Abstract
We have recently described a specialized subset of human natural killer (NK) cells with a CD56dimCD57+NKG2C+ phenotype that expand specifically in response to cytomegalovirus (CMV) reactivation in hematopoietic cell transplant (HCT) recipients and exhibit properties characteristic of adaptive immunity. We hypothesize that these cells mediate relapse protection and improve post-HCT outcomes. In 674 allogeneic HCT recipients, we found that those who reactivated CMV had lower leukemia relapse (26% [17–35%], p=0.05) and superior disease-free survival (DFS) (55% [45–65%] p=0.04) 1 year after reduced intensity conditioning (RIC) compared to CMV seronegative recipients who experienced higher relapse rates (35% [27–43%]) and lower DFS (46% [38–54%]). This protective effect was independent of age and graft-versus-host disease (GvHD) and was not observed in recipients who received myeloablative (MA) regimens. Analysis of the reconstituting NK cells demonstrated that CMV reactivation is associated with both higher frequencies and greater absolute numbers of CD56dimCD57+NKG2C+ NK cells, particularly after RIC HCT. Furthermore, expansion of these cells at 6 months post-transplant independently trended toward a lower 2-year relapse risk. Together, our data suggest that the protective effect of CMV reactivation on post-transplant relapse is in part driven by adaptive NK cell responses.
Keywords: cytomegalovirus, NK cell, adaptive, transplant, relapse, memory
Introduction
Natural killer (NK) cells are the predominant lymphocyte population to reconstitute early after hematopoietic cell transplantation (HCT) and have the potential to influence post-HCT outcomes1. However, their graft vs. leukemia (GvL) activity is limited by delayed NK cell functional maturation throughout the first year after HCT2–4. The immature phenotype of reconstituting donor NK cells is associated with significant impairments in NK cell-mediated cytotoxicity and interferon (IFN)-γ production in response to tumor cell lines and primary AML blasts ex vivo4,5. Overall, the phenotypic and functional immaturity of donor NK cells reconstituting early after HCT limits their clinical benefit. Thus, there is considerable interest in identifying factors that drive NK cell maturation and function in the HCT setting.
We have shown that NK cells expressing high levels of the activating receptor NKG2C robustly expand in HCT recipients after CMV reactivation, preferentially acquire the maturation marker CD57 and persist for at least 1 year post-HCT. In many respects, CD56dimCD57+NKG2C+ NK cells appear to represent a human analogue of Ly49H+ memory NK cells that participate in the clearance of murine CMV (MCMV) infections. Thus, CMV reactivation has a powerful effect in HCT recipients and drives the maturation of NK cells with heightened effector functions. Given the similarities between human CD56dimCD57+NKG2C+ NK cells and mouse Ly49H+ memory NK cells6, we elect to refer to CD56dimCD57+NKG2C+ NK cells as adaptive.
Several recent studies have reported an association between CMV reactivation and reduced risk of relapse after HCT7–9, but a specific mechanism for this observation has not been described. We hypothesized that CMV-induced CD56dimCD57+NKG2C+ NK cells with enhanced function and long-term persistence may promote cancer control in transplant recipients. In this study, we sought to define the relevant transplant-related variables that influence the protective effect of CMV reactivation on relapse and to determine whether CD56dimCD57+NKG2C+ NK cells are directly associated with clinical outcomes post-HCT.
Patients and Methods
Transplant Procedures
Myeloablative (MA) conditioning was used in 366 patients with malignant hematologic diseases and consisted of cyclophosphamide (60 mg/kg × 2) and total body irradiation (13.2 Gy, 165 cGy twice daily × 4 days). For some, this regimen also included fludarabine (25 mg/m2/day on day −8 through −6 and mycophenolate mofetil (1 g every 12 hours from day −3 to day +30). All patients also received cyclosporine A starting at day −3 and continuing through 180 days post-HCT. Reduced intensity conditioning (RIC) was used in 308 patients and consisted of cyclophosphamide (50 mg/kg) and fludarabine (200 mg/m2) and total body irradiation (2 Gy). Following conditioning, stem cells from bone marrow, peripheral blood or cord blood (single or double) were infused. Table 1 describes the HCT patient demographics stratified by recipient CMV status (seronegative, seropositive without reactivation and seropositive with reactivation).
Table I.
Demographics by CMV serostatus and reactivation
| Variable | CMV seronegative | CMV seropositive | CMV reactivation | p* | |
|---|---|---|---|---|---|
| n | 270 | 214 | 190 | 0.07 | |
| Age | Median (range) | 42 (2–72) | 37 (1–74) | 45 (1–71) | |
| IQR | (22–57) | (15–54) | (25–56) | ||
| Gender | Male | 163 (60%) | 120 (56%) | 107 (56%) | 0.56 |
| Female | 107 (40%) | 94 (44%) | 83 (44%) | ||
| Diagnosis | ALL | 81 (30%) | 57 (26%) | 49 (26%) | 0.97 |
| AML | 123 (46%) | 99 (46%) | 91 (48%) | ||
| CML | 14 (5%) | 8 (4%) | 6 (3%) | ||
| MDS | 31 (12%) | 29 (14%) | 25 (13%) | ||
| NHL | 16 (6%) | 14 (7%) | 12 (6%) | ||
| Hodgkin’s | 4 (2%) | 5 (2%) | 5 (3%) | ||
| Multiple Myeloma | 1 (<1%) | 2 (1%) | 2 (1%) | ||
| Diagnosis Risk | Standard risk | 211 (78%) | 160 (75%) | 145 (76%) | 0.68 |
| High risk | 59 (22%) | 54 (25%) | 45 (24%) | ||
| Prior Auto | Yes | 14 (5%) | 9 (4%) | 13 (7%) | 0.50 |
| CMV Serostatus R/D | neg/neg | 247 (92%) | 7 (4%) | ||
| neg/pos | 23 (8%) | 3 (2%) | |||
| pos/neg or pos/pos | 214 (100%) | 180 (95%) | |||
| Conditioning Intensity | MA | 140 (52%) | 127 (59%) | 99 (52%) | 0.20 |
| RIC | 130 (48%) | 87 (41%) | 91 (48%) | ||
| GvHD prophylaxis | Csa or Tac w/MTX | 51 (19%) | 56 (26%) | 33 (17%) | 0.08 |
| Csa or Tac w/MMF | 206 (76%) | 151 (71%) | 153 (81%) | ||
| Other | 13 (5%) | 7 (3%) | 4 (2%) | ||
| Donor type | Matched sibling | 75 (28%) | 81 (38%) | 48 (25%) | 0.01 |
| Single UCB | 50 (19%) | 39 (18%) | 27 (14%) | ||
| Double UCB | 145 (54%) | 94 (44%) | 115 (61%) | ||
| Date of transplant | 2001–2007 | 139 (52%) | 121 (57%) | 98 (52%) | 0.48 |
| 2008–2013 | 131 (49%) | 93 (44%) | 92 (48%) | ||
| Days to CMV reactivation | Median (range) | 44 (1–100) | |||
| IQR | (33–58) | ||||
A p-value for between-treatment comparisons. Continuous variables were analyzed by a general Wilcoxon test. Categorical variables were analyzed by chi-square
CMV Screening and Treatment
Prior to conditioning, all recipients were assessed for CMV exposure by serology using enzyme-linked immunosorbent assays: CMV IgG antibody level > 10.0 EU/ml was considered seropositive. After transplant, all recipients underwent weekly screening for CMV reactivation by either pp65 antigenemia (prior to 2006) or quantitative real-time polymerase chain reaction (PCR) (after 2006) until day +100 post-transplant. CMV prophylaxis included high-dose acyclovir (500 mg/m2 [10–12 mg/kg] i.v. every 8 hours or 800 mg [18 mg/kg pediatric] orally 5 times daily) until day 100. CMV reactivation was defined as CMV antigenemia (≥ 2 pp65-positive cells/50,000), DNAemia (≥ 500 copies by quantitative real-time PCR) or culture of CMV from blood, body fluid or tissue and was treated with ganciclovir or foscarnet.
Data Collection
The University of Minnesota Blood and Marrow Transplant program prospectively collected all data regarding patient characteristics and outcomes. The University of Minnesota institutional review board approved all protocols, and all patients (and/or their legal guardians) provided informed consent in accordance with the Declaration of Helsinki.
Phenotypic Analysis of Reconstituting NK Cells in HCT Recipients
Peripheral blood mononuclear cells (PBMCs) from HCT recipients were isolated from peripheral blood samples by density gradient centrifugation and analyzed by fluorescence-activated cell sorting (FACS) using an LSR II (BD Biosciences). PBMCs from recipients that reactivated CMV were collected at viral diagnosis, at 2, 4 and 8 weeks after antiviral therapy and at 6 months and 1 year post-transplant. For recipients that were CMV seronegative or were CMV seropositive without viral reactivation, PBMCs were collected at day 100, 6 months and 1 year post-transplant. The following fluorescently conjugated antibodies were used for phenotypic analysis: ECD-conjugated anti-CD3 (Beckman Coulter; IM2705U), PECy7-conjugated anti-CD56 (BioLegend; 318318), Pacific Blue-conjugated anti-CD57 (BioLegend; 322316) and PE-conjugated NKG2C (R&D Systems FAB138P-025). For statistical comparisons of adaptive NK cell percentages and absolute counts between RIC and MA recipients, unpaired, two-sided t-tests calculated using GraphPad were used. Error bars represent SEM. GraphPad was used to calculate R2 values and associated p values for the correlation between absolute monocyte and lymphocyte counts and adaptive NK cell expansion in 28 CMV seropositive recipients.
Statistical Analysis of Clinical Associations in the HCT Cohort
Kaplan-Meier curves were used to estimate the probability of disease free survival (DFS) through 1-year post-HCT10, and the log-rank test was used for comparisons. Adjusted survival curves were calculated based on a stratified Cox model11. Cox regression was used to examine the independent effect of factors on DFS, and proportional hazards were checked using Martingale residuals. The cumulative incidence of relapse was assessed treating non-relapse mortality (NRM) as a competing risk. The Fine and Gray proportional hazards model12 was used to determine the independent effect of CMV reactivation on relapse and to calculate adjusted relapse curves. The primary covariates of interest were CMV reactivation post-HCT, treated as a time-dependent covariate, and conditioning regimen intensity. Potential confounders included donor type, diagnosis, year of transplant (<2008 versus ≥2008), GvHD prophylaxis, gender, disease risk and prior autologous transplant. Disease risk at the time of HCT was classified into standard risk or high risk based on the ASBMT RFI 2006 risk scoring schema (http://www.asbmt.org). Variance was similar between groups being compared. Recursive partitioning was used to determine the optimal cut points for the percentages and absolute numbers of adaptive CD56dimCD57+NKG2C+ NK cells in association with relapse. All clinical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
NK cell function assays
Buffy coats collected from 5 healthy CMV seropositive donors were obtained from Memorial Blood Bank (Minneapolis, MN). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque (GE Healthcare) and cultured with K562 cells at a 2:1 (effector:target) ratio for 5 hours in RPMI media supplemented with 10% fetal bovine serum (Gibco). GolgiStop and GolgiPlug protein transport inhibitors (BD Biosciences) were added 1 hour into the assay. The following antibodies were used for functional analysis of NK cell subsets: BV785-conjugated anti-CD3 (clone OKT3; Biolegend), PECy7-conjugated anti-CD56 (BioLegend; 318318), PE-CF594-conjugated anti-CD57 (Biolegend; 359620), PE-conjugated NKG2C (R&D Systems; FAB138P-025), PerCP-Cy5.5-conjugated anti-CD107a (Biolegend; 328616), AF700-conjugated anti-TNF (Biolegend; 502928) and BV605-conjugated IFN-γ (Biolegend; 502536). The K562 cell line was purchased from ATCC (Manassas, Virginia) and is screened monthly for mycoplasma contamination. The experiment was performed at 2 independent times. Two-sided, paired t-tests in GraphPad were used to determine significance. Error bars represent SEM.
Results
Lower relapse risk post-HCT in RIC recipients that reactivate CMV
We analyzed 1 year relapse risk and DFS in 674 allogeneic HCT recipients with AML, (n=313), ALL (n=187), MDS (n=85) NHL (n=42) CML (n=28), Hodgkin’s (n=14) and multiple myeloma (n=5) treated at the University of Minnesota between 2001 and 2013. 516 patients were classified as standard risk, 148 patients as high risk and 36 patients had a prior autologous transplant. 37 patients received bone marrow, 166 patients received peripheral blood stem cells and 471 patients received cord blood grafts. The entire cohort was stratified by recipient CMV serostatus (CMV seronegative [n=270] vs. CMV seropositive without reactivation [n=214] vs. CMV seropositive with reactivation [n=190]) and by conditioning regimen (reduced intensity [n=308] vs. myeloablative [n=366]). Disease type and treatment-related variables were balanced across groups stratified by CMV serostatus (Table 1).
Following RIC (n=308), CMV reactivation was associated with a lower risk of relapse 1 year post-HCT (26% [17–35%], p=0.05) compared to CMV seropositive recipients without reactivation (30% [20–40%]) or CMV seronegative recipients (35% [27–43%]) (Figure 1A). Similarly, in RIC transplants CMV reactivation was associated with improved DFS (55% [45–65%] p=0.04) compared to CMV seropositive recipients without reactivation (45% [35–55%]) or CMV seronegative recipients (46% [38–54%]) (Figure 1B). Following myeloablative conditioning (n=366), CMV serostatus or reactivation did not influence either relapse or DFS post–HCT (Figure 1C, D).
Figure 1. CMV reactivation is associated with reduced relapse risk and superior disease-free survival in RIC, but not MA HCT recipients.
Kaplan-Meier curves of (A) relapse rates and (B) DFS stratified by CMV status in RIC recipients. (C) Relapse rates and (D) DFS stratified by CMV status in MA recipients. Blue dashed lines represent trends calculated for CMV seronegative recipients. Green dotted lines represent trends calculated for CMV seropositive recipients that did not experience viral reactivation. Red solid lines represent trends calculated for CMV seropositive recipients that experienced viral reactivation. p values shown in each plot were calculated for trends.
In regression analyses, CMV reactivation, but not seropositivity without reactivation, trended toward a lower risk of relapse (RR=0.6 [0.4–1.0], p=0.06) and was associated with significantly better DFS (RR=0.7 [0.6–1.0], p=0.04) in RIC recipients. Importantly, there was no statistically significant effect of GvHD and age on relapse and non-relapse mortality (NRM) in multivariate models within the RIC group (Table 2). In contrast, for the MA cohort, grade II–IV acute GVHD and lower age were associated with both relapse protection and higher rates of NRM. While CMV reactivation or recipient seropositivity had no effect on relapse in the MA cohort, patients who were CMV positive but did not reactivate had lower disease free survival. Regression analyses were also performed separately for myeloid (AML and MDS) and other diagnoses (ALL, CML, NHL, Hodgkin’s and multiple myeloma). Though power was compromised and thus confidence intervals were wider with this further subsetting of the data, similar trends towards a lower risk of relapse in RIC recipients with CMV reactivation were observed in all disease groups (data not shown).
Table II.
Multiple variable regression analysis of relapse and NRM post-transplant
| Conditioning Intensity | Outcome | Recipient CMV Status | n | RR | p |
|---|---|---|---|---|---|
| RIC | Relapse | seronegative | 130 | 1.0 | |
| seropositive | 87 | 0.8 (0.5–1.4) | 0.46 | ||
| reactivation | 91 | 0.6 (0.4–1.0) | 0.06 | ||
| NRM | seronegative | 130 | 1.0 | ||
| seropositive | 87 | 1.0 (0.7–1.5) | 0.89 | ||
| reactivation | 91 | 0.7 (0.5–1.0) | 0.04 | ||
| MA | Relapse | seronegative | 140 | 1.0 | |
| seropositive | 127 | 1.2 (0.7–2.2) | 0.49 | ||
| reactivation | 99 | 1.0 (0.5–1.7) | 0.76 | ||
| No aGvHD | 210 | 1.0 | |||
| Grade II–IV aGVHD | 156 | 0.5 (0.3–0.9) | 0.02 | ||
| NRM | seronegative | 140 | 1.0 | ||
| seropositive | 127 | 1.5 (1.0–2.6) | 0.04 | ||
| reactivation | 99 | 0.8 (0.5–1.3) | 0.35 | ||
| <21 years old | 171 | 1.0 | |||
| ≥21 years old | 195 | 1.6 (1.2–2.3) | <0.01 | ||
| No aGvHD | 210 | 1.0 | |||
| Grade II–IV aGvHD | 156 | 0.6 (0.4–0.8) | <0.01 |
Covariates tested included CMV reactivation as a time-dependent variable (no vs. yes), donor type (sibling vs. UCB), diagnosis (AML vs. others), year of transplant (<2008 vs. ≥2008), conditioning (MA vs. RIC), GvHD prophylaxis (MTX vs. MMF vs. other), gender (male vs. female), disease risk (standard vs. high), age (<21 vs. ≥21), grade II–IV aGvHD as a time-dependent variable (no vs. yes) and prior autologous transplant (no vs. yes).
The primary beneficial effect of CMV reactivation occurs early (when it is most often detected), as the protective effect is less apparent for late relapses. There was no observed protection against late relapse (occurring after day 100) in survivors with earlier CMV reactivation (RR=1.0 [0.5–1.9], p=0.98). Similarly, there was no association between previous CMV reactivation and DFS in survivors beyond day 100. Since CMV reactivation after 100 days post-HCT is uncommon and asymptomatic reactivation is less often detected because it occurs beyond the window of routine monitoring, we did not have enough events to fully evaluate the association between late CMV reactivation and late relapse. Together, our results show that the beneficial effect of CMV reactivation in the HCT setting is observed early after transplant and is evident only in recipients of RIC HCT.
CD56dimCD57+NKG2C+ NK cells preferentially expand in RIC HCT recipients after CMV reactivation
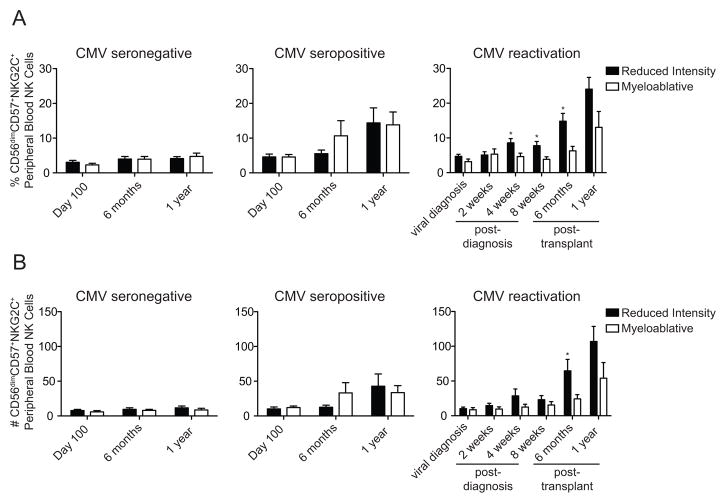
To further evaluate the association between CMV reactivation, relapse protection and improved DFS after RIC HCT, we analyzed the phenotype of donor-derived peripheral blood NK cells post-HCT. As previously reported13, we observed increases in the frequency of CD56dimCD57+NKG2C+ NK cells in CMV seropositive, but not seronegative recipients at 6 months and 1 year (Figure 2A left and middle panels). Recipients who reactivated CMV exhibited the highest proportions of CD56dimCD57+NKG2C+ NK cells. Importantly, further analysis revealed that the frequencies of CD56dimCD57+NKG2C+ NK cells were significantly higher in RIC vs. MA recipients at 4 weeks (8.58% vs. 4.64%, p=0.02), 8 weeks (7.77% vs. 3.84%, p=0.02) and 6 months post-reactivation (14.80% vs. 6.29%, p=0.01) (Figure 2A right panel). Similarly, we observed an association towards greater absolute numbers of CD56dimCD57+NKG2C+ NK cells in CMV seropositive recipients relative to CMV seronegative recipients at 6 months and 1 year (Figure 2B left and middle panels). The absolute numbers of CD56dimCD57+NKG2C+ NK cells were highest in CMV seropositive recipients who reactivated CMV and were also significantly higher in RIC vs. MA recipients at 6 months (22.2 vs. 10.44 cells/μl, p=0.04) post-transplant. A similar trend in absolute numbers was observed at 1 year. The higher rate of CD56dimCD57+NKG2C+ NK cell expansion in RIC recipients was not explained by differences in acute GvHD rates at day 100, as these rates were not significantly different (p=0.46) between RIC (43% [38–43%]) and MA (42% [36–48%]) preparative regimens. The CD56dimCD57+NKG2C+ NK cells expanding from day 100 onward likely differentiated from cells of donor origin, as 91% of survivors had ≥ 90% donor chimerism at day 100, and 93% of survivors at 6 months had ≥ 90% donor chimerism. Thus, the expansion of donor-derived CD56dimCD57+NKG2C+ NK cells in response to CMV reactivation correlated with reduced relapse risk and superior DFS, but only among RIC recipients.
Figure 2. Preferential expansion of CD56dimCD57+NKG2C+ adaptive NK cells in RIC HCT recipients that experience CMV reactivation.
Average percentage (A) and (B) absolute number (cells/μl of blood) of CD56+ NK cells with an adaptive CD56dimCD57+NKG2C+ phenotype in CMV seronegative recipients at day 100 (RIC n=44, MA n=32), 6 months (RIC n=35, MA n=23), and 1 year (RIC=31, MA=21) post-transplant. Values for CMV seropositive recipients without CMV reactivation at day 100 (RIC n=22, MA n=12), 6 months (RIC=13, MA=14) and 1 year (RIC=11, MA=8) post-transplant are shown in the middle panels. Values for CMV seropositive recipients that reactivated CMV at the time of viral diagnosis (RIC n=28, MA n=18), 2 weeks post-diagnosis (RIC n=26, MA n=14), 4 weeks post-diagnosis (RIC n=29, MA=23), 8 weeks post-diagnosis (RIC n=24, MA n=15), 6 months post-transplant (RIC n=29, MA n=17) and 1 year post transplant (RIC n=26, MA n=10) are shown in the right panels. *p ≤ 0.05 comparing RIC to MA. Error bars represent SEM.
Expansion of CD56dimCD57+NKG2C+ NK cells at 6 months post-HCT is directly associated with lower 2 year relapse rates
We hypothesized a direct association between CD56dimCD57+NKG2C+ NK cell expansion at 6 months post-HCT and lower relapse risk. At 6 months post-HCT (n=68), we analyzed absolute CD56dimCD57+NKG2C+ NK cell counts in recipients independently of CMV serostatus or reactivation. At this time point, nearly all patients were > 90% donor engrafted. We used recursive partitioning to determine the optimal cut point for absolute counts of CD56dimCD57+NKG2C+ NK cells. We found that recipients in the expanding group based on CD56dimCD57+NKG2C+ NK cell numbers (> 2.5 cells/μl, n=54) trended toward a lower 2-year relapse rates (16% [6–26%], p=0.06) compared with the non-expanding group (0.1–2.5 cells/μl, n=14), who had higher 2-year relapse rates (46% [10–82%]) (Table 3). Thus, our data suggest that CD56dimCD57+NKG2C+ NK cells that expand in response to CMV reactivation early after transplant protect against relapse.
Table III.
Relapse rates stratified by CD56dimCD57+NKG2C+ NK cell absolute counts at 6 months post-transplant
| All Patients | ||||
|---|---|---|---|---|
| Absolute Counts | n | 2 year relapse (95% CI) | p (2 year estimate) | TRM (2 year estimate) |
| 0.06 | ||||
| 0.1–2.5 cells/μl | 14 | 46% (10–82%) | 7% | |
| >2.5 | 54 | 16% (6–26%) | 7% | |
Recursive partitioning was used to determine optimal cut points of the absolute counts (cells/μl of blood) of CD56dimCD57+NKG2C+ NK cells at 6 months post-transplant in association with 2 year relapse rates.
Higher absolute monocyte counts at viral diagnosis are associated with the subsequent expansion of CD56dimCD57+NKG2C+ NK cells
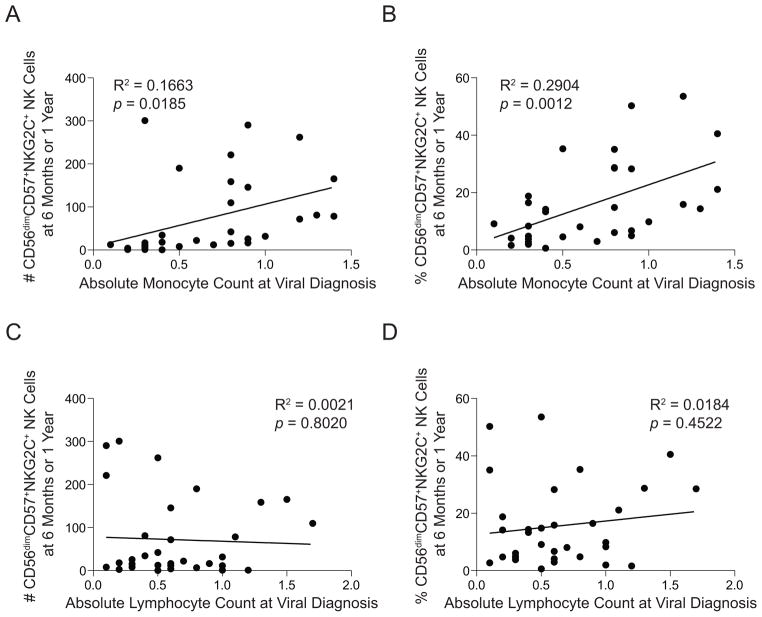
In vitro studies that mimic CMV infection have shown that monocytes play a key role in promoting the expansion of NK cells expressing NKG2C14. Thus, we hypothesized that RIC recipients might have higher absolute monocyte counts (AMC) at viral diagnosis, accounting for the preferential expansion of CD56dimCD57+NKG2C+ NK cells relative to MA recipients (Figure 2). Within the HCT cohort, there were 28 recipients (16 RIC and 12 MA) that experienced CMV reactivation for which we had AMC and absolute lymphocyte counts (ALC) at viral diagnosis and NK cell phenotypic data at 6 months and/or 1 year. At viral diagnosis, the average AMC trended higher in RIC compared to MA recipients (0.71 vs. 0.54 × 109 cells/L, p=0.08). In contrast, the average ALC was similar between RIC and MA recipients (0.65 vs. 0.61 × 109 cells/L, p=0.39). To determine whether an association exists between the number of monocytes present in the blood of recipients at the time of CMV reactivation and subsequent adaptive NK cell expansion, we plotted AMC values at viral diagnosis for each recipient against the absolute number and percentage of CD56dimCD57+NKG2C+ NK cells at either 6 months (if no 1 year phenotype was available) or 1 year. We observed a significant positive correlation between AMC at viral diagnosis and both the absolute number (Figure 3A, p=0.02) and relative percentage (Figure 3B, p<0.01) of CD56dimCD57+NKG2C+ NK cells at 6 months or 1 year. No such correlation was observed between ALC at viral diagnosis and CD56dimCD57+NKG2C+ NK cell expansion (Figure 3C, D). Thus, a greater number of monocytes at the time of CMV may account for the preferential expansion of CD56dimCD57+NKG2C+ NK cells in RIC recipients.
Figure 3. Absolute monocyte counts at the time of CMV reactivation are associated with CD56dimCD57+NKG2C+ NK cell expansion.
Absolute monocyte counts from 28 CMV seropositive recipients at the time of viral reactivation were plotted against either (A) the absolute number or (B) the percentage of CD56dimCD57+NKG2C+ NK cells in peripheral blood samples from these recipients at either 6 months or 1 year. Absolute lymphocyte counts at the time of viral diagnosis from the same recipients were also plotted against either (C) the absolute number or (D) the percentage of CD56dimCD57+NKG2C+ NK cells in peripheral blood samples at either 6 months or 1 year.
CD56dimCD57+NKG2C+ NK cells are highly functional against the K562 myeloid leukemia cell line
Given the observed association between the expansion of CD56dimCD57+NKG2C+ NK cells and relapse protection, we sought to determine whether these cells mediate heightened effector functions against leukemia targets relative to other NK cell subsets. To this end, we isolated peripheral blood mononuclear cells (PBMCs) from healthy CMV seropositive blood donors and cultured these cells at a 2:1 ratio with K562 myeloid leukemia cells. Tumor necrosis factor (TNF) and IFN-γ production were analyzed in immature CD56bright NK cells, early mature CD56dimCD57−NKG2C− NK cells, late mature CD56dimCD57+NKG2C− NK cells and adaptive CD56dimCD57+NKG2C+ NK cells. We found that, relative to all other subsets analyzed, CD56dimCD57+NKG2C+ NK cells exhibited a higher frequency of both TNF and IFN-γ production in response to K562 cells (Figure 3A, B). No significant differences were observed for NK cell degranulation, as determined by CD107a surface expression (not shown). Thus, CD56dimCD57+NKG2C+ NK cells likely contribute to relapse protection post-HCT directly through enhanced inflammatory cytokine production upon direct recognition of tumor targets.
Discussion
Several groups have reported on the potential association between CMV reactivation and relapse protection post-HCT. In 266 adult AML patients who underwent allogeneic HCT, Elmaagacli et al reported a substantial reduction in relapse in recipients with early CMV reactivation7. Ito et al reported similar findings in a cohort of 110 CML patients8. In a large similar analysis in HCT patients with AML, ALL, lymphoma, CML and MDS the authors observed a 53% decreased risk of relapse by day 100 and a 32% decreased risk of relapse by 1 year in recipients that experienced CMV reactivation9. The mechanisms underlying the association between CMV reactivation and relapse protection have remained obscure. One hypothesis is that CMV-specific T cells with cross-reactive T cell receptors, which expand in response to viral antigenemia, might be responsible. However, in a series of cellular immunotherapy studies where CMV-specific primary T cells or T cell lines were infused early after transplantation, the authors found no evidence for a protective effect of CMV-specific T cells against leukemia relapse15. Another hypothesis is that NK cells expanding in response to CMV reactivation are responsible for relapse protection.
Here, we show that CD56dimCD57+NKG2C+ NK cells expand preferentially in reduced intensity recipients after CMV reactivation, and the expansion of these cells is directly associated with lower leukemia relapse. Our results contrast somewhat with those of Manjappa et al who reported a protective effect of CMV reactivation specifically in allogeneic HCT patients that received myeloablative conditioning16. The discrepancy in results between studies may be due to differences in the hematopoietic progenitor cell source. The majority of the transplants in our cohort were performed with either single or double umbilical cord blood units. The cohort studied by Manjappa et al consisted entirely of T cell-replete matched sibling or unrelated donor transplantations16. Our study also used a higher DNA copy number threshold to define CMV reactivation. Regardless of the differences between hematopoietic cell source and definitions for precisely how CMV reactivation is defined, both studies conclude a protective effect of CMV reactivation post-HCT.
In our analysis, CMV seropositive RIC recipients had moderately higher AMC at viral reactivation relative to MA recipients. Furthermore, AMC at viral diagnosis correlated with subsequent CD56dimCD57+NKG2C+ NK cell expansion. This is in agreement with a recent HCT cohort study by DeCook et al who reported that AMC were higher in RIC relative to MA recipients early post-transplant, and higher AMC was independently associated with overall survival17. One way in which monocytes likely promote adaptive NK cell differentiation and expansion is through production of IL-12. Ly49H+ memory NK cells fail to expand after MCMV challenge in mice lacking components of the IL-12 receptor complex18, and inflammatory monocytes producing IL-12 are necessary for the expansion of NKG2C+ NK cells from CMV seropositive individuals in vitro14. Other inflammatory cytokines, such as IL-18 and type-I IFN (IFN-α and IFN-β), produced by monocyte-derived dendritic cells can enhance NK cell function19 and may contribute to the differentiation or maturation of adaptive NK cells.
Although the expansion of CD56dimCD57+NKG2C+ NK cells is associated with CMV infection or reactivation post-transplant, they do not appear to have strict specificity for CMV antigen. In fact, our in vitro experiments demonstrated that, relative to other NK cell subsets, CD56dimCD57+NKG2C+ NK cells exhibit markedly elevated TNF and IFN-γ production in response to K562 myeloid leukemia cells. Similar to virally infected cells, cancer cells can down-regulate classical class I HLA molecules while retaining expression of HLA-E3,20,21. The switch in receptor usage for HLA-E recognition from predominantly inhibitory NKG2A to activating NKG2C may be a key mechanism by which adaptive NK cells mediate GvL effects.
Our group, along with Kim and colleagues, has recently shown that the phenotype of CMV-induced adaptive NK cells extends well beyond expression of NKG2C and includes distinct subsets of NK cells lacking expression of the proximal signaling molecules FcεR1γ, EAT-2 and SYK individually or in combination22,23. These NK cell subsets have distinct genome-wide epigenetic profiles and unique functional attributes with respect to immunoregulation and antibody-dependent cellular cytotoxicity (ADCC). Importantly, FcεR1γ−, EAT-2− and SYK− adaptive NK cells are enriched for the expression of DAP12-coupled activating receptors, but frequently lack NKG2C22. Given the considerable heterogeneity of the human NK cell response to CMV infection, further high-resolution analyses that incorporate these novel adaptive NK cell markers may allow better definition of the association between adaptive NK cells and relapse protection, as well as the mechanisms underlying this effect.
Additional work is necessary to identify the precise mechanisms underlying the adaptive NK cell response to CMV, define the ontogeny of adaptive NK cells and to understand factors that promote their maturation. However, our results suggest that CMV-induced adaptive NK cells are clinically relevant and have anti-tumor properties. Novel strategies, such as vaccination, that mimic the unique immunomodulatory effects of acute CMV infection may reproduce these favorable HCT outcomes, particularly for RIC recipients at high risk for relapse.
Figure 4. CD56dimCD57+NKG2C+ NK cells produce TNF and IFN-γ at high frequencies relative to other NK cell subsets.
PBMCs from CMV seropositive donors were cultured with or without K562 target cells at a 2:1 ratio, and functional responses were analyzed in subsets of CD56dim NK cells. (A) Histograms of TNF expression (open black lines) and intracellular IFN-γ expression (open black lines) in NK cells cultured with K562 targets compared to effector cells cultured alone (shaded grey lines) for a representative donor. (B) Cumulative TNF and IFN-γ expression data in NK cells cultured with K562 targets from 5 donors. Two independent experiments were performed. * =p≤ 0.05, ** =p≤ 0.005. Two-sided, paired t-tests were used to determine significance. Error bars represent SEM.
Acknowledgments
We would like to thank the following shared resources within the Masonic Cancer Center at the University of Minnesota: The Translational Therapy Laboratory, Flow Cytometry Shared Resource, Clinical Trials Office, Oncology Medical Informatics and Services and the Biostatistical Core.
This work was supported by NIH P30 CA77598, R01 CA72669 (B.R.B.), 1R01 CA181045 (D.J.D), 5R01 CA077544 (D.J.D.), P30 CA033572 (City of Hope Cancer Center). F.C. is an Amy Strelzer Manasevit Research Program Scholar and is supported by a National Marrow Donor Program Award (CON000000052310).
Footnotes
Authorship Contributions
F.C. contributed to the study design, analyzed and interpreted data and wrote the manuscript. S.C. contributed to the study design, analyzed and interpreted data and wrote the manuscript. Z.D. analyzed data. T.E.D. performed the biostatistical analyses for the study. H.S. contributed to the conception of the study. B.Z. performed NK cell function assays. C.G.B., B.R.B., J.S. and M.R.V. contributed to the study design and sample procurement. D.J.D. reviewed and edited the manuscript. Y.T.B. contributed to the conception of the study and interpretation of results. D.J.W. and J.S.M contributed to the study design and sample procurement, analyzed and interpreted data and wrote the manuscript.
Conflict-of-Interest Statement
The authors declare no competing financial interest
References
- 1.Cooley S, Weisdorf DS. Natural killer cells and tumor control. Curr Opin Hematol. 2010;17:514–521. doi: 10.1097/MOH.0b013e32833f10f1. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs R, Stoll M, Stratmann G, Leo R, Link H, Schmidt RE. CD16− CD56+ natural killer cells after bone marrow transplantation. Blood. 1992;79:3239–3244. [PubMed] [Google Scholar]
- 3.Nguyen S, Dhedin N, Vernant JP, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105:4135–4142. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- 4.Foley B, Cooley S, Verneris MR, et al. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118:2784–2792. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vago L, Forno B, Sormani MP, et al. Temporal, quantitative, and functional characteristics of single-KIR-positive alloreactive natural killer cell recovery account for impaired graft-versus-leukemia activity after haploidentical hematopoietic stem cell transplantation. Blood. 2008;112:3488–3499. doi: 10.1182/blood-2007-07-103325. [DOI] [PubMed] [Google Scholar]
- 6.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34:251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 8.Ito S, Pophali P, Co W, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013;48:1313–1316. doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 11.Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35:669–674. doi: 10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 12.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 13.Foley B, Cooley S, Verneris MR, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolle A, Pollmann J, Ewen EM, et al. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J Clin Invest. 2014;124:5305–5316. doi: 10.1172/JCI77440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomson KJ, Mackinnon S, Peggs KS. CMV-specific cellular therapy for acute myeloid leukemia? Blood. 2012;119:1088–1090. doi: 10.1182/blood-2011-10-383943. author reply 1090-1081. [DOI] [PubMed] [Google Scholar]
- 16.Manjappa S, Bhamidipati PK, Stokerl-Goldstein KE, et al. Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol Blood Marrow Transplant. 2014;20:46–52. doi: 10.1016/j.bbmt.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCook LJ, Thoma M, Huneke T, et al. Impact of lymphocyte and monocyte recovery on the outcomes of allogeneic hematopoietic SCT with fludarabine and melphalan conditioning. Bone Marrow Transplant. 2013;48:708–714. doi: 10.1038/bmt.2012.211. [DOI] [PubMed] [Google Scholar]
- 18.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin R, Ruiz-Cabello F, Pedrinaci S, et al. Analysis of HLA-E expression in human tumors. Immunogenetics. 2003;54:767–775. doi: 10.1007/s00251-002-0526-9. [DOI] [PubMed] [Google Scholar]
- 21.Lo Monaco E, Tremante E, Cerboni C, et al. Human leukocyte antigen E contributes to protect tumor cells from lysis by natural killer cells. Neoplasia. 2011;13:822–830. doi: 10.1593/neo.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus Infection Drives Adaptive Epigenetic Diversification of NK Cells with Altered Signaling and Effector Function. Immunity. 2015;42:443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Zhang T, Hwang I, et al. Epigenetic Modification and Antibody-Dependent Expansion of Memory-like NK Cells in Human Cytomegalovirus-Infected Individuals. Immunity. 2015;42:431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]