |
|---|---|
| Male/female | 45/58 |
| Age (years) | 42.8±12.5 |
| Cough duration (months) | 29.0 (36.0) |
| Cough symptom score | |
| Daytime | 3 (1.0) |
| Nighttime | 1 (1.0) |
| DeMeester score | 62.6±12.8 |
| SAP for acidic reflux (%) | 78.1 (45.2) |
| SAP for nonacidic reflux (%) | 63.0 (73.8) |
| FEV1 (% predictive value) | 94.5±9.3 |
| FVC (% predictive value) | 98.1±10.9 |
| FEV1/FVC (%) | 83.2±8.6 |
Data for cough duration, cough symptom score, and SAP are expressed as median (interquartile). GERC, gastroesophageal reflux-induced chronic cough; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; SAP, symptom association probability.
Figure 1.
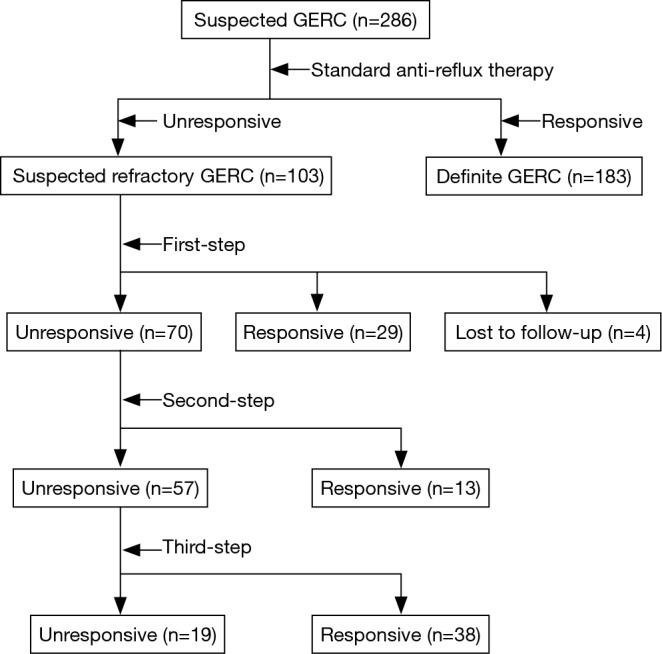
Consort flow diagram of the study for the treatment of refractory gastroesophageal reflux-induced chronic cough (GERC) with sequential stepwise anti-reflux therapy.
Except for four patients with abnormal nonacid reflux who were lost to follow-up after 2 weeks of treatment and were considered as failed, 99 patients (96.1%) completed the first step of the protocol. Of this latter group, 29 patients (28.1%) responded favorably (controlled in 16 patients and improved in 13 patients). The treatment duration for these responders was 11.52±1.98 weeks. Of the 70 patients (68.0%) who enrolled in the second tier of the stepwise protocol, 13 patients (12.6%) had their cough resolved (controlled in 6 patients and improved in 7 patients). The treatment duration for these responders was 10.08±1.70 weeks. The remaining 57 patients (55.3%) were included in the third step of the protocol, which proved effective in 38 patients (36.9%) (controlled in 20 patients and improved in 18 patients). The treatment duration for this last group of protocol responders was 10.96±1.72 weeks.
In total, 80 patients (77.6%) benefited from this stepwise anti-reflux protocol, including 49 cases of acid GERC and 31 cases of nonacid GERC. Cough was controlled in 52.5% of patients (26 cases of acid GERC and 16 cases of nonacid GERC) and improved in 47.5% of patients (23 cases of acid GERC and 15 cases of nonacid GERC). In 23 patients resistant to the therapy (including 4 patients lost to follow-up), 7 presented with abnormal acid reflux and 16 with abnormal nonacid reflux; and the underlying causes of chronic cough in these patients remain unclear.
As detected by MII-pH, the DeMeester score and the number of acid reflux episodes were significantly higher in the patients responsive to the first-step therapy than in the patients responsive to the second- or third-step therapy; while these values were comparable between the responders to the second- and third-step therapies. The numbers of weakly acidic reflux episodes were similar between the patients who benefited from the first- and second-step therapies but were higher than those of the responders to the third step of the protocol (Table 2).
Table 2. Comparison of MII-pH variables between patients with refractory GERC who responded to each step of the therapy.
| Variable | First step (n=29) | Second step (n=13) | Third step (n=38) |
|---|---|---|---|
| DeMeester score | 85.1±18.8*∆ | 36.3±15.2 | 29.9±9.2 |
| SAP for acidic reflux (%) | 82.1 (33.4) | 76.7 (70.7) | 75.3 (39.0) |
| SAP for nonacidic reflux (%) | 61.0 (80.3) | 95.5 (18.5) | 69.1 (53.0) |
| Acid reflux (n) | 63.7±13.7*∆ | 20.7±3.2 | 21.6±4.1 |
| Weakly acidic reflux (n) | 46.8±6.8∆ | 45.2±14.6∆ | 27.7±7.4 |
| Weakly alkaline reflux (n) | 10.7±2.8 | 16.6±5.0 | 12.8±4.6 |
| Gas reflux (n) | 23.9±8.1 | 23.9±11.6 | 28.9±13.9 |
| Liquid reflux (n) | 25.1±7.1 | 21.6±3.4 | 18.5±3.5 |
| Mixed reflux (n) | 76.4±14.7 | 45.2±12.3 | 55.4±18.8 |
| Proximal extent (n) | 19.3±4.7 | 18.0±4.3 | 18.5±4.8 |
| Bolus exposure (%) | 1.4±0.2 | 1.2±0.1 | 1.3±0.2 |
| Bolus clearance (s) | 9.2±2.1 | 8.4±1.9 | 7.5±1.7 |
*, P<0.05 vs. second step; ∆, P<0.05 vs. third step. The data are expressed as mean ± SD except for SAP, which was expressed as median (interquartile). GERC, gastroesophageal reflux-induced chronic cough; SAP, symptom association probability.
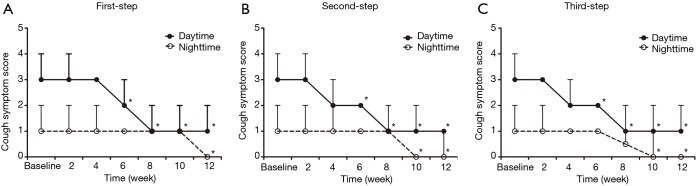
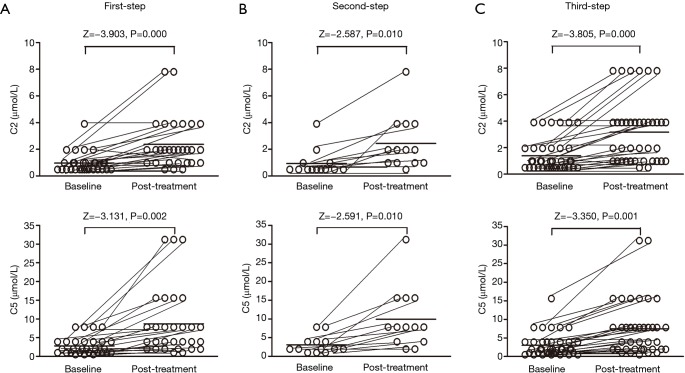
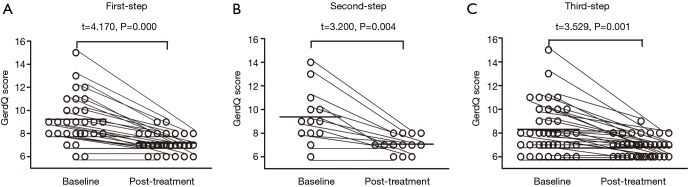
For the 80 patients whose cough was controlled or improved by the protocol, the cough symptom score decreased from 3 [1] to 1 [0] for the daytime (Z=6.316, P=0.000) and from 1 [1] to 0 [1] for the nighttime (Z=–4.511, P=0.000), with a corresponding reduction in the GerdQ score from 8.6±1.7 to 6.8±0.7 (t=3.612, P=0.000). Conversely, the cough threshold C2 to capsaicin was increased from 0.49 (0.49) µmol/L to 1.95 (2.92) µmol/L (Z=–5.892, P=0.000) and the cough threshold C5 to capsaicin was increased from 1.95 (2.92) µmol/L to 7.8 (5.85) µmol/L (Z=–5.171, P=0.000). The changes in the cough symptom score, cough threshold, and GerdQ score for responders at each step of the therapy are shown in Figures 2,3,4.
Figure 2.
Changes of the cough symptom score in the patients with refractory gastroesophageal reflux-induced chronic cough (GERC) responsive to each step of the therapy. *, P<0.05 vs. baseline.
Figure 3.
Changes of the cough thresholds C2 and C5 to capsaicin in the patients with refractory gastroesophageal reflux-induced chronic cough (GERC) responsive to each step of the therapy.
Figure 4.
Changes of the GerdQ score in the patients with refractory gastroesophageal reflux-induced chronic cough (GERC) responsive to each step of the therapy.
Adverse effects of the protocol were mainly reported by patients who were escalated to the third step, including somnolence (n=21, 36.8%), dizziness (n=7, 12.3%), and drowsiness (n=12, 21.1%). These adverse effects were tolerable and did not interrupt the treatment.
Discussion
Our study showed that refractory GERC accounted for about a third of all patients with GERC, which was consistent with the estimated prevalence of 10–40% for refractory GERD, defined as failing to respond symptomatically, either partially or completely, to a standard-dose PPI (16).
With our stepwise protocol, cough was eliminated or improved in 77.6% of patients with suspected refractory GERC, and these results were confirmed by a corresponding decrease in the cough symptom score, GerdQ score, and cough sensitivity to capsaicin. Therefore, the sequential and stepwise anti-reflux protocol appears to be a useful therapeutic strategy for refractory GERC.
Despite substantial uncontrolled data have supported the efficacy of acid suppressive therapy including PPIs, histamine-2 receptor antagonists and prokinetics in resolving cough due to reflux (1), the randomized controlled trials have consistently failed to confirm any significant improvement in cough with high-dose of esomeprazole (4,5). Therefore, it is conceivable that the benefit of PPIs on GERC has been debating. However, PPIs are currently recommended as the standard anti-reflux therapy for GERC (1,7,17,18), especially for the patients with chronic cough who also have typical classical symptoms or objective evidence of abnormal reflux as indicated by reflux monitoring (17). A recent systematic review concludes the use of PPIs is favored for treating GERC in the patients with pathogenic esophageal acid exposure (19). At present, PPIs are still a usual and first-line therapeutic option for the management of GERC even though further study is needed to clarify the issue.
The major mechanism underlying refractory GERC may be incomplete acid suppression (18). Several lines of evidence have demonstrated that an abnormal esophageal pH is found in 4−16% of GERD patients with refractory symptoms and taking PPIs twice daily (20,21). The residual acid reflux can continue to elicit cough through microaspiration or an esophageal-tracheobronchial reflex (1). The resistance to PPIs may be due to rapid PPI metabolism; thus PPI serum levels allowing adequate acid suppression cannot be reached (22). Moreover, the severe esophageal acid exposure may require a higher dose of PPI to control gastric acid secretion effectively (20,21). Therefore, cough resolution in the patients responding to the first-step therapy appears to be related to more adequate acid suppression as a consequence of the increased omeprazole dose. In apparent agreement, more pronounced acid reflux was identified in these patients indicated by MII-pH.
Our results also revealed that further escalation of acid suppression by adding a histamine H2 receptor antagonist to the first-step therapy resulted in cough resolution in an additional subfraction of patients. Nocturnal acid breakthrough, a phenomenon of the total time of pH <4 at night longer than 60 min, occurred in 75% of patients with GERD, irrespective of PPI therapy (23), and has been proposed as one of the causes of PPI failure (7,17,18). It has been reported that the addition of a histamine H2 receptor antagonist at bedtime improves nocturnal gastric acid control and attenuates overall symptoms in patients with GERD (24). In this study, we did not attempt to target possible nocturnal acid breakthrough, since ranitidine was not used at bedtime and an accumulating body of evidence does not support a significant role for nocturnal acid breakthrough in precipitating the occurrence of refractory GERD (17). The therapeutic gain was probably derived from the more effective acid suppression due to the synergistic action of omeprazole and ranitidine through inhibiting the different target points on the parietal cell in the stomach.
Surprisingly, the patients responsive to the second tier of the protocol therapy presented with less severe esophageal acid exposure than those benefiting from the first-step treatment, as shown by a relatively lower DeMeester score and a fewer number of acid reflux episodes. Esophageal hypersensitivity could be a possible explanation for these patients (18). Local acidification due to distal esophageal reflux can trigger tissue injury, dilate the epithelial intercellular space, permit some acid penetration, and expose the subepithelial nerve to the acid refluxate (25). Furthermore, inflammatory mediators released during inflammation may upregulate vanilloid 1 receptor expression on the sensory nerve terminals innervating the esophagus, which in turn may modify the release and responsiveness to neurotransmitters and thus reduce the transduction threshold of primary afferents (26,27). The final result will be the strengthening of the esophageal-tracheobronchial reflex, thus causing the cough to become persistent (1). By adding a histamine H2 receptor antagonist to the high-dose PPI, the acidity of the refluxate might be further decreased. Even for weakly acidic reflux, which accounts for the majority of nonacid reflux (28), a mild-grade increase in the pH of the refluxate might result in an obvious decrease of acid signal transduction in the hypersensitive esophagus, leading to cough resolution. Thus, our results reinforce the fact that total acid control is extremely important in the management of refractory GERC, even when the cough is caused by nonacid reflux.
Transient lower esophageal sphincter relaxation can explain all types of reflux (29). PPIs reduce the acidity and volume of the refluxate, decrease reflux episodes when combined with the prokinetic agents including domperidone or metoclopramide, and thus resolve cough in patients with GERC (7). Nevertheless, they are unable to rectify more frequent transient lower esophageal sphincter relaxation in GERC patients and cannot eliminate reflux episodes (7,18). Moreover, PPIs are less effective for nonacid GERC (30). In this case, baclofen may help to control cough since it inhibits transient lower esophageal sphincter relaxations in addition to its nonspecific antitussive activity recognized for long time (31). In the present study, baclofen was effective in 66.7% of the patients with refractory GERC who failed the first two steps of the protocol, with a success rate higher than that reported by us previously (6,8). In contrast to our previous study in which baclofen was the first line of therapy, in the present study, patients were first provided with initial intensified acid suppression prior to baclofen application. The delay in baclofen therapy in our protocol may have resulted in the selection of patients most likely to respond to baclofen treatment.
There were several limitations to the present study, the open interventional nature being the most prominent. However, it is very difficult to design a randomized, double-blind, and placebo-controlled trial for the sequential stepwise anti-reflux protocol. Although a coincidental cough relief or a placebo effect of the therapy cannot be excluded, we believe that they do not explain the high efficacy of the therapeutic strategy, since these patients had had a persistent cough for a median period of more than 2 years and had received diverse therapies specific to the various etiologies of chronic cough before being recruited into this study. Ideally, the MII-pH analysis should have been repeated after each step of the treatment, but the invasive nature of MII-pH precluded this analysis for most of the subjects except for the five patients with acid GERC, who accepted a repeated MII-pH after their cough resolved with the first-step therapy. As predicted, acid reflux was nearly completely inhibited in these five patients. However, we cannot definitely conclude how acid or nonacid reflux was directly inhibited by the therapeutic strategy because of the lack of enough data. Finally, one can criticize the cough severity evaluation and outcome measures because they are mainly based on subjective questionnaires (32). The cough symptom score is a reliable tool, and it has been validated against a 24-h continuous cough recording (11). Moreover, the use of acoustic cough monitoring to measure the changes in cough frequency as a function of anti-reflux therapy is not feasible because of its commercial unavailability.
In conclusion, the sequential stepwise anti-reflux therapy, initially with a high-dose PPI followed by the addition of a histamine H2 receptor antagonist or baclofen, may be an appropriate therapeutic strategy for the management of refractory GERC.
Acknowledgements
The authors thank AstraZeneca AB, Sweden, for its kind permission to use a Chinese version of GerdQ in this study.
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81470276 and 81400071) and the project of Science and Technology Commission of Shanghai Municipality (No. 14411971700 and 15411965500) and the project of Shanghai Municipal Health Bureau (No. 20134Y004).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Irwin RS. Chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest 2006;129:80S-94S. [DOI] [PubMed] [Google Scholar]
- 2.Morice AH, Fontana GA, Sovijarvi AR, et al. The diagnosis and management of chronic cough. Eur Respir J 2004;24:481-92. [DOI] [PubMed] [Google Scholar]
- 3.Xu X, Yang Z, Chen Q, et al. Comparison of clinical characteristics of chronic cough due to non-acid and acid gastroesophageal reflux. Clin Respir J 2015;9:196-202. [DOI] [PubMed] [Google Scholar]
- 4.Faruqi S, Molyneux ID, Fathi H, et al. Chronic cough and esomeprazole: a double-blind placebo-controlled parallel study. Respirology 2011;16:1150-6. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Crockett SD, Bright SD, et al. Randomised clinical trial: high-dose acid suppression for chronic cough - a double-blind, placebo-controlled study. Aliment Pharmacol Ther 2011;33:225-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X, Chen Q, Liang S, et al. Successful resolution of refractory chronic cough induced by gastroesophageal reflux with treatment of baclofen. Cough 2012;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ates F, Francis DO, Vaezi MF. Refractory gastroesophageal reflux disease: advances and treatment. Expert Rev Gastroenterol Hepatol 2014;8:657-67. [DOI] [PubMed] [Google Scholar]
- 8.Xu XH, Yang ZM, Chen Q, et al. Therapeutic efficacy of baclofen in refractory gastroesophageal reflux-induced chronic cough. World J Gastroenterol 2013;19:4386-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei W, Yu L, Lu H, et al. Comparison of cause distribution between elderly and non-elderly patients with chronic cough. Respiration 2009;77:259-64. [DOI] [PubMed] [Google Scholar]
- 10.Xu XH, Chen Q, Yu L, et al. Efficacy of sequential stepwise anti-reflux therapy on refractory chronic cough due to gastroesophageal reflux (Abstract) Lung 2014;192:1-7. Available online: http://link.springer.com/article/10.1007/s00408-013-9552-7 [Google Scholar]
- 11.Hsu JY, Stone RA, Logan-Sinclair RB, et al. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur Respir J 1994;7:1246-53. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Chen Q, Liang S, et al. Comparison of gastroesophageal reflux disease questionnaire and multichannel intraluminal impedance pH monitoring in identifying patients with chronic cough responsive to antireflux therapy. Chest 2014;145:1264-70. [DOI] [PubMed] [Google Scholar]
- 13.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2009;30:1030-8. [DOI] [PubMed] [Google Scholar]
- 14.Fujimura M, Sakamoto S, Kamio Y, et al. Effects of methacholine induced bronchoconstriction and procaterol induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid. Thorax 1992;47:441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007;29:1256-76. [DOI] [PubMed] [Google Scholar]
- 16.Hershcovici T, Fass R. Management of gastroesophageal reflux disease that does not respond well to proton pump inhibitors. Curr Opin Gastroenterol 2010;26:367-78. [DOI] [PubMed] [Google Scholar]
- 17.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-28; quiz 329. [DOI] [PubMed] [Google Scholar]
- 18.Lv HJ, Qiu ZM. Refractory chronic cough due to gastroesophageal reflux: Definition, mechanism and management. World J Methodol 2015;5:149-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahrilas PJ, Howden CW, Hughes N, et al. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest 2013;143:605-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bautista JM, Wong WM, Pulliam G, et al. The value of ambulatory 24 hr esophageal pH monitoring in clinical practice in patients who were referred with persistent gastroesophageal reflux disease (GERD)-related symptoms while on standard dose anti-reflux medications. Dig Dis Sci 2005;50:1909-15. [DOI] [PubMed] [Google Scholar]
- 21.Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005;100:283-9. [DOI] [PubMed] [Google Scholar]
- 22.Klotz U. Impact of CYP2C19 polymorphisms on the clinical action of proton pump inhibitors (PPIs). Eur J Clin Pharmacol 2009;65:1-2. [DOI] [PubMed] [Google Scholar]
- 23.Peghini PL, Katz PO, Bracy NA, et al. Nocturnal recovery of gastric acid secretion with twice-daily dosing of proton pump inhibitors. Am J Gastroenterol 1998;93:763-7. [DOI] [PubMed] [Google Scholar]
- 24.Xue S, Katz PO, Banerjee P, et al. Bedtime H2 blockers improve nocturnal gastric acid control in GERD patients on proton pump inhibitors. Aliment Pharmacol Ther 2001;15:1351-6. [DOI] [PubMed] [Google Scholar]
- 25.Park S, Lee EJ, Chun HJ, et al. Electron microscopic study of intercellular space: correlation analysis of bronchial asthma and gastroesophageal reflux disease. J Gastroenterol Hepatol 2011;26:104-7. [DOI] [PubMed] [Google Scholar]
- 26.Harnett KM, Rieder F, Behar J, et al. Viewpoints on Acid-induced inflammatory mediators in esophageal mucosa. J Neurogastroenterol Motil 2010;16:374-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews PJ, Aziz Q, Facer P, et al. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol 2004;16:897-902. [DOI] [PubMed] [Google Scholar]
- 28.Boeckxstaens GE, Smout A. Systematic review: role of acid, weakly acidic and weakly alkaline reflux in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2010;32:334-43. [DOI] [PubMed] [Google Scholar]
- 29.Hershcovici T, Mashimo H, Fass R. The lower esophageal sphincter. Neurogastroenterol Motil 2011;23:819-30. [DOI] [PubMed] [Google Scholar]
- 30.Sifrim D, Mittal R, Fass R, et al. Review article: acidity and volume of the refluxate in the genesis of gastro-oesophageal reflux disease symptoms. Aliment Pharmacol Ther 2007;25:1003-17. [DOI] [PubMed] [Google Scholar]
- 31.Dicpinigaitis PV, Dobkin JB. Antitussive effect of the GABA-agonist baclofen. Chest 1997;111:996-9. [DOI] [PubMed] [Google Scholar]
- 32.Vaezi MF. Chronic cough and gastroesophageal reflux disease: how do we establish a causal link? Chest 2013;143:587-9. [DOI] [PubMed] [Google Scholar]