Abstract
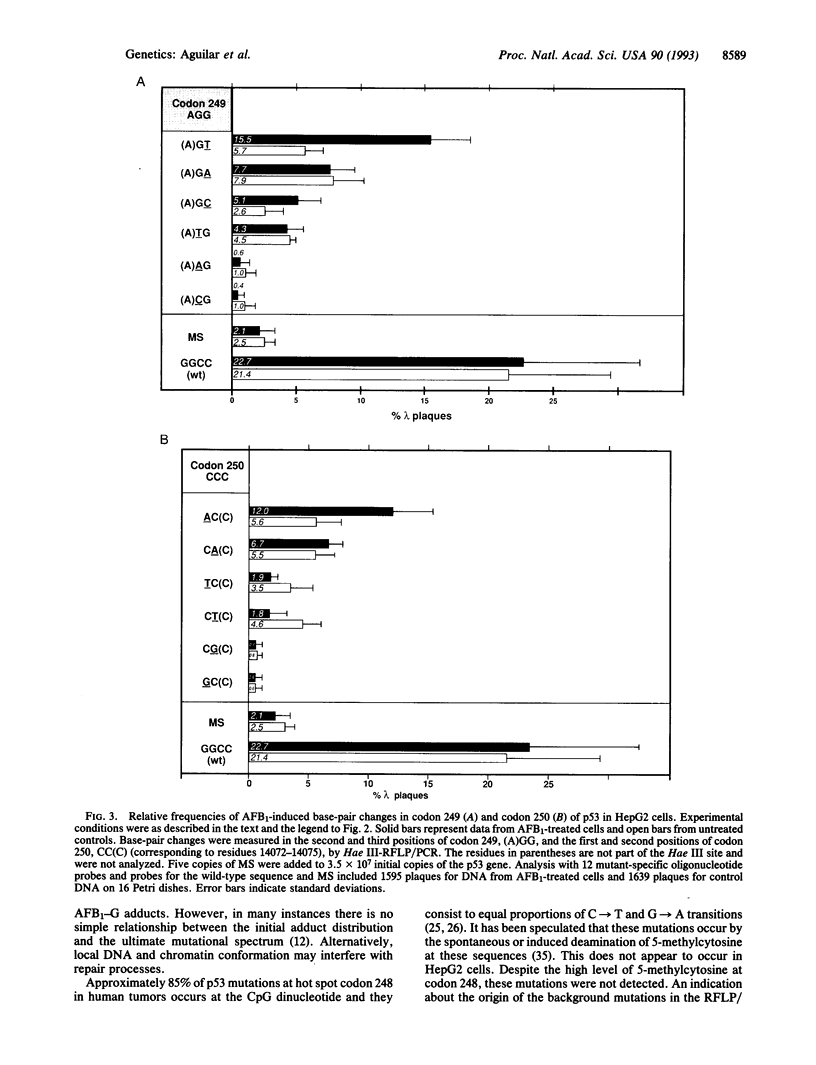
Approximately half of hepatocellular carcinoma (HCC) from regions in the world with high contamination of food with the mycotoxin aflatoxin B1 (AFB1) contain a mutation in codon 249 of the p53 tumor suppressor gene. The mutation almost exclusively consists of a G-->T transversion in the third position of this codon, resulting in the insertion of serine at position 249 in the mutant protein. To gain insight into the mechanism of formation of this striking mutational hot spot in hepatocarcinogenesis, we studied the mutagenesis of codons 247-250 of p53 by rat liver microsome-activated AFB1 in human HCC cells HepG2 by restriction fragment length polymorphism/polymerase chain reaction genotypic analysis. AFB1 preferentially induced the transversion of G-->T in the third position of codon 249. However, AFB1 also induced G-->T and C-->A transversions into adjacent codons, albeit at lower frequencies. Since the latter mutations are not observed in HCC it follows that both mutability on the DNA level and altered function of the mutant serine 249 p53 protein are responsible for the observed mutational hot spot in p53 in HCC from AFB1-contaminated areas. Our results are in agreement with an etiological role of AFB1 in hepatocarcinogenesis in regions of the world with AFB1-contaminated food.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bressac B., Kew M., Wands J., Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991 Apr 4;350(6317):429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- Caron de Fromentel C., Soussi T. TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer. 1992 Jan;4(1):1–15. doi: 10.1002/gcc.2870040102. [DOI] [PubMed] [Google Scholar]
- Chiocca S. M., Sandy M. S., Cerutti P. A. Genotypic analysis of N-ethyl-N-nitrosourea-induced mutations by Taq I restriction fragment length polymorphism/polymerase chain reaction in the c-H-ras1 gene. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5331–5335. doi: 10.1073/pnas.89.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert K. A., Kunkel T. A. High fidelity DNA synthesis by the Thermus aquaticus DNA polymerase. Nucleic Acids Res. 1990 Jul 11;18(13):3739–3744. doi: 10.1093/nar/18.13.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felley-Bosco E., Pourzand C., Zijlstra J., Amstad P., Cerutti P. A genotypic mutation system measuring mutations in restriction recognition sequences. Nucleic Acids Res. 1991 Jun 11;19(11):2913–2919. doi: 10.1093/nar/19.11.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E., Miller J. H. Base substitution mutations induced by metabolically activated aflatoxin B1. Proc Natl Acad Sci U S A. 1983 May;80(9):2695–2698. doi: 10.1073/pnas.80.9.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman J. D., Croy R. G., Wogan G. N. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5445–5449. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman J. D., Croy R. G., Wogan G. N. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5445–5449. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hsu I. C., Metcalf R. A., Sun T., Welsh J. A., Wang N. J., Harris C. C. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991 Apr 4;350(6317):427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- Kress S., Jahn U. R., Buchmann A., Bannasch P., Schwarz M. p53 Mutations in human hepatocellular carcinomas from Germany. Cancer Res. 1992 Jun 1;52(11):3220–3223. [PubMed] [Google Scholar]
- Lamb P., Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986 May;6(5):1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadon S. A., Tyrrell R. M., Cerutti P. A. Excision repair of aflatoxin B1-DNA adducts in human fibroblasts. Cancer Res. 1981 Dec;41(12 Pt 1):5125–5129. [PubMed] [Google Scholar]
- Levy D. D., Groopman J. D., Lim S. E., Seidman M. M., Kraemer K. H. Sequence specificity of aflatoxin B1-induced mutations in a plasmid replicated in xeroderma pigmentosum and DNA repair proficient human cells. Cancer Res. 1992 Oct 15;52(20):5668–5673. [PubMed] [Google Scholar]
- Li D., Cao Y., He L., Wang N. J., Gu J. R. Aberrations of p53 gene in human hepatocellular carcinoma from China. Carcinogenesis. 1993 Feb;14(2):169–173. doi: 10.1093/carcin/14.2.169. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Preston B. D. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- Lundberg K. S., Shoemaker D. D., Adams M. W., Short J. M., Sorge J. A., Mathur E. J. High-fidelity amplification using a thermostable DNA polymerase isolated from Pyrococcus furiosus. Gene. 1991 Dec 1;108(1):1–6. doi: 10.1016/0378-1119(91)90480-y. [DOI] [PubMed] [Google Scholar]
- Muench K. F., Misra R. P., Humayun M. Z. Sequence specificity in aflatoxin B1--DNA interactions. Proc Natl Acad Sci U S A. 1983 Jan;80(1):6–10. doi: 10.1073/pnas.80.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Hayashi K., Hirohashi S., Sekiya T. Aberrations of the tumor suppressor p53 and retinoblastoma genes in human hepatocellular carcinomas. Cancer Res. 1991 Oct 15;51(20):5520–5525. [PubMed] [Google Scholar]
- Oda T., Tsuda H., Scarpa A., Sakamoto M., Hirohashi S. p53 gene mutation spectrum in hepatocellular carcinoma. Cancer Res. 1992 Nov 15;52(22):6358–6364. [PubMed] [Google Scholar]
- Pourzand C., Cerutti P. Genotypic mutation analysis by RFLP/PCR. Mutat Res. 1993 Jul;288(1):113–121. doi: 10.1016/0027-5107(93)90213-y. [DOI] [PubMed] [Google Scholar]
- Puisieux A., Lim S., Groopman J., Ozturk M. Selective targeting of p53 gene mutational hotspots in human cancers by etiologically defined carcinogens. Cancer Res. 1991 Nov 15;51(22):6185–6189. [PubMed] [Google Scholar]
- Rideout W. M., 3rd, Coetzee G. A., Olumi A. F., Jones P. A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990 Sep 14;249(4974):1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- Ross R. K., Yuan J. M., Yu M. C., Wogan G. N., Qian G. S., Tu J. T., Groopman J. D., Gao Y. T., Henderson B. E. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992 Apr 18;339(8799):943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- Sagher D., Strauss B. Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry. 1983 Sep 13;22(19):4518–4526. doi: 10.1021/bi00288a026. [DOI] [PubMed] [Google Scholar]
- Sambamurti K., Callahan J., Luo X., Perkins C. P., Jacobsen J. S., Humayun M. Z. Mechanisms of mutagenesis by a bulky DNA lesion at the guanine N7 position. Genetics. 1988 Dec;120(4):863–873. doi: 10.1093/genetics/120.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorsone K. A., Zhou Y. Z., Butel J. S., Slagle B. L. p53 mutations cluster at codon 249 in hepatitis B virus-positive hepatocellular carcinomas from China. Cancer Res. 1992 Mar 15;52(6):1635–1638. [PubMed] [Google Scholar]
- Van Rensburg S. J., Cook-Mozaffari P., Van Schalkwyk D. J., Van der Watt J. J., Vincent T. J., Purchase I. F. Hepatocellular carcinoma and dietary aflatoxin in Mozambique and Transkei. Br J Cancer. 1985 May;51(5):713–726. doi: 10.1038/bjc.1985.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. C., Cerutti P. A. Effect of formation and removal of aflatoxin B1:DNA adducts in 10T 1/2 mouse embryo fibroblasts on cell viability. Cancer Res. 1980 Aug;40(8 Pt 1):2904–2909. [PubMed] [Google Scholar]
- Wang T. C., Cerutti P. A. Formation and removal of aflatoxin B1-induced DNA lesions in epithelioid human lung cells. Cancer Res. 1979 Dec;39(12):5165–5170. [PubMed] [Google Scholar]
- Wang T. V., Cerutti P. Spontaneous reactions of aflatoxin B1 modified deoxyribonucleic acid in vitro. Biochemistry. 1980 Apr 15;19(8):1692–1698. doi: 10.1021/bi00549a027. [DOI] [PubMed] [Google Scholar]
- Wogan G. N. Markers of exposure to carcinogens. Environ Health Perspect. 1989 May;81:9–17. doi: 10.1289/ehp.89819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Kinds of mutations formed when a shuttle vector containing adducts of (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9, 10-tetrahydrobenzo[a]pyrene replicates in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3787–3791. doi: 10.1073/pnas.84.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F. S., Yu M. C., Mo C. C., Luo S., Tong M. J., Henderson B. E. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989 May 1;49(9):2506–2509. [PubMed] [Google Scholar]