Abstract
The Wada test is widely used in the presurgical evaluation of potential temporal lobectomy patients to predict postoperative memory function. Expected asymmetry (EA), defined as Wada memory lateralized to the nonsurgical hemisphere, or a higher score after injection of the surgical hemisphere would be considered favorable in terms of postoperative memory outcome. However, in some cases, nonlateralized memory (NM) results, with no appreciable asymmetry, may occur because of impaired scores after both injections, often leading to denial of surgery. The reason for such nonlateralized Wada memory in patients with intractable temporal lobe epilepsy (TLE) remains unclear. Given that quantitative morphometric magnetic resonance imaging studies in TLE patients have shown bilateral regional atrophy in temporal and extratemporal structures, we hypothesized that the volume loss in contralateral temporal structures could contribute to nonlateralized Wada memory performance. To investigate this, we examined the relationship between the volume changes of temporal structures and Wada memory scores in patients with intractable TLE with mesial temporal sclerosis (MTS) using an age- and gender-matched control group. Memory was considered nonlateralized if the absolute difference in the total correct recall scores between ipsilateral and contralateral injections was <11%. Among 21 patients, Wada memory was lateralized in 15 and nonlateralized in 6 patients, with all the nonlateralized scores being observed in left TLE. The recall scores after ipsilateral injection were significantly lower in patients with an NM profile than an EA profile (23±14% vs. 59±18% correct recall, p < 0.001). However, the recall scores after contralateral injection were low but similar between the two groups (25±17% vs. 25±15% correct recall, p=0.97). Compared to controls, all the patients showed greater volume loss in the temporal regions. However, patients with a NM profile showed significantly more volume loss than those with a lateralized memory profile in both contralateral and ipsilateral temporal regions (p<0.05). Left hemispheric Wada memory performance correlated positively with the size of the left mesial and neocortical temporal structures (r = 0.45–0.63, p = 0.005–0.02). Our study suggests that volume loss in the nonsurgical temporal structures is associated with nonlateralized Wada memory results in patients with intractable TLE.
Keywords: Regional Atrophy, Temporal Lobe Epilepsy, Wada test, mesial temporal sclerosis
1. INTRODUCTION
Verbal memory decline is one of the major concerns associated with surgery for temporal lobe epilepsy (TLE), especially after dominant anterior temporal lobectomy (ATL). Accurate prediction of memory decline prior to ATL is challenging. The Wada test (also known as the intracarotid amobarbital procedure) was originally developed to determine hemispheric language dominance in prospective ATL candidates to prevent significant language disturbances after surgery (Wada and Rasmussen, 2007). Subsequently, the test was adopted for memory assessment before resective surgery (Milner and Rasmussen, 1962). The Wada test simulates the effects of surgery by temporarily inactivating the surgical hemisphere using amobarbital, a short-acting pharmaceutical agent, to evaluate the ability of the nonsurgical hemisphere to maintain memory functions. The results of the Wada memory assessment may be classified into three categories: expected asymmetry (EA), when the memory score after ipsilateral injection (surgical side) is higher than contralateral injection (nonsurgical side); reversed asymmetry (RA), when the memory score after contralateral injection (nonsurgical side) is higher than ipsilateral injection (surgical side); and nonlateralized memory (NM), when there is no appreciable asymmetry in the memory scores after the two injections. Patients with EA are usually offered surgery under the assumption that the nonsurgical hemisphere can support memory after the removal of the intended surgical temporal lobe. On the contrary, patients with RA may be denied surgery because such patients have been shown to have significantly lower scores on neuropsychological measures (Diaz-Arrastia et al., 2002), greater risk for postoperative memory impairment, and unfavorable seizure outcomes compared to patients with EA (Sabsevitz et al., 2001). However, patients with NM are challenging, and the decision to proceed to surgery in such patients remains controversial. For NM patients, the surgical decision depends upon various factors, including the presence of lesions, seizure localization, results of neuropsychological assessment, and the experience of the epilepsy center.
The practice of performing anterior temporal resections based on results of the Wada test is controversial. The underlying reasons for RA and NM in patients with intractable TLE also remain unclear. Thus, a more complete understanding of the substrates of memory in TLE patients is needed to better predict postoperative memory outcome after ATL. Prior quantitative volumetric magnetic resonance imaging (MRI) studies in TLE patients have shown bilateral regional atrophy in temporal and extratemporal structures (Bernhardt et al., 2010; Ding et al., 2014; Labate et al., 2011; McDonald et al., 2008; Mueller et al., 2009a, b), suggesting that poor memory performance in the nonsurgical hemisphere may be related to unrecognized hippocampal damage or dysfunction in the nonsurgical temporal lobe. The objective of this study was to further investigate the relationship between regional brain volumes within the temporal structures and presurgical Wada memory test performance in TLE patients. We hypothesized that greater temporal lobe volume loss in the nonsurgical hemisphere (contralateral to the seizure focus) is associated with impaired Wada memory performance after ipsilateral amobarbital injection.
2. METHODS
2.1. Subjects
This is a retrospective, cross-sectional study of patients that were recruited over a 5-year period (January 2005 to June 2010) from the Epilepsy Monitoring Unit at Parkland Memorial Hospital and the University of Texas Southwestern Medical Center, Dallas, Texas. Demographic and clinical data were obtained through a prospectively maintained electronic database. Seizure localization, lateralization and TLE diagnosis were made based on prolonged ictal and interictal video-electroencephalography (EEG) recordings. The inclusion criteria were: 1) unilateral temporal seizure onset documented by video-EEG recording, 2) radiological diagnosis of mesial temporal sclerosis (MTS), and 3) at least one 3.0 Tesla (T) MRI with high-resolution 3D T1 sequence. Exclusion criteria were: 1) presence of focal lesions other than MTS, such as encephalomalacia, vascular malformations or lesions, brain tumor, neurocysticercosis and brain abscess, and 2) other conditions which may result in abnormal MRI findings and compromise cognitive functions (e.g., multiple sclerosis, encephalitis/meningitis, Alzheimer’s disease/mild cognitive impairment, history of developmental delay and psychiatric disease). Twenty-two age- and gender-matched healthy volunteers were selected from our traumatic brain injury database (Wang et al., 2008) as controls to determine the MRI volumetric measurements of the patients. University of Texas Southwestern Medical Center Institutional Review Board approval was obtained prior to data collection for this study.
2.2. The Wada memory test
The Wada protocol used at our center has been described in detail in the elsewhere (Diaz-Arrastia et al., 2002). Briefly, under continuous video-EEG monitoring, each patient underwent angiographic visualization of the carotid circulation bilaterally to assess vascular anatomy and exclude abnormalities. Subsequently, 90–120 mg of sodium amobarbital, at a concentration of 20 mg/ml, was injected over 10 seconds into the internal carotid artery. The presumptive surgical side was injected first (ipsilateral injection), and 30–45 minutes later, the nonsurgical side was injected (contralateral injection). The duration of arm weakness, hemianopia, aphasia and EEG slowing were recorded. Tests were considered invalid if, in the opinion of the epileptologist, obtundation or inattention was sufficient to interfere with registration of items during the presentation phase. Invalid tests were not included in the analysis. Items were presented within the first 3 minutes of injection in the ipsilateral visual field during the period of arm weakness and EEG slowing. English-speaking subjects were administered an 18-item memory test while the Spanish-speaking subjects were administered a 17-item test. In each test, half of the items were composed of written words that alternated with pictorial items, including both abstract shapes and everyday items. After recovery of arm weakness and return of the EEG to baseline (approximately 10 minutes), item recognition was tested by presenting the target and seven similar foils simultaneously, and forcing the patients to choose one among the eight items. The test was scored as the fraction of items recalled correctly, with no deduction for errors. A second similar, but separate, set of targets and foils was used for the second injection. A third set of targets and foils was presented the day prior to the test in the absence of anesthetic to obtain a baseline score. For the purposes of this, the Wada memory asymmetry was arbitrarily determined by the difference between ipsilateral and contralateral injection scores. A difference of more than +11% (equivalent to non-recognition of ≥2 targets after contralateral injection compared to ipsilateral injection) was considered an EA profile, whereas a difference of more than −11% (equivalent to non-recognition of ≥2 targets after ipsilateral injection compared to contralateral injection) was considered an RA profile. If the difference in memory scores between the 2 injections was <11%, the test was considered an NM profile. Patients with correct recall of ≤33% with ipsilateral injection or bilateral injections were considered to have failed the Wada memory test (Diaz-Arrastia et al., 2002).
2.3. MRI acquisition and processing
Structural MRI was performed using a General Electric Signal Excite 3.0T MR scanner (GE, Milwaukee, WI). Three-dimensional (3D) T1-weighted structural images were obtained with slice thickness of 1 mm. The Digital Imaging and Communications in Medicine (DICOM) files were transferred to a Macintosh workstation for analysis with FreeSurfer analysis suite (Version 4.5.0; Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA) as described elsewhere (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2002; Fischl et al., 1999). Briefly, the FreeSurfer analysis included averaging of multiple volumetric T1-weighted images, removal of nonbrain tissue, conversion to Talairach coordinates, automated segmentation of subcortical white and deep gray matter structures, tessellation of the gray-white matter junction, surface deformation along intensity gradients for optimal placement of gray-white and gray-cerebrospinal fluid borders, and cortical parcellation with sub-millimeter precision into units based on gyral and sulcal structure. These procedures resulted in high-resolution quantification of thickness, surface area and volume over the entire brain in addition to delineating the atlas-derived subcortical and cortical brain regions. All the images were processed and analyzed using the same version of Freesurfer on a single Macintosh workstation.
2.4. Statistical analysis
Z-scores (standardized scores defined by the number of standard deviations away from the mean of the respective control group) were calculated for all the subcortical and neocortical regions in each subject based on the mean and standard deviation for each region among the controls. The relative differences between healthy and patient group volumes for these regions were compared using 2-tailed t tests. The correlations between regional volume and Wada memory scores were calculated using Spearman’s rank correlation coefficients. A false discovery rate (FDR) of 0.05 was used for all the analyses to correct for multiple comparisons.
Morphometric analyses were conducted by 2 independent raters (K.D. and Y.G.) and inter-rater reliability was determined by having each rater analyze 20 brain images for determination of intra-class correlation coefficients using 2-way analysis of variance with mixed effects. Statistical analyses were performed with SPSS (version 11.5; SPSS Inc., Chicago, Illinois).
3. RESULTS
3.1. Demographics
Twenty-one patients were included in the study (Table 1; see Supplementary Table 1S for detailed information). There were 12 men and 9 women, all right-handed, 22–57 years of age (median 30 years; mean 32 years). The duration of epilepsy was 8–57 years (median 24 years, mean 26 years). Language lateralization was left hemispheric in 20 patients and bilateral in 1 patient. Fourteen out of 21 patients (67%) patients had left MTS on MRI and left temporal seizure onset on scalp EEG. Seven patients (33%) had right MTS on MRI and right temporal seizure onset on scalp EEG. Seizure lateralization based on scalp monitoring was clear enough that none of the patients required depth electrode monitoring. The left- and right-sided seizure onset groups were similar with respect to age and epilepsy duration (p=0.50).
Table 1.
Demographic data
| Seizure lateralization | Total (N) | Male (N) | Handedness (Right/Left) | Age (Y) (mean ± SD) | Epilepsy duration(Y) (mean ± SD) | Wada asymmetry (EA/RA/NM) |
|---|---|---|---|---|---|---|
| Left | 14 | 8 | 14/0 | 32 ± 9.5 | 23 ± 13 | 8/0/6 |
| Right | 7 | 4 | 7/0 | 33 ± 10 | 31 ± 11 | 7/0/0 |
| Total | 21 | 12 | 20/0 | 32 ± 10 | 26 ± 12 | 15/0/6 |
EA, expected asymmetry; RA, reverse asymmetry; NM, nonlateralized memory.
3.2. Wada memory lateralization
The baseline memory recall scores were 74 ±22% and 88±13% in the left and right seizure onset groups respectively (p = 0.16). Wada memory lateralization showed EA, RA and NM profiles in 15 (71%), 0 (0%) and 6 (29%) patients respectively (Table 1). Among the 15 patients with an EA profile, 8 had left MTS and 7 had right MTS. Of note, all patients with NM profile had left MTS. All the 6 patients with NM memory profile failed the Wada test, with correct recall ≤33% on bilateral injections. All the patients with EA memory profile passed the test, with correct recall >33% on ipsilateral injection.
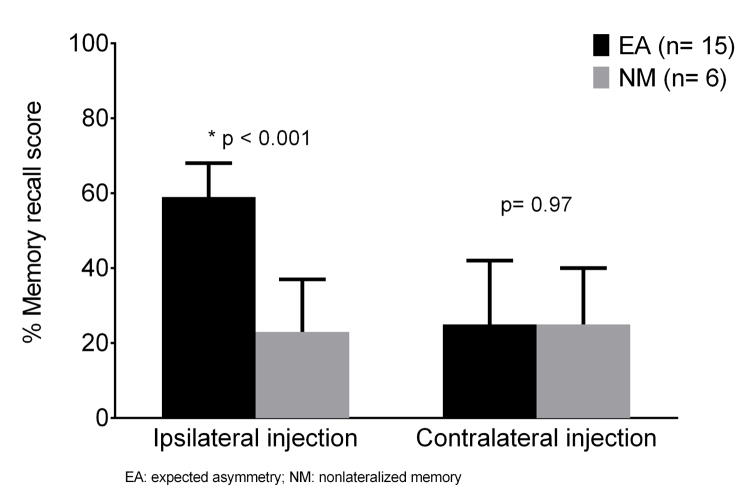
We compared the EA and NM Wada memory profiles (Figure 1 and Table 2S). The recall scores after ipsilateral injection were significantly lower in patients with an NM profile than an EA profile (23±14% vs. 59±18% correct recall, p < 0.001). However, the recall scores after contralateral injection were low but similar between the two groups (25±17% vs. 25±15% correct recall, p=0.97). These findings suggest that bilateral memory impairment, rather than bilateral memory preservation, underlies nonlateralized Wada memory.
Figure 1.
Wada memory lateralization profiles in patient with mesial temporal sclerosis. The recall scores after ipsilateral injection were significantly lower in patients with an NM profile than an EA profile (23±14% vs. 59±18% correct recall, p < 0.001). However, the recall scores after contralateral injection were low but similar between the two groups (25±17% vs. 25±15% correct recall, p=0.97).
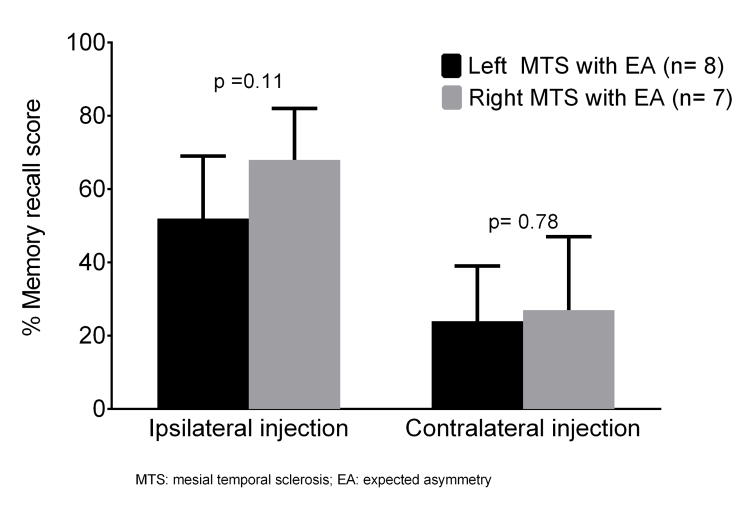
The NM profile on the Wada test was exclusively seen in left MTS patients who showed low memory scores after both ipsilateral (24±14% correct recall) and contralateral injections (25 ±15% correct recall). Among patients with EA profile, the memory recall after ipsilateral injection tended to be lower in left MTS patients compared to right MTS patients (52±17% vs. 68±14% correct recall score, p=0.11). As expected, memory scores were uniformly low (24±15% vs. 27±20% correct recall, p=0.78) between the two groups after contralateral injection (Figure 2 and Table 3S). These findings suggest that left MTS patients are likely to have NM (due to bilateral impairment) or poorly lateralized memory (due to contralateral impairment) compared to right MTS patients.
Figure 2.
Comparison of Wada memory scores in patients with expected asymmetry profile. Among patients with EA profile, the memory recall after ipsilateral injection tended to be lower in left MTS patients compared to right MTS patients (52±17% vs. 68±14% correct recall score, p=0.11). As expected, memory scores were uniformly low (24±15% vs. 27±20% correct recall, p=0.78) between the two groups after contralateral injection.
3.3. Volumetric measurements
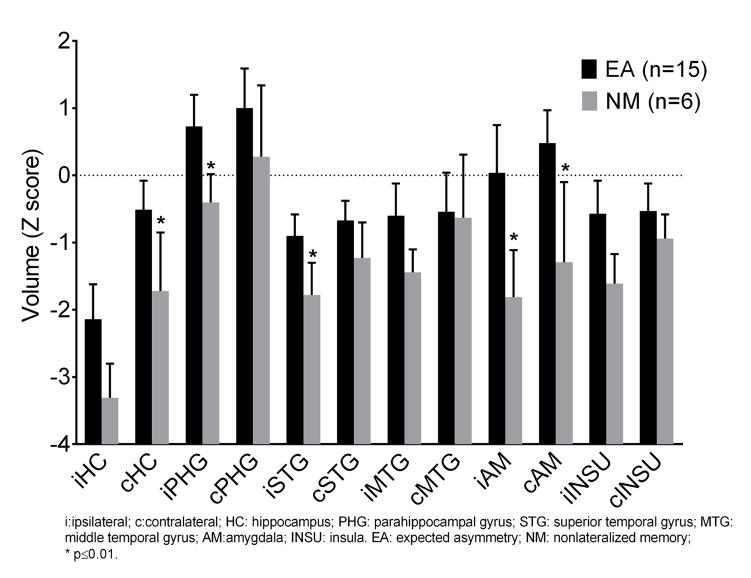
We analyzed the volume and cortical thicknesses of the various limbic regions (Z scores) in the left and right MTS groups. In contrast to patients with right MTS, the patients with left MTS showed significant atrophy in the left hippocampus (−2.66 vs. −0.31), left amygdala (−0.96 vs. 0.93), and left fusiform (−0.78 vs. 0.56) (p< 0.05) (Table 4S). In order to compare the volume and cortical thicknesses in the EA and NM groups, we combined the left and right MTS patients to obtain an adequate sample size. Compared with the controls, the MTS patients showed significant widespread volume loss bilaterally, involving the hippocampi, temporal neocortex, and insula. Furthermore, the NM profile patients showed significantly more volume loss than the EA profile patients not only in the ipsilateral hippocampus (−3.31 vs −2.14), amygdala (−1.81 vs 0.04), parahippocampus (−0.40 vs 0.73), and superior temporal gyrus (−1.78 vs −0.90)], but also in the contralateral hippocampus (−1.72 vs −0.51) and amygdala (−1.29 vs 0.48) (Figure 3 and Table 5S). These findings suggest that the bilateral memory impairment seen on nonlateralized Wada test (as discussed above) may have an anatomic basis, perhaps related to bitemporal volume loss.
Figure 3.
Regional Atrophy in MTS with expected asymmetry Wada memory(EA) and nonlateralized Wada memory(NM). The NM profile patients showed significantly more volume loss than the EA profile patients not only in the ipsilateral hippocampus (−3.31 vs −2.14), amygdala (−1.81 vs 0.04), parahippocampus (−0.40 vs 0.73), and superior temporal gyrus (−1.78 vs −0.90)], but also in the contralateral hippocampus (−1.72 vs −0.51) and amygdala (−1.29 vs 0.48)
3.4. Correlation between Wada memory scores and volumetric measurements
To determine if Wada memory scores correlated with volumetric measurements of temporal and extratemporal structures, we calculated the Spearman’s rank correlation coefficients (Table 2). After right hemispheric injection, which tests left hemispheric memory, the Wada memory recall scores showed significant positive correlation with the size (i.e., volume or thickness) of left hippocampus (r= 0.63, p=0.005), left amygdala (r=0.49, p= 0.04), left superior temporal gyrus (r=0.60, p=0.009), left middle temporal gyrus (r=0.49, p=0.03) and left fusiform gyrus (r= 0.55, p =0.02). After left hemispheric injection, which tests right hemispheric memory, there were no statistically significant correlations between the Wada memory recall scores and volume of temporal regions. Thus, left hemispheric memory correlates positively with the size of the left temporal structures whereas right hemispheric memory has no such correlation with the size of right temporal structures in this cohort of MTS patients.
Table 2.
Correlation between Wada memory scores and volumetric measurements
| Memory score after left injection | Memory score after right injection | |||
|---|---|---|---|---|
| r | p | r | p | |
| L Hippocampus | −0.16 | 0.52 | 0.63 | 0.005 |
| R Hippocampus | 0.26 | 0.27 | −0.37 | 0.13 |
| L Parahippocampal | −0.26 | 0.28 | 0.39 | 0.10 |
| R Parahippocampal | −0.21 | 0.39 | −0.14 | 0.57 |
| L Superior temporal | −0.10 | 0.68 | 0.60 | 0.009 |
| R Superior temporal | −0.14 | 0.58 | 0.20 | 0.41 |
| L Middle temporal | 0.01 | 0.96 | 0.49 | 0.03 |
| R Middle temporal | −0.25 | 0.29 | 0.13 | 0.61 |
| L Inferior temporal | −0.33 | 0.16 | 0.45 | 0.06 |
| R Inferior temporal | −0.11 | 0.66 | 0.07 | 0.77 |
| L Transverse temporal | 0.21 | 0.40 | −0.03 | 0.91 |
| R Transverse temporal | 0.05 | 0.83 | 0.26 | 0.30 |
| L Temporal pole | 0.04 | 0.86 | 0.37 | 0.14 |
| R Temporal pole | −0.32 | 0.18 | −0.11 | 0.69 |
| L Amygdala | −0.08 | 0.74 | 0.49 | 0.04 |
| R Amygdala | 0.02 | 0.93 | 0.27 | 0.27 |
| L Entorhinal | −0.25 | 0.32 | 0.32 | 0.22 |
| R Entorhinal | −0.18 | 0.46 | 0.22 | 0.38 |
| L Fusiform | −0.24 | 0.32 | 0.55 | 0.02 |
| R Fusiform | −0.12 | 0.63 | 0.06 | 0.82 |
L: left; R: right.
4. DISCUSSION
In this study, we investigated the relationship between regional brain volumes within the temporal structures and presurgical Wada memory performance in patients with MTS. We found that: 1) patients with nonlateralized Wada memory showed significantly greater memory impairment in the nonsurgical hemisphere, 2) bilateral memory impairment is likely in left MTS patients regardless of the Wada memory profile, 3) bilateral memory impairment on nonlateralized Wada test may have an anatomic basis, perhaps related to bitemporal volume loss, and 4) left hemispheric memory correlates positively with the size of the left temporal structures whereas right hemispheric memory has no such correlation with the size of right temporal structures.
In general, it is desirable to have a higher Wada memory score after ipsilateral injection than contralateral injection to expect a favorable postoperative memory outcome after temporal resection, i.e., EA. However, nonlateralized Wada memory is often encountered in practice where the memory scores are similar after the two injections without allowing for a clear determination of memory dominance. Although such a scenario may result from bilaterally impaired or bilaterally preserved memory, our current findings and prior data (Diaz-Arrastia et al., 2002) support the idea that bilateral memory impairment, rather than preservation, underlies nonlateralized Wada memory in MTS patients. In addition, we demonstrated that patients with nonlateralized Wada memory showed more volume loss and cortical thinning in the contralateral temporal regions. Taken together, these findings indicate that the MTS patients with nonlateralized Wada memory have more widespread impairment involving the memory network. This idea is in alignment with recent studies speculating that structural abnormalities in the contralateral temporal lobe might contribute to postoperative amnesia in patients who failed the Wada test after ipsilateral injection and subsequently underwent ATL (Weber et al., 2007; Weber et al., 2006).
In our cohort, we did not encounter the nonlateralized Wada memory profile in right MTS patients, which could be related to the small sample size. In other words, the NM profile on the Wada test was exclusively seen in left MTS patients who showed low memory scores after both ipsilateral and contralateral injections. Among patients with EA profile, the memory scores after ipsilateral injection (which reflect the memory function of the contralateral hemisphere) tended to be lower in left MTS patients compared with right MTS patients while the memory scores were uniformly low in both two groups after contralateral injection. Taken together, these findings suggest the likelihood of bilateral memory impairment in left MTS regardless of the Wada memory profile, which is concordant with our previous experience (Diaz-Arrastia et al., 2002) and that of others (Alessio et al., 2006; Baxendale et al., 1998).
Compared with controls, the left MTS patients, but not the right MTS patients, demonstrated volume loss in the temporal regions not only ipsilateral to the seizure focus but also contralaterally. The contralateral volume loss was more prominent in patients with a nonlateralized Wada memory profile. For example, the Z scores of contralateral hippocampal volumes and superior temporal gyrus were decreased by 340% and 180%, respectively, in the nonlateralized group versus the lateralized group. However, the left hemispheric memory function in left MTS patients showed significant positive correlation only with the size of the left mesial and neocortical temporal structures. These findings are consistent with structural connectivity studies showing that left TLE has a more significant impact on network function than right TLE (Besson et al., 2014).
Our study is limited by its small sample size. In addition to the size of the temporal structures, the Wada memory results could have been influenced by other variables, such as duration of epilepsy, seizure frequency and antiepileptic medications. Because of the retrospective nature of the study, we were unable to evaluate the impact of these variables. It would be worthwhile evaluating the association between structural volume and reverse Wada asymmetry. Unfortunately, we did not have such patients in our cohort. Similarly, we were unable to investigate the relationship between nonlateralized Wada memory and postoperative memory outcome because many patients with nonlateralized Wada memory were deemed to be at high risk for memory decline, and therefore, not offered surgery. Along this line, however, Kubu et al. reported a series of 10 patients who failed the Wada memory test bilaterally and underwent unilateral temporal resection but did not experience adverse postoperative memory outcome (Kubu et al., 2000).
5. Conclusions
This study explored the neuroanatomic basis for Wada memory performance. Our findings suggest that the MTS patients with nonlateralized Wada memory performance have significantly greater memory impairment in contralateral hemisphere and have more volume loss and cortical thinning in contralateral temporal region. Our study supports the notion that the size of the left temporal structures is an important determinant of the Wada memory function regardless of the side of injection. Future studies in a larger sample, and investigation of the relationship between neuropsychological test performance and Wada memory and volume loss, will be important to further understand the implications of our findings.
Supplementary Material
We measured the temporal lobe volume using Freesurfer
Nonlateralized Wada profile is defined as a difference in memory recall between bilateral injections < 11%
Nonlateralized Wada profile is associated with more atrophy in bilateral temporal lobes
The left temporal lobe volume correlated with both hemisphere Wada performance
Acknowledgments
The project was supported by NIH grants R01HD48179, U01HD42652, NIDRR H133A0252604 (RDA). We thank Charlene Supnet, PhD and Carlos Marquez De la Plata, PhD for assistance with paper preparation.
Footnotes
Disclosures
None of the authors have any conflicts of interest. We confirm that we have read the journal’s position on the issue involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kan Ding, Email: Kan.ding@utsouthwestern.edu.
Yunhua Gong, Email: Yunhua.gong@nih.gov.
Pradeep Modur, Email: pmodur@gmail.com.
Ramon Diaz-Arrastia, Email: ramon.diaz-arrastia@nih.gov.
Mark Agostini, Email: Mark.agostini@utsouthwestern.edu.
Puneet Gupta, Email: puneet.gupta@utsouthwestern.edu.
Roderick McColl, Email: Roderick.McColl@utsouthwestern.edu.
Ryan Hays, Email: ryan.hays@utsouthwestern.edu.
Paul Van Ness, Email: Paul.van_ness@utsouthwestern.edu.
References
- Alessio A, Bonilha L, Rorden C, Kobayashi E, Min LL, Damasceno BP, Cendes F. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav. 2006;8:593–600. doi: 10.1016/j.yebeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Baxendale SA, van Paesschen W, Thompson PJ, Connelly A, Duncan JS, Harkness WF, Shorvon SD. The relationship between quantitative MRI and neuropsychological functioning in temporal lobe epilepsy. Epilepsia. 1998;39:158–166. doi: 10.1111/j.1528-1157.1998.tb01353.x. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology. 2010;74:1776–1784. doi: 10.1212/WNL.0b013e3181e0f80a. [DOI] [PubMed] [Google Scholar]
- Besson P, Dinkelacker V, Valabregue R, Thivard L, Leclerc X, Baulac M, Sammler D, Colliot O, Lehericy S, Samson S, Dupont S. Structural connectivity differences in left and right temporal lobe epilepsy. NeuroImage. 2014;100:135–144. doi: 10.1016/j.neuroimage.2014.04.071. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Frol AB, Garcia MC, Agostini MA, Chason DP, Lacritz LH, Cullum CM, Van Ness PC. Bilateral Memory Dysfunction in Epilepsy Surgery Candidates Detected by the Intracarotid Amobarbital Procedure (Wada Memory Test) Epilepsy Behav. 2002;3:82–91. doi: 10.1006/ebeh.2001.0298. [DOI] [PubMed] [Google Scholar]
- Ding K, Gong Y, Gupta P, Frol A, McColl R, Agostini M, Modur P, Van Ness P, Diaz-Arrastia R. Regional Atrophy in Temporal Lobe Epilepsy: Correlations with Cognitive Impairment. Journal of Neurological Disorder & Stroke. 2014;2:1053. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci US A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der KA, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Kubu CS, Girvin JP, McLachlan RS, Pavol M, Harnadek MC. Does the intracarotid amobarbital procedure predict global amnesia after temporal lobectomy? Epilepsia. 2000;41:1321–1329. doi: 10.1111/j.1528-1157.2000.tb04612.x. [DOI] [PubMed] [Google Scholar]
- Labate A, Cerasa A, Aguglia U, Mumoli L, Quattrone A, Gambardella A. Neocortical thinning in “benign” mesial temporal lobe epilepsy. Epilepsia. 2011;52:712–717. doi: 10.1111/j.1528-1167.2011.03038.x. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Hagler DJ, Jr, Ahmadi ME, Tecoma E, Iragui V, Gharapetian L, Dale AM, Halgren E. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008;49:794–803. doi: 10.1111/j.1528-1167.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- Milner BBC, Rasmussen T. Study of short-term memory after intracarotid injection of sodium Amytal. Trans Am Neurol Assoc. 1962;87:224–226. [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Subfield atrophy pattern in temporal lobe epilepsy with and without mesial sclerosis detected by high-resolution MRI at 4 Tesla: preliminary results. Epilepsia. 2009a;50:1474–1483. doi: 10.1111/j.1528-1167.2009.02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. Neuroimage. 2009b;46:353–359. doi: 10.1016/j.neuroimage.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Morris GL, Mueller WM, Seidenberg M. Memory outcome after left anterior temporal lobectomy in patients with expected and reversed Wada memory asymmetry scores. Epilepsia. 2001;42:1408–1415. doi: 10.1046/j.1528-1157.2001.38500.x. [DOI] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. 1960. J Neurosurg. 2007;106:1117–1133. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- Wang JY, Bakhadirov K, Devous MD, Sr, Abdi H, McColl R, Moore C, Marquez de la Plata CD, Ding K, Whittemore A, Babcock E, Rickbeil T, Dobervich J, Kroll D, Dao B, Mohindra N, Madden CJ, Diaz-Arrastia R. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008;65:619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- Weber B, Fliessbach K, Lange N, Kugler F, Elger CE. Material-specific memory processing is related to language dominance. NeuroImage. 2007;37:611–617. doi: 10.1016/j.neuroimage.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, Ruhlmann J, Elger CE, Fernandez G. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain : a journal of neurology. 2006;129:346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.