Abstract
Approximately 12% of histone H2B in mammalian brain contains an unusual D-aspartate residue in its N-terminal tail. Most of this D-aspartate is linked to the C-flanking glycine via an isopeptide bond. To explore the possible significance of these modifications, we generated an antibody the D-isoaspartyl form of H2B, and used it to assess its levels in H2B associated with “active” vs. “silent” chromatin. We found that the D-isoaspartyl form of H2B appears to be highly enriched in the former. This irreversible modification could serve a novel regulatory function in gene expression.
Keywords: D-aspartate, chromatin, histone, isoaspartate, isomerization, methylation
Introduction
Covalent modification of the N-terminal tails of core histones plays a major role in controlling the readout of genetic information. In 2005 we reported a highly unusual modification in mammalian histone H2B; the presence of a D-aspartate residue in place of the normal L-aspartate residue position 25 (Young et al. 2005). In mouse brain, this modification is present in approximately 12% of the H2B, a level well within the range of more commonly known histone modifications. The racemization of aspartate in H2B arises as a two-step process; spontaneous formation of a succinimide at the Asp25-Gly26 junction, followed by hydrolysis of the succinimide to a mixture of L-aspartyl, D-aspartyl, L-isoaspartyl, and D-isoaspartyl variants, with L-isoaspartyl as the predominant product (typically 60–80%), and the D-forms as minor products (Figures 1 and 2). The enzyme PIMT (protein L-isoaspartyl methyltransferase; product of the pcmt1 gene) catalyzes repair of L-isoaspartyl sites by converting them back to normal L-aspartyl sites, but in the process (which also involves a succinimide intermediate) contributes to additional accumulation of the two D-forms, neither of which are efficiently repaired by this enzyme. Model studies with pure, synthetic L-isoaspartyl peptides indicate D-isoaspartyl as the major component of the two D-Asp-containing by-products of succinimide racemization (Johnson et al. 1987; McFadden and Clarke 1987). Because this modification of H2B is unusual, site selective, and relatively abundant, we wondered if it might have some significance with regard to chromatin function. To explore this issue, we generated an antibody that is relatively specific for the D-isoaspartyl form of mouse brain H2B and used it to estimate the levels of D-isoaspartyl H2B in “active” vs. “repressed” regions of chromatin. Our results suggest that the D-isoaspartyl form of H2B is significantly more abundant in active chromatin than in repressed chromatin.
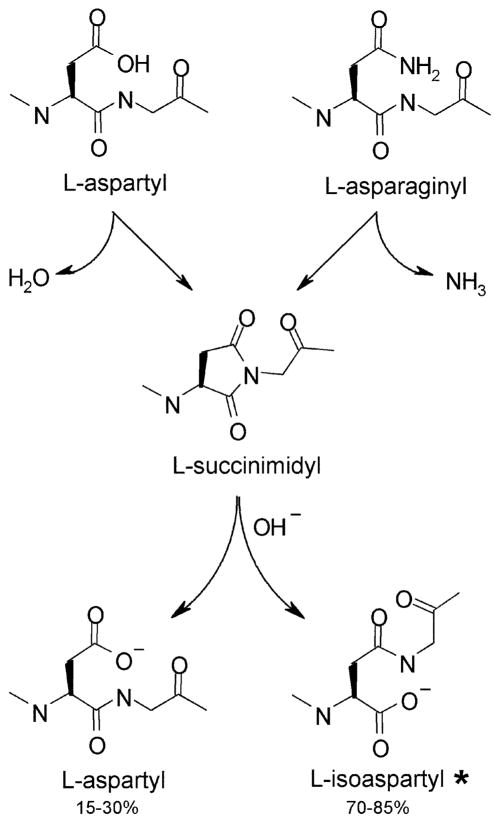
Fig. 1. Mechanism by which L-isoaspartyl sites arise from L-aspartyl and L-asparginyl sites in peptides and proteins.
This spontaneous intramolecular rearrangement occurs most readily at Asn-Gly, Asn-Ser and Asp-Gly sequences in flexible regions of polypeptides. The L-isoaspartyl form, lower right, typically accounts for 60–85% of the succinimide hydrolysis product.
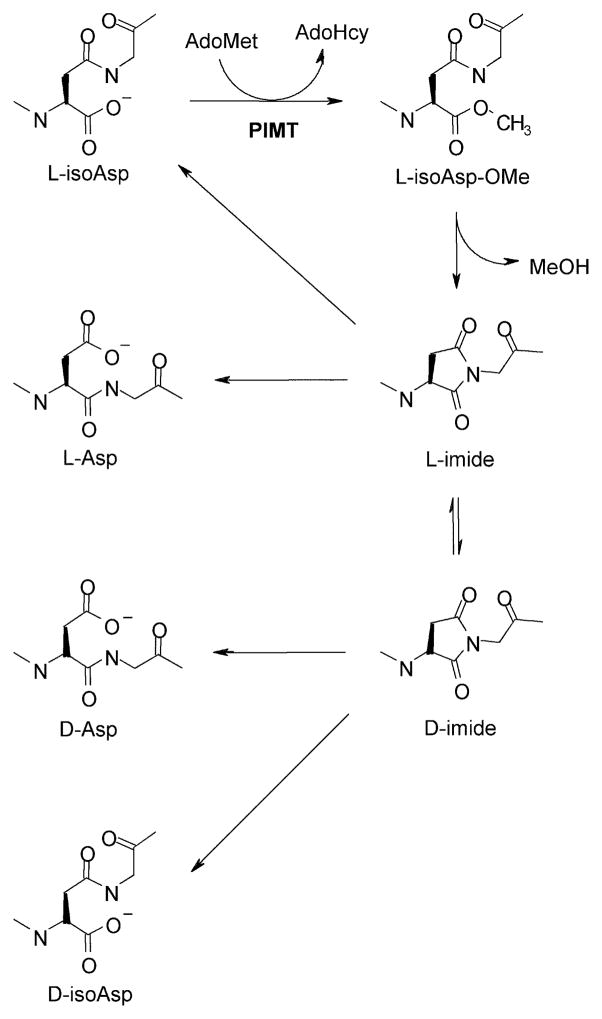
Fig. 2. Mechanism for PIMT-dependent repair and racemization of L-isoaspartyl sites.
PIMT catalyzes the methylation of L-isoaspartyl sites (top left) to form α-aspartyl O-methyl esters (top right). At physiological pH and temperature, the methyl esters spontaneously demethylate with a half-life of ca. 5–15 min to form the more stable L-succinimide (L-imide, middle right) which has a half-life of several hours. Hydrolysis of the L-succinimide generates a mixture of L-aspartyl and L-isoaspartyl peptides, the former representing completion of one repair cycle. Several additional cycles of methylation and demethylation convert nearly all of the original L-isoaspartyl sites to L-aspartyl sites. In the succinimide form, the acidity of the aspartate α-carbon markedly increases, thereby promoting production of D-succinimide (bottom right) via spontaneous racemization. Both the L-succinimide and the D-succinimide hydrolyze to form the corresponding isoaspartyl and aspartyl peptides. Although succinimide racemization is slow compared to its hydrolysis, its relative stability, combined with its constant replenishment during repeated repair cycles, provide ample opportunity for accumulation of D-isoaspartyl and D-aspartyl sites, which are poor substrates for PIMT.
Materials and methods
Animals
C57BL/6 PCMT1 +/− (HZ) males used to start our mouse colony were kindly provided by Prof. Mark Mamula (Yale University School of Medicine) and were originally generated by inserting a neomycin resistance cassette into exon one of the pcmt1 gene (Kim et al. 1997). KO and WT mice were obtained by intercrossing the HZ mice. Genotyping from tail clips was carried out by PCR at Transnetyx, Inc. (Cordova, TN) with probes for the neo cassette and the PIMT gene. Mice were monitored by on-site veterinarians, with all protocols undertaken in strict accordance with the recommendations for the Care and Use of Laboratory Animals, and approved by the University of California at Irvine Institutional Animal Care and Use Committee. Mice were anesthetized with Euthasol® and sacrificed by decapitation at an age of 4–5 weeks.
Generation of an anti-(D-isoaspartyl-25)-H2B polyclonal antibody
Two peptides containing residues 21–31 of mouse/human H2B (P10853/P62807) were synthesized and purified by AnaSpec, Inc. (San Francisco, CA). The “control peptide” (Asp-H2B) had the sequence Ac-AQKKDGKKRKR-ARGC-NH2 and the D-isoaspartyl peptide (D-isoAsp-H2B) was identical except that the L-Asp in position 25 was replaced by a D-Asp that was linked to Gly-26 via a β-aspartyl isopeptide bond in place of the normal α-aspartyl linkage. A rabbit antiserum against D-isoAsp-H2B conjugated to KLH was also made by Anaspec. The antibody was purified from this serum in our own lab by affinity chromatography on a column of the D-isoAsp-H2B peptide liked to agarose.
Histone purification
Core histones were purified from individual freshly excised mouse brains following procedures described previously (Young et al. 2001). Individual brains were homogenized in a Teflon/glass Potter-Elvehjem homogenizer in 9 vol of cold HB (5 mM K-Hepes, pH 7.6, containing 0.5 mM EDTA, 0.5 mM dithiothreitol, 10% (w/v) sucrose, and a 1/100 dilution of mammalian protease inhibitor (Sigma P8340). Homogenates were centrifuged at 800 × g for 30 min. The nuclear pellet fraction was dispersed by repeated passage through a 25-gauge needle, re-pelleted at 3,000 × g for 10 min, and then resuspended in 0.5 ml of 0.4 N H2SO4. After gently stirring for 1 h, the acidic solutions were centrifuged at 14,000 g for 10 min. Histones were recovered from the supernatant after precipitation with a final concentration of 25% w/v trichloroacetic acid followed by centrifugation at 14,000 g for 10 min. The final histone pellets were washed with cold acetone, then dissolved in 10 mM sodium phosphate pH 7.4.
Electrophoresis and Western blotting
Core histones or chromatin immunoprecipitates were subjected to electrophoresis in 10% NuPAGE® Bis-Tris gels (Life Technologies) after heating at 70°C for 10 min in SDS sample buffer. After semi-dry transfer to PVDF, the membrane was blocked with 5% non-fat milk in TBS-T (Tris-buffered saline, with 0.01% (v/v) Tween-20) and phosphatase inhibitors (50 mM NaF and 1 mM Na3VO4). Western blotting utilized HRP-conjugated goat anti-rabbit IgG (1:15,000, GE Heathcare) as a secondary antibody. Light signals were generated with ECL-Plus (Thermo-Pierce) and acquired in the linear range using a Nikon D700 camera (Khoury et al. 2010). Band densities were quantified with NIH ImageJ 1.43r and corrected for background.
Our custom D-isoAsp-H2B antibody was diluted into TBS-T containing 0.5 μg/ml of the Asp-H2B control peptide, prior to use. In the absence of this control peptide, the ability of D-isoAsp-H2B antibody to discriminate between H2B derived from pcmt1 WT vs. KO mice was greatly diminished. The pan-H2B antibody for Fig. 3 was from Cell Signaling Tech. (cat. # 8135). Western blots in Fig. 4 used pan-H2B from Cell Signaling (cat. # 12364) and pan-H3 from EMD/Millipore (cat. # 05-928)
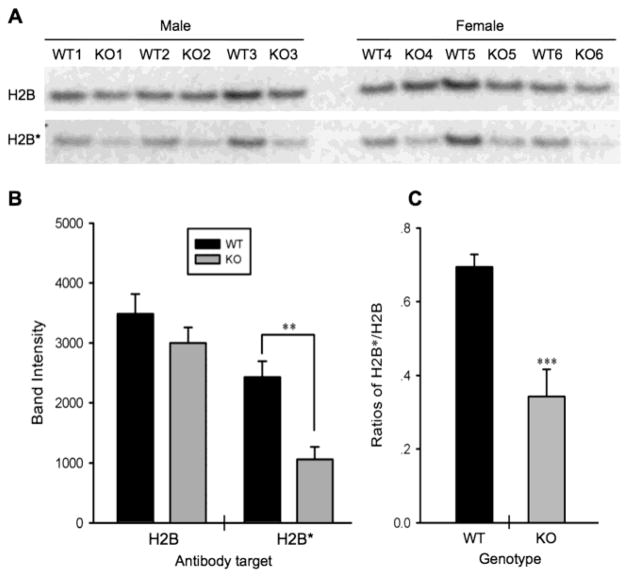
Fig. 3. Demonstration of antibody specificity to D-isoAsp-H2B.
(A) Western blots of histones from pcmt1 WT or KO mouse brain using a commercial pan-H2B antibody (H2B, top row), and our custom antibody made against the D-isoAsp-H2B synthetic peptide (H2B*, bottom row). Because the same histone preps were used for both blots, the top row serves as a loading control for the bottom row. (B) There is little difference (p = 0.235) between the WT and KO brain extracts when the pan-H2B antibody is used for blotting, but a large and significant (p = 0.003) difference when the H2B* antibody is used. Panel C shows that, when normalized to the pan-H2B signals, immuno-reactivity with the H2B* antibody is 2X stronger (p = 0.001) in the WT vs. KO extracts.
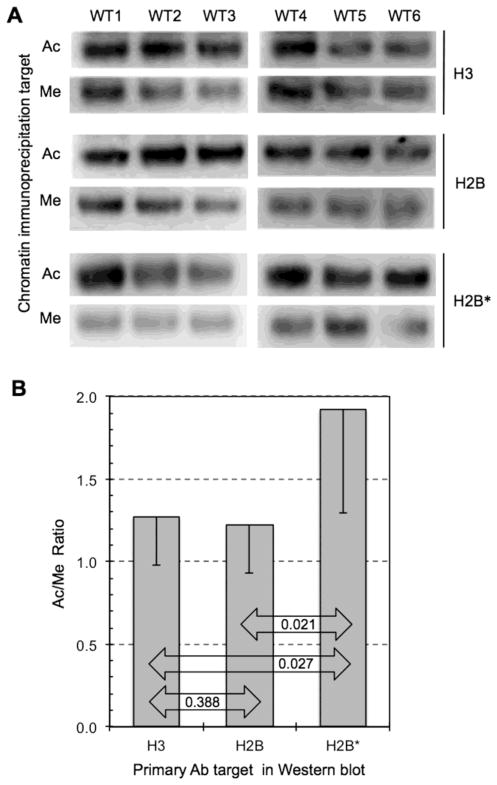
Fig. 4. D-isoAsp-H2B is enriched in active chromatin.
Chromatin was prepared from all 6 WT mouse brains (WT1-3 males, WT4–6 females) and each preparation was subjected to immunoprecipitations with antibody to acetylated histone H3 (H3K9; Ac) and with methylated histone H3 (H3K9Me3; Me). (A) The immunoprecipitates were than subjected to Western blotting using a pan-H3 antibody (H3), a pan-H2B antibody (H2B), and our D-isoAsp-H2B antibody (H2B*). (B) The Ac/Me band ratios are the same, as expected, when immunoblotting for pan-H3 or pan-H2B, whereas the ratio when using H2B* is about 55% higher.
Chromatin immunoprecipitation
Mouse brains were disaggregated by chopping with a razor and passing sequentially through 18- and 21-gauge needles in 5 vol of ice-cold PBS. The disaggregated tissue was treated with formaldehyde at a final concentration of 1% for 10 min, then quenched with glycine to a final concentration of 125 mM. The cross-linked chromatin was washed twice in PBS and resuspended in ice-cold cell lysis buffer and nuclear lysis buffer as provided in the EZ-Magna ChIP A/G kit (Millipore), then fragmented by sonication into pieces ranging from 200 and 500 bp as verified by agarose gel electrophoresis. Antibodies H3K9ac (Millipore cat. 06-942) and H3K9me3 (Millipore cat. 07-442) were used for ChIP. Antibody (2 μg) and 20 μL of fully resuspended protein A/G magnetic beads were incubated with sonicated chromatin at 4°C in ChIP dilution buffer with shaking for 4 h. The beads were washed with cold buffer contained in the EZ-Magna kit. Finally, the beads were resuspended in lithium dodecyl sulfate sample buffer (Invitrogen) plus DTT. Immunoprecipitates were boiled for 10 min at 70°C, prior to electrophoresis.
Protein Assays
Protein concentrations in brain extracts and histone preparations were determined with the Pierce BCA Protein Assay Kit (Life Technologies) using the provided bovine serum albumin as a standard.
Statistical Analysis
The paired student T-test (2-tailed; heteroscedastic) was used for all data comparisons. Additional details are provided in the figure legends.
Results
Validation of an antibody to D-isoaspartyl-25 H2B
Our validation is based on a previous finding that the level of D-isoaspartate at the Asp-25 position in histone H2B from mouse brain is known to be four-times higher in pcmt1 WT (+/+) mice than in pcmt1 KO (−/−) mice (Young et al. 2005). We isolated core histones from 12 mice (6 WT and 6 KO) with equal numbers of male and female mice in each genotype. We then carried out Western blots using a custom antibody to D-isoaspartyl-H2B (αH2B*) or a commercial pan-H2B antibody (αH2B). As shown in Fig. 3A,B, αH2B* reacts 2.3X stronger (p = 0.003) with histone from WT mice, while αH2B shows only a minor (1.17X; p = 0.235) apparent preference for WT histone. The previous HPLC analysis of acid hydrolysates of the N-terminal region of H2B from WT and KO mice yielded D-Asp/L-Asp ratios of 0.14 and 0.035 respectively, a 4-fold difference. The more modest difference between WT and KO seen in Fig. 3C suggests that the epitope specificity of our αH2B* polyclonal antibody is not restricted to the D-isoaspartyl moiety of the antigen.
D-Isoaspartyl H2B is preferentially associated with active chromatin
We were interested to see if the D-isoaspartyl version of H2B is preferentially associated with either active or repressed chromatin. Our approach is based on the fact that acetylation (Ac) of lysine-9 in histone H3 is typically found on actively transcribed genes, while trimethylation (Me) of lysine-9 in H3 is typically associated with repressed or silent genes. Sheared fragments of chromatin (200–500 bp) were therefore prepared from brain extracts of 6 WT mice (WT1-3 males; WT4-6 females). Each chromatin preparation was then immuno-precipitated with antibodies specific to either of the aforementioned (Ac or Me) histone H3 modifications. Finally, each immunoprecipitation was subjected to Western analysis with pan-H3, pan-H2B, or the H2B* antibody (Fig. 4).
To properly interpret the data shown in panel A of Fig. 4, it is important to understand how the Western blots were carried out. As an example, in the first two rows, all 6 immuno-precipitates (3 Ac and 3 Me) from WT1-3 were run on the same gel to justify a direct comparison of the Ac vs. Me blot intensities from a given set of immunoprecipitates. Although all 6 were run on the same gel, the final blot image was cut and cropped to reposition the Me bands directly below the corresponding Ac bands in order to facilitate visual assessment of the Ac/Me band ratios. The same was done for all other sample sets in Figure 2.
A quantitative summary of the Ac/Me blot ratios is shown in panel B of Fig. 4. Importantly, this confirms the expectation that the relative abundance of histones H3 and H2 are virtually identical in the Ac and Me immunoprecipitates. In marked contrast, the D-isoaspartyl variant of H2B is a least 55% richer in the Ac immunoprecipitates than in the Me immunoprecipitates. We say “at least” here because, as discussed above, we know that the H2B* antibody shows significant cross-reaction with normal H2B molecules. These observations strongly suggest that the D-isoaspartyl form of H2B is preferentially associated with active chromatin.
Discussion
The selective enrichment of D-isoaspartyl-H2B in active chromatin raises interesting questions with regard to its possible significance. The propensity of an aspartyl residue to form racemization/isomerization-prone succinimides depends on both its C-flanking neighbor (with glycine providing the greatest enhancement) along with the local flexibility and conformation of the polypeptide chain (Aswad et al. 2000; Clarke 1987; Sydow et al. 2014). The enrichment of D-isoAsp-H2B in active chromatin thus implies that the N-terminal region of H2B is more flexible, and probably more accessible to PIMT, than H2B associated with repressed genes. Enhanced flexibility could be related to the fact that H2A/H2B dimers undergo rapid exchange in nucleosomes present in regions of active transcription (Venkatesh and Workman, 2015 ). Frequent and/or prolonged activation of a gene could foster succinimide formation in histone H2B, resulting in the irreversible accumulation of D-isoaspartyl variants that would tend to keep the associated chromatin in an active state. In this view, D-isoaspartyl formation could serve a regulatory modification that enhances the expression of a gene based on its history of prior activation. The observations reported here will hopefully promote future studies to more fully explore this and other possible functions of this unusual histone modification. The development of a monoclonal antibody that is highly selective for the D-Asp form of H2B would be good first step toward this goal.
Acknowledgments
We thank the laboratory of Prof. Mark J. Mamula at the Yale University School of Medicine for providing us with the founder mice from which our colony was generated. Some of this work was funded by NIH grant NS17269 to DWA.
The abbreviations used are
- ECL
enhanced chemiluminescence
- isoAsp
isoaspartyl
- KO
knockout (PIMT−/−)
- PIMT
protein L-isoaspartyl methyltransferase
- PVDF
polyvinylidene difluoride
- TBS
Tris-buffered saline
- WT
wild type (PIMT+/+)
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest with the contents of this article.
References
- Aswad DW, Paranandi MV, Schurter BT. Isoaspartate in peptides and proteins: formation, significance, and analysis. J Pharm Biomed Anal. 2000;21:1129–1136. doi: 10.1016/s0731-7085(99)00230-7. [DOI] [PubMed] [Google Scholar]
- Clarke S. Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Peptide Protein Res. 1987;30:808–821. doi: 10.1111/j.1399-3011.1987.tb03390.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Murray ED, Jr, Clarke S, Glass DB, Aswad DW. Protein carboxyl methyltransferase facilitates conversion of atypical L-isoaspartyl peptides to normal L-aspartyl peptides. J Biol Chem. 1987;262:5622–5629. [PubMed] [Google Scholar]
- Khoury MK, Parker I, Aswad DW. Acquisition of chemiluminescent signals from immunoblots with a digital single-lens reflex camera. Anal Biochem. 2010;397:129–131. doi: 10.1016/j.ab.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Lowenson JD, MacLaren DC, Clarke S, Young SG. Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice Proc. Natl Acad Sci U S A. 1997;94:6132–6137. doi: 10.1073/pnas.94.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden PN, Clarke S. Conversion of isoaspartyl peptides to normal peptides: Implications for the cellular repair of damaged proteins. Proc Natl Acad Sci U S A. 1987;84:2595–2599. doi: 10.1073/pnas.84.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow JF, et al. Structure-based prediction of asparagine and aspartate degradation sites in antibody variable regions. PLoS One. 2014;9:e100736. doi: 10.1371/journal.pone.0100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh S, Workman JL. Histone exchange, chromatin structure and the regulation of transcription. Nature reviews. 2015;16:178–189. doi: 10.1038/nrm3941. [DOI] [PubMed] [Google Scholar]
- Young AL, Carter WG, Doyle HA, Mamula MJ, Aswad DW. Structural integrity of histone H2B in vivo requires the activity of protein L-isoaspartate O-methyltransferase, a putative protein repair enzyme. J Biol Chem. 2001;276:37161–37165. doi: 10.1074/jbc.M106682200. [DOI] [PubMed] [Google Scholar]
- Young GW, Hoofring SA, Mamula MJ, Doyle HA, Bunick GJ, Hu Y, Aswad DW. Protein L-isoaspartyl methyltransferase catalyzes in vivo racemization of Aspartate-25 in mammalian histone H2B. J Biol Chem. 2005;280:26094–26098. doi: 10.1074/jbc.M503624200. [DOI] [PubMed] [Google Scholar]