Abstract
Purpose
Post-contrast myocardial T1 (T1myo,c) values have been shown to be sensitive to myocardial fibrosis. Recent studies have shown differences in results obtained from T1myo,c and extracellular volume fraction (ECV) with respect to percentage fibrosis. By exploring the relationship between blood plasma volume and T1myo,c, the underlying basis for the divergence can be explained. Furthermore, dose administration based on BMI, age and gender can mitigate the divergence in results.
Methods
Inter-subject comparison of T1myo,c required adjustment for dose (in mmol/kg), time and glomerular filtration rate (GFR). Further adjustment for effective dose based on lean muscle mass reflected by blood/plasma volume (PV) was performed. A test case of 605 subjects from the MESA study who had undergone pre and post-contrast T1 mapping was studied. T1myo,c values were compared between subjects with and without metabolic syndrome (MetS), between smoking and non-smoking subjects, and subjects with and without impaired glucose tolerance, before and after dose adjustment based on plasma volume. Comparison with ECV (which is dose independent), pre-contrast myocardial T1 and blood normalized myocardial T1 values was also performed to validate the correction.
Results
There were significant differences in T1myo,c (post plasma volume correction) and ECV between current and former smokers (p-value 0.017 and 0.01, respectively) but not T1myo,c prior to correction (p = 0.12). Prior to dose adjustment for plasma volume, p-value was < 0.001 for T1myo,c between MetS and non-MetS groups and was 0.13 between subjects with and without glucose intolerance; after adjustment for PV, p value was 0.63 and 0.99. Corresponding ECV p values were 0.44 and 0.99, respectively. Overall, ECV results showed the best agreement with PV corrected T1myo,c (mean absolute difference in p values = 0.073) and pre-contrast myocardial T1 in comparison with other measures (T1myo,c prior to correction and blood/plasma T1 value normalized myocardium).
Conclusions
Weight-based contrast dosing administered in mmol/kg results in a bias in T1 values which can lead to erroneous conclusions. After adjustment for lean muscle mass based on plasma volume, results from T1myo,c were in line with ECV derived results. Furthermore, the use of a modified equivalent dose adjusted for body mass index (BMI), age, sex and hematocrit can be adopted for quantitative imaging.
Keywords: T1 mapping, cardiac, dose correction, blood volume, plasma volume
Introduction
Non-ischemic cardiomyopathy is frequently associated with diffuse myocardial fibrosis. Although late gadolinium enhancement (LGE) is excellent for depicting focal myocardial scar, diffuse fibrosis may show only minimal variation in intensity on LGE images since each voxel contains normal and abnormal myocardium. Recent studies have reported shorter post-contrast T1 myocardial times (T1myo,c) in the case of diffuse fibrosis [1,2]. Prior and subsequent studies have shown significant differences in T1myo,c between normal population and subjects with heart failure [1], chronic aortic regurgitation [3], cardiac amyloidosis [4] and idiopathic dilated cardiomyopathy [5] among other conditions. T1myo,c continues to be used as a marker for cardiac disease alongside ECV and pre-contrast myocardial T1, especially when pre-contrast T1 values are highly inaccurate as in legacy studies where post-contrast T1 values derived from TI scout scans (inversion recovery Look Locker with 2 R-R interval acquisition) are only available.
Although several studies have shown T1 (and other derived values such as ECV) differences between healthy and fibrotic myocardium, there have been only a handful of studies showing histological correlation. The difficulty in obtaining histological correlates can be easily appreciated in human studies. Nevertheless, T1myo,c has been histologically validated with percentage change in fibrosis [1,2,6]. One recent histological correlation study performed in segmented sections of six explanted hearts of heart transplantation patients showed a strong statistically significant linear relationship between ECV and intra- and inter-patient histological collagen fraction but only showed a strong statistically significant inverse linear relationship between T1myo,c and intra-patient histological collagen fraction [7]. Animal models have shown moderate (ECV vs collagen volume fraction, p = 0.013, [8]) to strong correlation (ECV vs fibrosis, p < 0.001) [9]. Another longitudinal study in canines found disagreement between percentage fibrosis vis-à-vis T1 and ECV [10]. While the mean change in collagen volume fraction was relatively small (from 0.9% to 1.9%), the change in T1 was relatively large (decreased by 24.9%) while ECV increased from 0.21 to 0.22 (a change of 4.5%). The authors observed the need to explain this discrepancy. Some of the variability in relating T1 (or ECV) can be attributed to inaccuracies in the mapping techniques used. The commonly used MOLLI sequence has been shown to exhibit dependency on B0 and B1 field homogeneity, T2 and heart rate [11,12]. Longer T1 values are typically underestimated and show a greater dependency on heart rate. Variations of the MOLLI acquisition scheme [13] can perform better at estimating longer pre-contrast blood and myocardium values. However, T1myo,c being shorter is more accurately determined and shows much reduced heart rate related variation. Other less commonly used cardiac T1 mapping schemes also exhibit shortcomings such as poorer precision or accuracy. On the other hand, T1 derived value such as ECV is relatively independent of dose which has a substantial impact on post-contrast T1 values.
Variability due to kinetics of the contrast agent exists in the case of post-contrast T1 mapping. In order to derive inferences regarding tissue composition of the myocardium using gadolinium contrast, it is necessary to normalize differences between subjects based on dose, time and glomerular filtration rate. A previous work explored the relationship between dose, time, GFR and T1myo,c [14]. An analytical solution was proposed which allowed for normalization between different subjects. It was shown that dose had a significant impact on post-contrast T1 values.
For example, assuming average physiological parameters and kinetics at time = 10 mins post-contrast, T1pre = 1220 ms [15] and r1 = 4.1 (mM.s)−1 [16], an increase in dose from 0.15 mmol/kg to 0.17 mmol/kg would result in T1post decreasing from 555 ms to 517 ms (a 7.3% decrease). Since many studies that compare healthy to fibrosed myocardium depend on mean differences between the two populations that are of the order of tens of milliseconds, it is easy to understand how dose variation can significantly impact end inferences.
In this study, we focus our attention on the use of equivalent dose between subjects based on millimoles per kilogram. When dose is standardized to body weight, this infers a linear relationship between body weight and blood plasma volume or lean muscle. However, it is well known that blood volume (BV) per unit weight decreases with increasing weight [17] so that obese subjects will have less blood volume than anticipated by standard dosing in mmol/kg. As a result, a higher concentration of dose in blood (and consequently plasma through the hematocrit) is delivered to heavier subjects. Since T1myo,c value depends on the exchange of contrast between the plasma compartment and the tissue compartment, the values in the myocardium will be biased to a lower value than expected for larger subjects. Such a bias can complicate interpretation of results. This is even more so since obese patients could typically be more risk prone and likelier to have cardiac disease. A lower T1 value in such subjects could then be misrepresented as reflecting fibrosis but in reality would be a result of the higher initial dose.
It is generally agreed that the normal human blood volume is about 5–6 liters. With progressive body fat, total BV increases as body mass increases but BV measured in ml/kg actually decreases in a non-linear manner with increasing weight. Although a dual isotope, dual tracer technique is considered the gold standard for BV measurement, the process is cumbersome and entails exposure to radioactivity. More recently, a FDA approved single isotope (131I) method is now commonly employed at major institutions to determine blood volume [18]. Indirect methods established based on body weight disregard body composition despite using different values for males and females. BV calculated using body surface area was found to be more accurate [19] although a ±25% variation from the normal predicted value was expected in the general population [20]. Later, Feldschuh and Enson [17] used data from 160 individuals (80M/80F) in the Metropolitan Life Insurance Company height and weight tables and showed that a curvilinear relationship of body weight deviation from ideal body weight to BV accurately estimates true blood volume as determined from nuclear studies. More recently, Lemmens et al. [21] successfully captured graphical information from different studies relating blood volume per kg to BMI in an analytical form and showed excellent correlation to the more accurate of earlier studies.
Here, we explore the relationship between blood plasma volume and T1myo,c. Examples of how erroneous contrast dosing based on standard mmol/kg can bias results are then presented. One test case was that of metabolic syndrome (MetS) because of its association with BMI as well as possible cardiac disease. MetS is defined as the co-occurrence of three out of five of the following risk factors: elevated blood pressure and plasma glucose, central obesity, high serum triglycerides and low high density cholesterol (HDL) levels. First a comparison between T1myo,c values (derived with standard dose in mmol/kg and corrected for physiological variations) in subjects with metabolic syndrome (MetS) against subjects without MetS from the MESA (Multi-Ethnic Study of Atherosclerosis) cohort was carried out. Next an adjustment for dose based on plasma volume was derived and normalized values were once again compared for the two groups. A similar comparison was carried out between smokers and non-smokers and subjects with and without impaired glucose tolerance. The results obtained with T1myo,c were compared with results using ECV. Other measures including pre-contrast myocardial T1, blood and plasma T1 normalized myocardial T1 were also calculated and compared with ECV.
Methods
Blood plasma volume calculation
From [21], BV can be calculated using the following two equations for males and females, respectively.
| (1) |
BVM and BVF are the blood volumes expressed in ml/kg in males and females, respectively. Plasma volume (PV) is then given by
| (2) |
where [Hct] is the hematocrit. Once the plasma volume is determined, the total contrast dose administered in ml is divided by the plasma volume to determine a scaling factor for the dose represented in mmol/kg.
The steps involved in achieving the correction are as follows:
Determine PV for males and females based on eqs. (1) and (2).
Calculate DPV = [Dose in ml] / PV.
Now correct the mmol/kg dose (D) using D’ = [D x DPV) / (DPVm)], where DPVm is the mean DPV across all subjects.
This D’ is then used to correct for differences in dose between subjects as detailed in [14].
Using equations 1–2, one can simulate the dose and T1 variation resulting from constant weight individuals with different BMIs, ages and for each gender. Other physiological parameters like GFR and [Hct] as well as the contrast kinetics are assumed constant to better understand the variation resulting from blood plasma volume. A study comparing T1myo,c values would then have to normalize for the different doses between the subjects.
Example Studies
A total of 605 subjects from the MESA study, who had undergone pre and post contrast (gadolinium dimeglumine) T1 mapping at 1.5T, and who had complete demographical and physiological parameters as well as hematocrit data available were studied. MESA is a multi-center study and the review boards of all participating centers approved the study and all participants gave written informed consent after the procedure had been fully explained. T1 mapping using the MOLLI sequence [22] was performed 12 min after a bolus dose of 0.15 mmol/kg based on subject weight was injected.
MetS Study
Of the 605 subjects, 177 were classified with MetS while the rest (N = 428) did not fall into the category and were used for comparison. The demographics for the two groups are provided in Table 1.
Table 1.
| MetS | No MetS | p-value* | |
|---|---|---|---|
| Parameter | Mean ± SD | Mean ± SD | |
| N | 177 | 428 | |
| Age | 67.7±9.0 | 67.9±8.6 | 0.8 |
| Sex - Male (%) | 40.7 | 49.5 | 0.05 |
| Body Mass Index (kg/m2) | 32.2 ± 5.0 | 27.6 ± 5.0 | < 0.001 |
| GFR (ml/min/1.73m2) | 82.1 ± 18.2 | 82.8 ± 18.8 | 0.7 |
| Heart Rate (beats per minute) | 67 ± 11 | 65 ± 11 | 0.052 |
| Hematocrit | 40.1 ± 3.6 | 39.5 ± 3.4 | 0.08 |
p-value is calculated using Student’s t-test for continuous variables and the χ2 test for categorical variables.
Post-contrast myocardial T1 values were normalized for differences in dose and GFR as described in [14]. The T1 values for the two groups were compared using Student’s t-test. This was done prior to and after dose adjustment based on blood plasma volume.
Smoking Study
Tables 2 and 3 provide relevant demographical and physiological information for current, former and non-smokers. Of the 605 subjects, 48 were current smokers, 265 were former smokers while 287 had never smoked. Five subjects were not included as information on smoking habits was not available.
Table 2.
| Current | Former | p-value | |
|---|---|---|---|
| Parameter | Mean ± SD | Mean ± SD | |
| N | 48 | 265 | |
| Age | 68.6±9.7 | 68.1±8.5 | 0.09 |
| Sex - Male (%) | 47.9 | 58.5 | 0.17 |
| BMI | 37.2 ± 5.0 | 29.2 ± 5.3 | 0.01 |
| GFR | 87.1 ± 18.7 | 82 ± 19 | 0.09 |
| Heart Rate | 69 ± 10 | 68 ± 9 | 0.71 |
| Hematocrit | 40.6 ± 3.5 | 40.1 ± 3.8 | 0.37 |
Table 3.
| Current | Never | p-value | |
|---|---|---|---|
| Parameter | Mean ± SD | Mean ± SD | |
| N | 48 | 287 | |
| Age | 68.6±9.7 | 68±8.9 | 0.11 |
| Sex - Male (%) | 47.9 | 35.9 | 0.11 |
| BMI | 37.2 ± 5.0 | 29 ± 5.5 | 0.014 |
| GFR | 87.1 ± 18.7 | 82.2 ± 18.0 | 0.1 |
| Heart Rate | 69 ± 10 | 66 ± 11 | 0.09 |
| Hematocrit | 40.6 ± 3.5 | 39.6 ± 3.4 | 0.07 |
Impaired Glucose Study
Subjects with untreated impaired glucose tolerance were compared to subjects with normal glucose tolerance. Demographical details are shown in Table 4.
Table 4.
| Impaired | Normal | p-value* | |
|---|---|---|---|
| Parameter | Mean ± SD | Mean ± SD | |
| N | 116 | 389 | |
| Age | 69.2±9.6 | 67.4±8.5 | 0.07 |
| Sex - Male (%) | 55.2 | 43.2 | 0.02 |
| BMI | 30.2 ± 5.8 | 27.6 ± 4.7 | < 0.001 |
| GFR | 81.3 ± 16.2 | 83.2 ± 18.9 | 0.3 |
| Heart Rate (bpm) | 67 ± 12 | 65 ± 10 | 0.06 |
| Hematocrit | 41 ± 3.8 | 39.9 ± 3.3 | 0.008 |
p-value is calculated using Student’s t-test for continuous variables and the χ2 test for categorical variables.
Comparison to ECV
To test our hypothesis that disparity in results obtained with ECV values against results from post-contrast myocardial T1 values is a result of incorrect dosing when calculated in mmol/kg, we compared ECV results with post-contrast T1 results prior to and after dose adjustment.
Normalization with blood pool
In the absence of availability of pre-contrast T1 values (and hence ECV), one technique that has been employed to normalize for the dose and physiological variation is to divide T1myo,c by T1bl,c (post-contrast T1 of blood at the same time point). Accordingly, this measure along with normalization with the hematocrit adjusted plasma T1pl,c (defined as T1bl,c × [1 – Hct]) was explored as an alternative to performing the correction as described in this work. Note that it is the contrast in the plasma which exchanges with the cardiac tissue and hence should provide more accurate normalization than T1bl,c.
Statistical analysis
All data were analyzed in Matlab®. Relative comparison of the measures was performed using a one-sided Student t-test. T values and degrees of freedom (df) were calculated for unequal sample sizes and unequal variances. P values were calculated based on the t value and df. A one-sided test was used to avoid misinterpretation of results. For example, post-contrast T1 value in fibrosed myocardium has been shown to be shorter than in normal myocardium while pre-contrast T1 value is expected to be longer in fibrosed myocardium. Similarly, ECV value is higher in fibrosed myocardium and lower in normal myocardium. In addition, Pearson correlation was employed to study correlation between p values obtained with ECV and with other measures.
Results
Simulation
Figure 1 shows the variation in dose for male and female subjects when the same amount of gadolinium dose (in ml) is given to individuals with the same weight but different ages and BMIs. Figure 2 shows the resulting variation in T1 values. In Figures 1 and 2, BMI varies from 10 kg/m2 to 50 kg/m2 while age varies from 40 to 80 years. For males and females in the example shown, a dose of 0.15 mmol/kg occurs at BMI = 27 kg/m2 and age 53 years. Although the plots for both genders look similar, the range of T1 values is slightly different for males (350–580 ms) as compared to females (350–585 ms) due to differences in blood volume in ml/kg.
Figure 1.
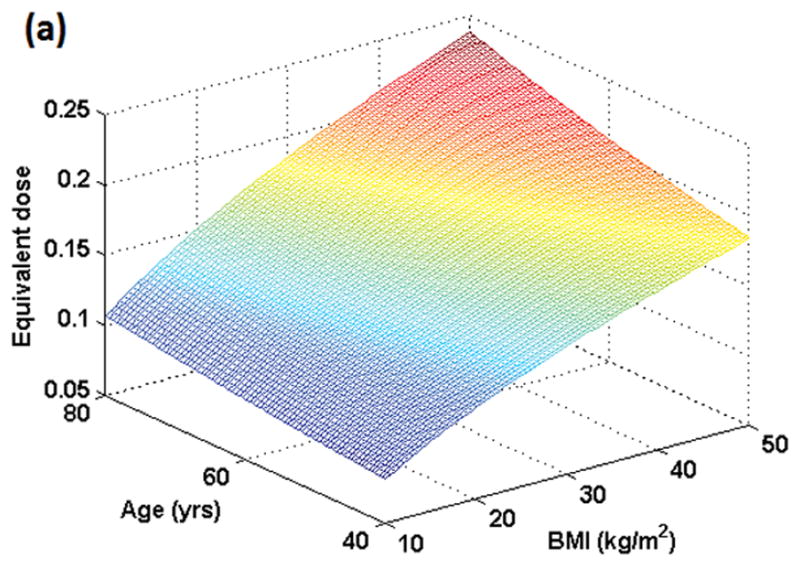
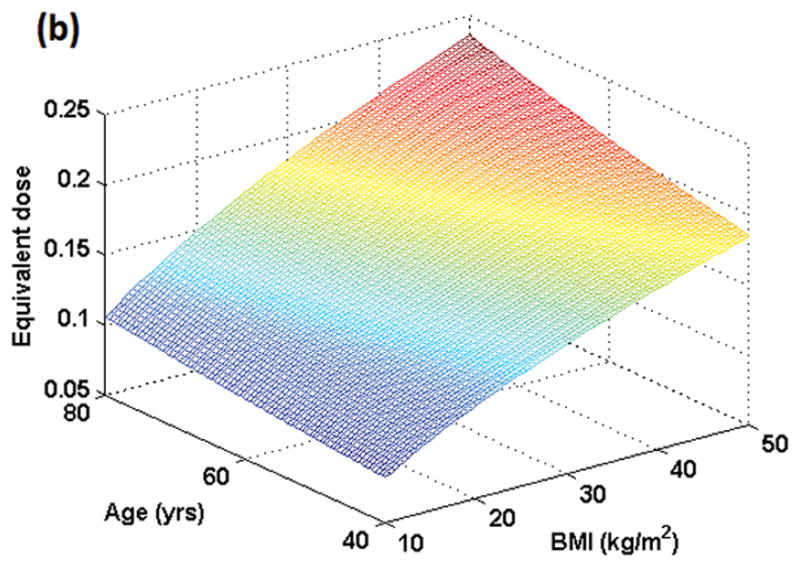
Figure 1 (a): Simulation of true dose in the female population resulting from dose administered in mmol/kg as a function of BMI and age. Maximum dose concentration in plasma occurs at the oldest age (80 yrs here) and highest BMI (50 kg/m2 here). All individuals who weigh the same but have different BMIs and ages would show different contrast concentrations in plasma as plotted here.
Figure 1 (b): Simulation of true dose in the male population resulting from dose administered in mmol/kg as a function of BMI and age. Maximum dose concentration in plasma occurs at the oldest age (80 yrs here) and highest BMI (50 kg/m2 here).
Figure 2.
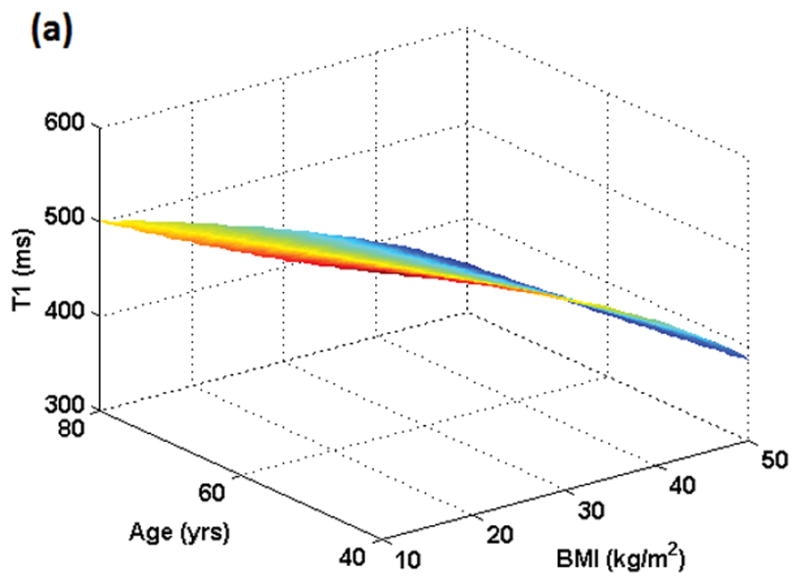
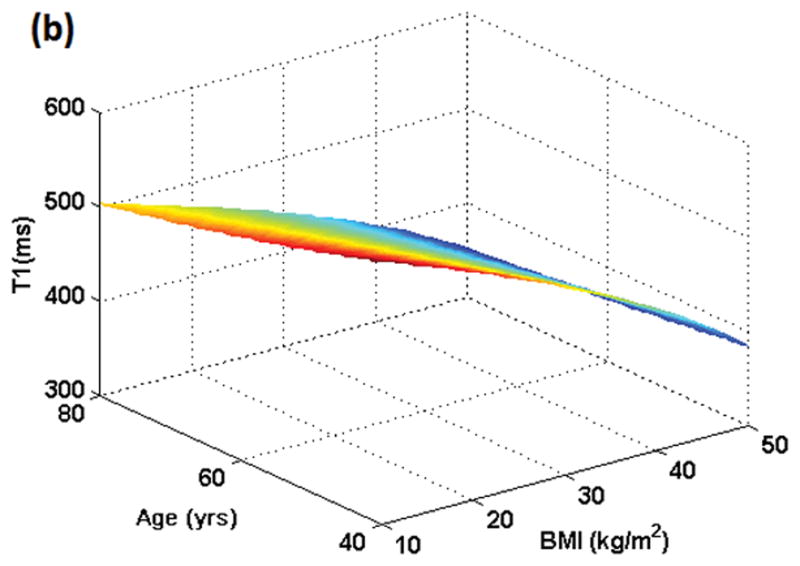
Figure 2 (a): Simulation of T1 variation in the female population resulting from dose administered in mmol/kg as a function of BMI and age. Minimum T1 value will result at the oldest age (80 yrs here) and highest BMI (50 kg/m2 here). All individuals who weigh the same but have different BMIs and ages would exhibit different T1 values post-contrast although the administered dose was the same based on mmol/kg.
Figure 2 (b): Simulation of T1 variation in the male population resulting from dose administered in mmol/kg as a function of BMI and age. Minimum T1 value will result at the oldest age (80 yrs here) and highest BMI (50 kg/m2 here). All individuals who weigh the same but have different BMIs and ages would exhibit different T1 values post-contrast although the administered dose was the same based on mmol/kg.
The mean dose change (|Doseuncorr – Dosecorr| / Doseuncorr) across the 605 MESA subjects after adjustment for blood plasma volume was 9.1% while the maximum dose adjustment was 29%. Mean T1 across 605 subjects was 451.9±37.3 ms prior to plasma volume related dose correction and 453.8±40.3 ms after correction. Mean change in post contrast myocardial T1 was 4.7% while the maximum change was 32.7%.
MetS
T1myo,c showed statistical difference between MetS and non-MetS subjects (p < 0.0001 subjects prior to correction. After correction for BV, myocardial T1 value in non-MetS was 452.5±37.8 ms and 456.1±38.1 ms in MetS subjects (p = 0.86). After including [Hct] to determine plasma volume variation, myocardial T1 value in non-MetS subjects was 453.4±40.9 ms and was 454.6±38.7 ms in subjects with MetS (p = 0.63).
Smoking
Prior to correction for plasma volume, T1myo,c showed no statistically significant difference between current and former smokers (T1myo,c = 449.5±34.4 ms vs 456±35.5 ms, p = 0.12) and between smokers and non-smokers (T1myo,c = 448.4 ± 39.2 ms, p = 0.42) but showed a statistically significant difference between smokers and former smokers (445.4±35.1 vs 457.6±39.3 ms, p = 0.017) after PV based correction; the difference between smokers and subjects who had never smoked (451.6 ± 41.6 ms) was not significant (p = 0.137).
Impaired Glucose
T1myo,c of patients with impaired tolerance to glucose showed no correlation to T1myo,c of subjects with normal tolerance (455.4±35.9 ms vs 451.1±37.9 ms, p = 0.86) prior to plasma volume related adjustment. After adjustment, they were strongly uncorrelated (p = 0.99, 1 tailed).
Comparison to ECV
There was no statistically significant difference in ECV values for subjects with and without MetS (0.270±0.026 vs 0.2696±0.032, p = 0.44) while showing significant differences between smokers and former smokers (0.279 ± 0.030 vs 0.268 ± 0.031, p = 0.01) and between smokers and non-smokers (0.271 ± 0.03, p = 0.043). Subjects with normal glucose had an ECV value of 0.271 ± 0.031 which was higher than subjects with impaired glucose, who had a ECV value of 0.262 ± 0.03 (p value = 0.99).
Normalization with blood pool
T1myo,c/T1bl,c values were 1.463 ± 0.094 for smokers and 1.5 ± 0.11 for former smokers (p = 0.008) while it was 1.515 ± 0.104 (p = 0.007) for subjects who had never smoked.
T1myo,c/T1bl,c values were 1.498 ± 0.107 and 1.522 ± 0.099 (p-value = 0.99) for subjects with non-METS and with METS, respectively. T1myo,c/T1bl,c values for subjects with normal glucose was 1.499 +/− 0.105 while it was 1.506 +/− 0.11 for those with impaired glucose (p = 0.253).
Table 5 shows the p-values obtained with the four different comparison studies for the six measures calculated. Mean absolute difference between ECV and the other measures showed differences of 0.45, 0.078, 0.073, 0.33 and 0.13 for T1myo,c, T1pre, T1myo,c (PV adjusted), T1myo,c / T1bl,c and T1myo,c / T1pl,c, respectively. When results obtained from the four comparison tests were analyzed, Pearson correlation between T1myo,c and ECV improved from −0.41 to 0.98 after correction. Other correlation values were 0.99, 0.35 and 0.94 for T1pre, T1myo,c / T1bl,c and T1myo,c / T1pl,c. Consequently, T1myo,c (PV adjusted) and T1pre showed the closest correspondence with ECV results and could be used instead of ECV.
Table 5.
| P values | ||||
|---|---|---|---|---|
| MetS | Smoking 1 | Smoking 2 | IM | |
| T1 | <0.0001 | 0.12 | 0.42 | 0.13 |
| ECV | 0.44 | 0.01 | 0.043 | 0.99 |
| T1pre | 0.33 | 0.003 | 0.037 | 0.8 |
| T1 (corr) | 0.63 | 0.017 | 0.137 | 0.99 |
| Ratio T1 | 0.99 | 0.008 | 0.007 | 0.253 |
| Ratio T1* | 0.81 | 0.11 | 0.10 | 0.99 |
P values are based on one-sided Student t-test for the four different comparison studies and for the six measures.
IM: Impaired glucose
T1: Values prior to adjustment for plasma volume
T1 (corr): T1 values after adjustment for PV.
Ratio T1 = T1myo,c / T1bl,c
Ratio T1* (corrected for plasma component) = T1myo,c / [T1bl,c × (1- Hct)]
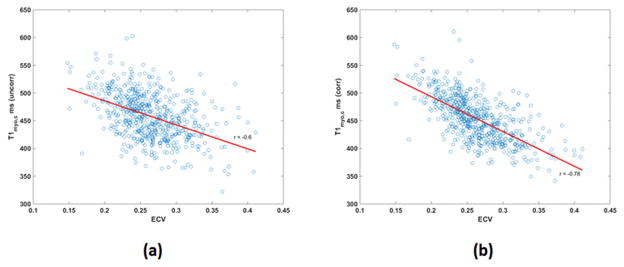
Overall, Pearson correlation between T1myo,c and ECV for the 605 subjects improved from r = −0.6 to r = −0.78 after dose adjustment and normalization (Figure 3).
Figure 3.
Figure 3 (a): Correlation between T1myo,c and ECV prior to correction (r = −0.6).
Figure 3 (b): Correlation between T1myo,c and ECV after correction (r = −0.78) as described in text.
Discussion
We have shown that the assumption of equivalent contrast dose based on mmol/kg between subjects leads to bias in post-contrast T1 values. Legacy T1 data are typically derived from available TI “scout” scans used for delayed enhancement [23] and not from dedicated T1 mapping sequences such as MOLLI as done here. Consequently, the inversion recovery Look-Locker sequence (IR-LL) using just 2 R-R intervals (as was done prior to the advent of newer cardiac T1 mapping techniques) for recovery is unsuitable for deriving longer native T1 values. Therefore, inferences are drawn based solely on post-contrast myocardial and blood T1 values in such studies.
ECV was used as the standard for comparison since ECV is relatively insensitive to the dose administered and to other physiological parameters. Although native (pre-contrast) T1 of myocardium showed better discrimination between smokers and non-smokers, it is more likely a result of dependencies on physiological parameter differences between the two groups [24]. For example, age, gender and BMI were different between current and former smokers (p < 0.2) while age, gender, BMI and heart rate were different between current smokers and non-smokers. In addition, ECV values showed anomalies in two cases where the mean value was higher in the lower risk group: former vs non-smoker and impaired vs subjects with normal glucose. This could be attributed the fact that there may be differences in medication, diet and exercise between the groups. In addition, myocardial fibrosis progresses unevenly based on disease load and treatment [25]. The cohort used here was asymptomatic. It’s important to note that similar anomalies were found with T1myo,c indicating agreement between the two measures.
The results obtained here were using the MOLLI sequence. It’s likely that they could be different with T1 values derived from other competing sequences such as ShMOLLI [26] or saturation recovery based T1 mapping [27,28] since each has its own accuracy and precision properties. The correction done here is based on differences in initial concentration of contrast in the blood pool and should be independent of the use of intra or extravascular contrast agnets. Contrast kinetics will vary based on the contrast agent and suitable correction can be applied for any time related variation [14].
Evaluation of ECV entails getting accurate T1 values in pre- and post-contrast blood in addition to myocardium. With each added measure, an increase in error is expected through error propagation theory. In addition, most techniques used for T1 mapping (IR-Look Locker, MOLLI etc) in the heart show reduced accuracy when insufficient dead time is available for regaining equilibrium magnetization. This is exacerbated at longer T1 values (such as in pre-contrast blood and myocardium) and higher heart rates and an ad-hoc correction is usually performed to correct for the bias. ECV calculation could also be sensitive to the different relaxivities of the gadolinium chelate in tissue and plasma [16,29]. Although, T1myo,c measurements are theoretically more accurate (being a single measure), the values show a dependency on dose and other kinetic parameters which require a correction prior to performing comparison studies. Nevertheless, T1myo,c values continue to be used for comparison studies, especially in legacy studies as previously discussed. Pre-contrast myocardial T1 values have also been used for discrimination. While there is no dependency on tissue kinetics, accuracy of measured longer T1 values is lower as discussed above. In addition, there can be dependencies on other physiological parameters as discussed in literature.
PV adjusted T1myo,c failed to show significance in one sub-group (current vs never smoked). Blood and plasma volume determined here were based on empirical measurements and further improvements in accuracy would result in improved correction for T1myo,c. However, it doesn’t necessarily follow that ECV and T1pre are more sensitive to fibrosis. Conclusive evidence in the form of hisotological correlates could answer that question definitively.
While normalizing T1myo,c with T1bl,c has been employed in literature as a surrogate for correction due to physiological and dose variations, our results show that such a correction falls short as indicated by the different results obtained when compared to ECV. After normalization with T1pl,c instead of T1bl,c, results better match those obtained using ECV.
Hypo- or hypervolemia as seen in patients with congestive heart failure, kidney failure and liver failure can show a variation of ±8% (mild) to severe (±24%) variation from normal blood volume. In addition, although the total blood volume decreases with age in healthy subjects of similar body size and chronic physical activity levels (equation (3)) [30], it has been shown that physical activity can negate some of the effects of aging on blood volume [31].
We have shown that the assumption of equivalent dose based on subject weight results in significant dose related variation between subjects of same weight but different BMI, sex and age. This is due to the different plasma volumes (and lean muscle mass) expected between such subjects. This relatively large variation in true dose results in a variation in the post-contrast T1 value. Consequently, when not accounted for, this can result in erroneous comparisons between subjects. We also demonstrated much improved agreement between results obtained using post-contrast myocardial T1 and ECV after applying the adjustment. A different method for calculating equal dose (or accounting for it through normalization as done here) based on the equations and nomograms presented here would rectify the situation.
Compliance with ethical standards: All human studies in this work were approved by the individual institutional review boards and were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects gave informed consent prior to their inclusion in the study.
Acknowledgments
This work was supported by the NIH Clinical Center intramural research program.
Footnotes
Conflict of interest: None.
References
- 1.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52 (19):1574–1580. doi: 10.1016/j.jacc.2008.06.049. S0735-1097(08)02798-8 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, Mudd JO, van der Geest RJ, Lima JA, Halushka MK, Bluemke DA. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265 (3):724–732. doi: 10.1148/radiol.12112721. radiol.12112721 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparrow P, Messroghli DR, Reid S, Ridgway JP, Bainbridge G, Sivananthan MU. Myocardial T1 mapping for detection of left ventricular myocardial fibrosis in chronic aortic regurgitation: pilot study. AJR Am J Roentgenol. 2006;187 (6):W630–635. doi: 10.2214/AJR.05.1264. 187/6/W630 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson. 2008;10:54. doi: 10.1186/1532-429X-10-54. 1532-429X-10-54 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schalla S, Bekkers SC, Dennert R, van Suylen RJ, Waltenberger J, Leiner T, Wildberger J, Crijns HJ, Heymans S. Replacement and reactive myocardial fibrosis in idiopathic dilated cardiomyopathy: comparison of magnetic resonance imaging with right ventricular biopsy. Eur J Heart Fail. 2010;12 (3):227–231. doi: 10.1093/eurjhf/hfq004. hfq004 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Ellims AH, Shaw JA, Stub D, Iles LM, Hare JL, Slavin GS, Kaye DM, Taylor AJ. Diffuse myocardial fibrosis evaluated by post-contrast t1 mapping correlates with left ventricular stiffness. J Am Coll Cardiol. 2014;63 (11):1112–1118. doi: 10.1016/j.jacc.2013.10.084. [DOI] [PubMed] [Google Scholar]
- 7.Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, Moon JC, Greiser A, Parker GJ, Schmitt M. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6 (3):373–383. doi: 10.1161/CIRCIMAGING.112.000192. CIRCIMAGING.112.000192 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Messroghli DR, Nordmeyer S, Dietrich T, Dirsch O, Kaschina E, Savvatis K, DOh-I, Klein C, Berger F, Kuehne T. Assessment of diffuse myocardial fibrosis in rats using small-animal Look-Locker inversion recovery T1 mapping. Circ Cardiovasc Imaging. 2011;4 (6):636–640. doi: 10.1161/CIRCIMAGING.111.966796. CIRCIMAGING.111.966796 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Neilan TG, Coelho-Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, Chen Y, Mandry D, Pierre-Mongeon F, Blankstein R, Kwong RY, Jerosch-Herold M. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013;6 (6):672–683. doi: 10.1016/j.jcmg.2012.09.020. S1936-878X(13)00270-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koopmann M, Hong K, Kholmovski EG, Huang EC, Hu N, Ying J, Levenson R, Vijayakumar S, Dosdall DJ, Ranjan R, Kim D. Post-contrast myocardial T and ECV disagree in a longitudinal canine study. NMR Biomed. 2014 doi: 10.1002/nbm.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gai ND, Stehning C, Nacif M, Bluemke DA. Modified Look-Locker T1 evaluation using Bloch simulations: human and phantom validation. Magn Reson Med. 2013;69 (2):329–336. doi: 10.1002/mrm.24251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kellman P, Herzka DA, Arai AE, Hansen MS. Influence of Off-resonance in myocardial T1-mapping using SSFP based MOLLI method. J Cardiovasc Magn Reson. 2013;15:63. doi: 10.1186/1532-429X-15-63. 1532-429X-15-63 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. doi: 10.1186/1532-429X-16-2. 1532-429X-16-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gai N, Turkbey EB, Nazarian S, van der Geest RJ, Liu CY, Lima JA, Bluemke DA. T1 mapping of the gadolinium-enhanced myocardium: adjustment for factors affecting interpatient comparison. Magn Reson Med. 2011;65 (5):1407–1415. doi: 10.1002/mrm.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Socolow J, Patel S, Pettigrew RI, Oshinski JN. Effect of Gd-DTPA-BMA on blood and myocardial T1 at 1.5T and 3T in humans. J Magn Reson Imaging. 2006;23 (3):323–330. doi: 10.1002/jmri.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donahue KM, Burstein D, Manning WJ, Gray ML. Studies of Gd-DTPA relaxivity and proton exchange rates in tissue. Magn Reson Med. 1994;32 (1):66–76. doi: 10.1002/mrm.1910320110. [DOI] [PubMed] [Google Scholar]
- 17.Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation. 1977;56 (4 Pt 1):605–612. doi: 10.1161/01.cir.56.4.605. [DOI] [PubMed] [Google Scholar]
- 18.Margouleff D. Blood volume determination, a nuclear medicine test in evolution. Clin Nucl Med. 2013;38 (7):534–537. doi: 10.1097/RLU.0b013e318292f370. [DOI] [PubMed] [Google Scholar]
- 19.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51 (2):224–232. [PubMed] [Google Scholar]
- 20.Manzone TA, Dam HQ, Soltis D, Sagar VV. Blood volume analysis: a new technique and new clinical interest reinvigorate a classic study. J Nucl Med Technol. 2007;35 (2):55–63. doi: 10.2967/jnmt.106.035972. quiz 77, 79. jnmt.106.035972 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Lemmens HJ, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes Surg. 2006;16 (6):773–776. doi: 10.1381/096089206777346673. [DOI] [PubMed] [Google Scholar]
- 22.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T-1 mapping of the heart. Magnet Reson Med. 2004;52 (1):141–146. doi: 10.1002/Mm.20110. [DOI] [PubMed] [Google Scholar]
- 23.Nacif MS, Turkbey EB, Gai N, Nazarian S, van der Geest RJ, Noureldin RA, Sibley CT, Ugander M, Liu S, Arai AE, Lima JA, Bluemke DA. Myocardial T1 mapping with MRI: comparison of look-locker and MOLLI sequences. J Magn Reson Imaging. 2011;34 (6):1367–1373. doi: 10.1002/jmri.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piechnik SK, Ferreira VM, Lewandowski AJ, Ntusi NA, Banerjee R, Holloway C, Hofman MB, Sado DM, Maestrini V, White SK, Lazdam M, Karamitsos T, Moon JC, Neubauer S, Leeson P, Robson MD. Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J Cardiovasc Magn Reson. 2013;15:13. doi: 10.1186/1532-429X-15-13. 1532-429X-15-13 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez B, Querejeta R, Varo N, Gonzalez A, Larman M, Martinez Ubago JL, Diez J. Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation. 2001;104 (3):286–291. doi: 10.1161/01.cir.104.3.286. [DOI] [PubMed] [Google Scholar]
- 26.Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song T, Stainsby JA, Ho VB, Hood MN, Slavin GS. Flexible cardiac T1 mapping using a modified Look-Locker acquisition with saturation recovery. Magn Reson Med. 2012;67 (3):622–627. doi: 10.1002/mrm.24137. [DOI] [PubMed] [Google Scholar]
- 28.Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T(1) mapping. Magn Reson Med. 2014;71 (6):2082–2095. doi: 10.1002/mrm.24878. [DOI] [PubMed] [Google Scholar]
- 29.Stanisz GJ, Henkelman RM. Gd-DTPA relaxivity depends on macromolecular content. Magn Reson Med. 2000;44 (5):665–667. doi: 10.1002/1522-2594(200011)44:5<665::AID-MRM1>3.0.CO;2-M. [pii] [DOI] [PubMed] [Google Scholar]
- 30.Davy KP, Seals DR. Total blood volume in healthy young and older men. J Appl Physiol (1985) 1994;76 (5):2059–2062. doi: 10.1152/jappl.1994.76.5.2059. [DOI] [PubMed] [Google Scholar]
- 31.Jones PP, Davy KP, DeSouza CA, van Pelt RE, Seals DR. Absence of age-related decline in total blood volume in physically active females. Am J Physiol. 1997;272 (6 Pt 2):H2534–2540. doi: 10.1152/ajpheart.1997.272.6.H2534. [DOI] [PubMed] [Google Scholar]