Abstract
Background
Vascular endothelial function declines with advancing age, due in part to increased oxidative stress and inflammation, and this age-related vascular dysfunction has been identified as an independent risk factor for cardiovascular diseases (CVD). This double-blind, placebo-controlled trial investigated the effects of a dietary supplement containing β-hydroxy-β-methylbutyrate (HMB), glutamine, and arginine on endothelial-dependent vasodilation of older adults.
Subjects/Methods
Thirty-one community-dwelling men and women aged 65-87 years were randomly assigned to two groups. The treatment group received two doses of the supplement daily (totaling 3g HMB, 14g glutamine, 14g arginine) for six months while the control group received an isocaloric placebo. At baseline and week 24, vascular endothelial function was measured by flow-mediated dilation of the brachial artery, and fasting blood samples were obtained to measure high-sensitivity C-reactive protein (hsCRP) and tumor necrosis factor-α (TNF-α).
Results
Paired samples t-tests revealed a 27% increase in flow-mediated dilation among the treatment group (p=0.003) while no change was observed in the placebo group (p=0.651). Repeated-measures ANOVA verified a significant time by group interaction (p=0.038). Although no significant changes were observed for hsCRP or TNF-α, a trend was observed for increasing hsCRP among the placebo group only (p=0.059).
Conclusions
These results suggest that dietary supplementation of HMB, glutamine, and arginine may favorably impact vascular endothelial function in older adults. Additional studies are needed to elucidate whether reduced inflammation or other mechanisms may underlie the benefits of supplementation.
Keywords: arginine, glutamine, beta-hydroxy-beta- methylbutyrate, endothelial function, aging
Background/Objectives
Aging is associated with impaired endothelial-dependent vasodilation1, and increased oxidative stress and inflammation are thought to be key contributors to this vascular dysfunction2, 3. Properties of certain dietary amino acids may ameliorate the age-related decline in vascular function. β-hydroxy-β-methylbutyrate (HMB) is a metabolite of the amino acid leucine that is best known for its inhibition of muscle protein breakdown 4. However, supplemental dosages of 3g daily of HMB have also been shown to attenuate circulating levels of pro-inflammatory cytokines such as C-reactive protein (CRP)5 and tumor necrosis factor alpha (TNF-α)6. Animal7 and in vitro studies8-10 further support the ability of HMB to reduce oxidative stress. Moreover, 3 g/day of supplemental HMB has been shown to reduce systolic blood pressure, providing limited evidence that HMB may also positively influence vascular function11. Glutamine is a precursor to glutathione, a principal endogenous antioxidant 12. Thus, supplemental glutamine may also decrease vascular damage by counteracting effects of reactive oxygen species. Arginine is another amino acid with antioxidant properties 13, 14. Arginine is also a precursor of nitric oxide, the primary vasodilatory molecule produced by endothelial cells13. In combination, these amino acids may act synergistically to attenuate endothelial dysfunction in older adults. The primary purpose of this study was to test the hypothesis that dietary supplementation of HMB, glutamine, and arginine would improve endothelial-dependent vasodilation in older adults. A secondary objective was to examine whether changes in vascular function would correspond to reductions in circulating levels of CRP and TNF-α.
Subjects/Methods
Participants were community-dwelling men and women, aged 65-87, with a body mass index of 22-39 kg/m2. Exclusion criteria included steroid use within the previous three months, tobacco use, cognitive impairment, or terminal illness.
Participants were randomized to supplement or placebo groups with stratification by sex. Supplement and placebo were provided in individual packets in powder form and were identical in packaging, appearance, dissolving characteristics, and weight. Packets were coded with product numbers so that participants and research staff were blinded. Participants ingested either two packets daily of the supplement (totaling 3g HMB, 14g glutamine, 14g arginine) or a calorically similar placebo (isomaltulose, citric acid, and flavoring) for six months. Dosages of the supplement and selection of placebo were based on previous studies with this mixture of amino acids15-19 as well as the manufacturer’s recommended dosage for this commercially-available supplement (Juven®; Abbott Nutrition, Columbus, OH). Participants were instructed to mix each packet into 240 ml of fluid and to take one dose with breakfast and another with dinner. Adherence was monitored using daily log forms, and minimal adherence was defined as 67% of recommended supplementation based on recommendations by the study’s data safety monitoring board. Over the 24-week study period, subjects came to the testing facility every four weeks to pick up supplement/placebo and obtain weight measurements. Participants were advised to keep physical activity patterns stable for the duration of the study. Dietary intake was monitored by 24-hour recalls at baseline and every four weeks afterwards. A single trained staff member administered each 24-hour recall using a multi-pass method that has been validated for use in older adults20. Data from 24-hour recalls were analyzed using Nutrition Data System for Research software (Nutrition Coordinating Center, Minneapolis, MN, 2013).
Vascular Endothelial Function
At baseline and week 24, vascular endothelial function was assessed by flow-mediated dilation (FMD) of the brachial artery according to published guidelines21. Subjects were instructed to fast from food and fluids other than water for at least eight hours prior to FMD measurements, and they were asked to hold their medications until after testing that morning. Participants also received a standardized form asking them to refrain from substances that could potentially affect the ultrasound such as caffeine, high-fat foods and vitamins for at least 12 hours before the study.
After the overnight fast, FMD was measured by high-resolution ultrasound with a 7.5 MHz linear-array probe (Philips HP Agilent Sonos 5500, Andover, MA). Scans of the brachial artery were taken at approximately five cm proximal from the elbow in longitudinal section on the right arm with the probe maintained in a fixed position at a fixed angle. Baseline artery diameter was recorded for one minute, blood flow was estimated by pulsed Doppler velocity, and brachial artery diameter was assessed every 5th cardiac cycle as determined by simultaneous ECG recording. Reactive hyperemia was induced by inflating a blood pressure cuff around the forearm to 50 mm Hg above resting systolic blood pressure. The cuff remained inflated for five minutes, and then it was rapidly deflated. Brachial arterial flow was determined using a pulsed-Doppler signal at baseline, 10-15 seconds after cuff release, and at the end of the study. The longitudinal image of the artery was recorded continuously from 30 seconds before to two minutes after cuff deflation. To assess hyperemic velocity, a mid-artery pulsed Doppler signal was obtained during the first 15 seconds after cuff deflation. Ultrasound images were recorded 30 seconds before and two minutes after cuff deflation. Measurements were taken from the anterior to the posterior interface between the media and adventitia. The five largest diameters after deflation were averaged, and FMD was expressed as the percentage increase in diameter from the baseline average to the peak average dilation. Arterial diameter was measured at end-diastolic phase (as confirmed by the incident R-wave on the synchronized ECG monitoring) from super-VHS recordings by a single physician blinded to the subject’s group assignment.
Biomarkers of Inflammation
A fasting blood sample was obtained at baseline and week 24 for measurement of serum high-sensitivity C-reactive protein (hsCRP) and tumor necrosis factor alpha (TNF-α). Blood was collected in 10ml vacutainer tubes. After holding at room temperature for 10 minutes, samples were centrifuged at 3000 rpm and 4 degrees Celsius for 10 minutes. Serum was aliquoted into cryovials and stored at −70° Celsius prior to measurement. Circulating hsCRP was assessed by a turbidometric assay run on Stanbio Sirrus Clinical Chemistry Analyzer using reagent from Pointe Scientific (Canton, MI). The volume size was 3 μl. Minimum sensitivity is 0.05 mg/l, inter-assay CV is 2.13%, and intra-assay CV is 7.49%. TNF-α concentrations were analyzed by chemiluminescence using a MSD imager (MSD, Gaithersburg, MD) and volume of 50 μl in duplicate. Minimum sensitivity is 0.507 pg/ml, inter-assay CV is 5.47%, and intra-assay CV is 7.61%. Serum cytokines for one participant from the treatment group and another from the placebo group were not obtained due to difficulty obtaining blood from the antecubital vein.
All testing and laboratory assays were conducted by the Core Laboratory of UAB’s Diabetes and Research Center, Nutrition Obesity Research Center, and Center for Clinical and Translational Science.
Statistical Analysis
Forty participants were recruited. Sample size for the primary outcome of FMD percent was based on guidelines for a parallel design from the Brachial Artery Reactivity Task Force22. Prior to analysis, hsCRP and TNF-α were log10-transformed due to non-normal distribution. Differences between the two groups at baseline and week 24 were compared by independent t-tests, chi-square tests, and the Mann-Whitney U test. Changes within each group from week 0 to week 24 were determined by paired t-tests and repeated-measures ANOVA with Bonferroni corrections. A separate variable of change in FMD% was calculated for each individual by subtracting FMD% at baseline from FMD% at week 24; independent t-tests were used to compare the mean ΔFMD between the two groups. All calculations were two-sided with a significance level of p<0.05 and were performed using SPSS software (version 20.0; Chicago, IL).
Results
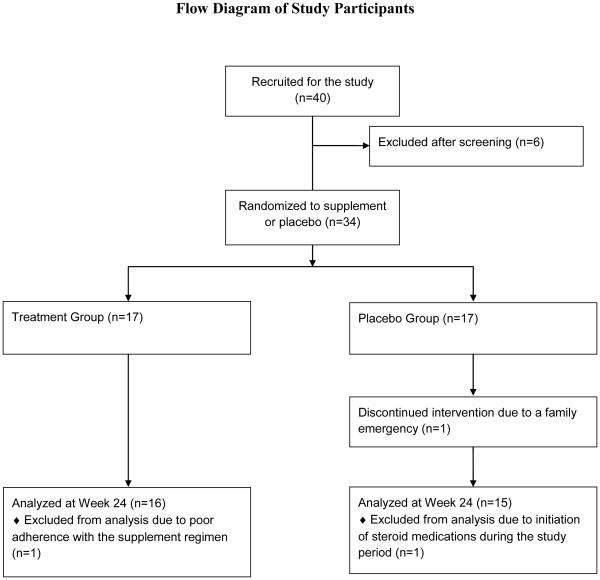
Of the initial cohort of 40 participants, six individuals were excluded after a screening visit. Another participant completed baseline testing but dropped out before the second visit. Data from one individual was excluded from statistical analysis due to self-reported poor adherence to the supplement regimen, and data from another person was excluded due to steroid therapy during the study period (Figure 1). Daily log forms revealed strong adherence to the supplement regimen by both treatment and placebo groups (98.3% and 97.9%, respectively).
Figure 1.
Flow diagram of study participants
The two groups were similar at baseline (Table 1). After 24 weeks, no significant changes in body mass index, weight, blood pressure, energy intake, or macronutrient intake were observed for either group (data not shown; dietary intake data at baseline and week 24 are available in Supplementary Information). Circulating levels of hsCRP and TNF-α didn’t differ between the two groups at baseline or week 24. Although paired t-tests revealed a trend for increased hsCRP over the study period in the placebo group only (p=0.059), time by treatment interactions were not significant (Table 2).
Table 1.
Baseline Characteristics (mean ± SD)
| Total Cohort (n=31) |
Treatment Group (n=16) |
Placebo group (n=15) |
p-value for difference between groups |
|
|---|---|---|---|---|
| Age (years) | 72 ± 6 | 72 ± 7 | 71 ± 5 | 0.451 |
| Sex (% male) | 45.2 | 43.8 | 46.7 | 0.870 |
| Race (% Caucasian) |
90.3 | 87.5 | 93.3 | 0.226 |
| Weight (kg) | 82.7 ± 15.6 | 85.4 ± 14.8 | 79.9 ± 16.3 | 0.328 |
| BMI (kg/m2) | 28.5 ± 4.3 | 29.4 ± 4.5 | 27.5 ± 4.0 | 0.230 |
| Systolic blood pressure (mm Hg) |
142 ± 22 | 147 ± 25 | 138 ± 16 | 0.257 |
| Diastolic blood pressure (mm Hg) |
81 ± 9 | 83 ± 10 | 79 ± 7 | 0.225 |
| FMD (%) | 8.6 ± 2.3 | 8.4 ± 1.8 | 8.9 ± 2.7 | 0.530 |
| Serum hsCRP (mg/l) | 2.3 ± 2.2* | 2.7 ± 2.6** | 1.9 ± 1.7*** | 0.389 |
| Serum TNF-α (pg/ml) | 7.8 ± 2.2* | 7.9 ± 2.2** | 7.7 ± 2.2*** | 0.101 |
| Average energy intake (kcals) |
1756 ± 396 | 1827 ± 408 | 1680 ± 381 | 0.308 |
| Average protein intake (% total kcals) |
17.9 ± 6.3 | 16.1 ± 4.6 | 19.8 ± 7.2 | 0.507 |
BMI = body mass index, kcals = kilocalories, FMD = flow mediated dilation
n=29,
n=15,
n=14
Table 2.
Serum Biomarkers of Inflammation
| Measure | Baseline | Month 6 | Change within group* |
Treatment effect** |
Time effect** |
Time × Treatment** |
|---|---|---|---|---|---|---|
| hsCRP (mg/l) Treatment Group Placebo Group |
2.7 ± 2.6 1.9 ± 1.7 |
3.5± 4.7 2.6± 2.4 |
p = 0.518 p = 0.059 |
p = 0.662 |
p = 0.045 |
p = 0.189 |
| TNF-α (pg/ml) Treatment Group Placebo Group |
7.9 ± 2.2 7.7 ± 2.2 |
7.8 ± 2.2 7.7 ± 2.1 |
p = 0.489 p = 0.966 |
p = 0.985 |
p = 0.661 |
p = 0.615 |
paired t-tests
RM ANOVA
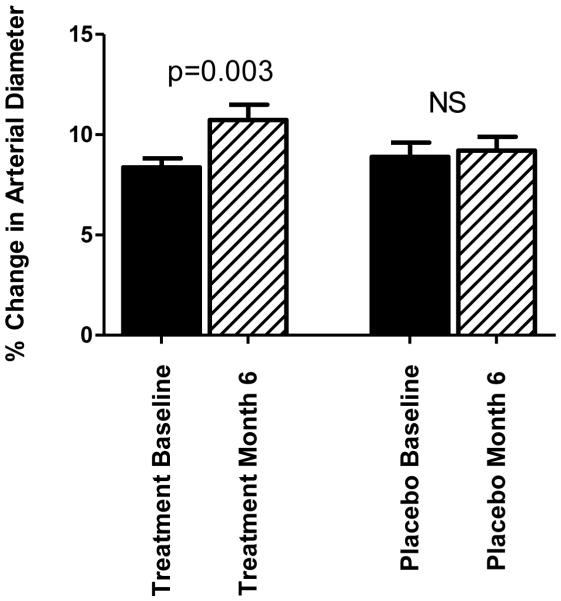
As shown in Figure 1, the treatment group exhibited a 27% increase in FMD percent from baseline to week 24 (p=0.003). However, no significant change in FMD was observed in the placebo group. Repeated measures ANOVA confirmed a significant time by group interaction (p=0.008 for Time; p=0.546 for Group; p=0.038 for Time x Group). Independent t-tests also confirmed a significant difference in ΔFMD between treatment and placebo groups (p=0.038).
Discussion
This study investigated whether dietary supplementation of HMB, glutamine, and arginine could improve endothelial function as measured by FMD. Participants receiving the supplement showed a significant increase in FMD, indicating improvement in endothelial-dependent vasodilation, while no change was observed among the placebo group. As vascular dysfunction is a key risk factor for CVD23, further investigation is warranted to determine whether this amino acid mixture may be cardioprotective.
Circulating cytokines such as hsCRP and TNF-α are known to increase with age24, and the age-related decline in vascular function is thought to be due in part to consequent chronic, low-grade inflammation2, 3. Animal and in vitro studies have demonstrated antioxidant properties of HMB7, 10, and one study with humans reported a positive effect of supplemental HMB on blood pressure11. As a precursor to glutathione, glutamine plays an important role in decreasing inflammation and oxidative damage in the body12. Glutamine stimulates heat shock proteins that reduce cellular inflammation25, and glutamine is a precursor to arginine26 which also suppresses reactive oxygen species and attenuates pro-inflammatory cytokines13, 14, 27. Although no previous studies have examined this combination of amino acids on vascular function, we hypothesized that the active ingredients of the supplement would act synergistically to improve endothelial function by reducing oxidative stress and inflammation. However, although we observed a trend for increasing hsCRP among the placebo group (p=0.059), no significant changes in hsCRP or TNF-α were observed for either group. Possibly, effects of the supplement on reducing oxidative stress and inflammation were subclinical, or the high variability in these biomarkers, particularly hsCRP, among our small sample could have precluded visible differences.
Alternatively, these results may indicate that the effects of the supplement on endothelial-dependent vasodilation relate more to other mechanisms such as the bioavailability of nitric oxide. Arginine is a precursor of nitric oxide, the main vasodilatory molecule produced by endothelial cells28. Researchers have reported favorable effects of supplemental arginine alone on vascular function in some28-30, but not all28, 31, studies. The supplement in this study provided a total of 14 grams of L-arginine per day. By comparison, typical daily dietary intake is approximately 5.4 grams28. Synthesis of nitric oxide from arginine is catalyzed by the enzyme endothelial nitric oxide synthase (eNOS)28. Previous studies have shown that eNOS in the endothelial cells of older adults is not reduced, but may instead be upregulated in an apparent compensatory attempt to generate more nitric oxide2, 32. Although investigation of this mechanism was beyond the scope of this study, it is feasible that the arginine in the supplement improved endothelial-dependent vasodilation by providing additional substrate for nitric oxide synthesis.
This study was limited by its small sample size, and participants were mostly Caucasian individuals of similar age and health status. While treatment and placebo groups were balanced by sex according to study design, the study lacked statistical power for further subgroup analysis by age. Additional studies are needed to confirm whether these results could be extrapolated to individuals of different ages and ethnicities. Participants were advised to maintain their usual dietary intake, and diet recalls showed no differences in total energy, protein, carbohydrate, or fat intake between or within groups. However, given the inherent limitations of retrospective dietary analysis methods and the potential influences of macronutrients such as dietary fats on vascular function, a controlled feeding study would be necessary to eliminate the possibility of confounding. Although biological data were not used to verify compliance, adherence with the supplement regimen was monitored by daily log forms and frequent contact with study staff. The study was also strengthened by the double-blind placebo-controlled design, a long intervention period, and a robust measure of vascular function under tightly-controlled conditions.
In conclusion, our results indicate that six months of dietary supplementation with HMB, glutamine, and arginine had a positive impact on vascular endothelial function in older adults. These results are clinically relevant because reduced endothelial-dependent vasodilation is a known risk factor for CVD2, 33, 34. Further investigation is warranted to elucidate mechanisms and confirm benefits of foods rich in these amino acids on cardiovascular outcomes.
Supplementary Material
Figure 2.
Percent change in flow mediated dilation (FMD) from baseline to week 24. (n=16 for the treatment group; n=15 for the placebo group)
Acknowledgments
This work was funded by the National Center for Complementary and Alternative Medicine (F31 AT005384-01). Core laboratory support and facilities were provided by the UAB Diabetes Research Center Human Physiology Core (P30DK079626-06). Abbott Nutrition provided supplement and placebo in coded packets.
Footnotes
ClinicalTrials.gov identifier: NCT01057082
Ethical Standard: All participants were informed about the purpose and risk associated with this study. Each person provided written consent, and the protocol was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham.
Conflict of interest: The authors declare no conflict of interest.
Supplementary information about average dietary intake of participants is available at the European Journal of Clinical Nutrition website.
References
- 1.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74(11):2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 2.Brandes R, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66(2):286–294. doi: 10.1016/j.cardiores.2004.12.027. doi: S0008-6363(05)00017-9 [pii] 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Yavuz B, Yavuz B, Sener D, Cankurtaran M, Halil M, Ulger Z, et al. Advanced age is associated with endothelial dysfunction in healthy elderly subjects. Gerontology. 2008;54(3):153–156. doi: 10.1159/000129064. doi: 000129064 [pii] 10.1159/000129064. [DOI] [PubMed] [Google Scholar]
- 4.Fitschen PJ, Wilson GJ, Wilson JM, Wilund KR. Efficacy of β-hydroxy-β-methylbutyrate supplementation in elderly and clinical populations. Nutrition. 2013;29(1):29–36. doi: 10.1016/j.nut.2012.05.005. doi: 10.1016/j.nut.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh LC, Chien SL, Huang MS, Tseng HF, Chang CK. Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr. 2006;15(4):544–550. [PubMed] [Google Scholar]
- 6.Townsend JR, Fragala MS, Jajtner AR, Gonzalez AM, Wells AJ, Mangine GT, et al. β-Hydroxy-β-methylbutyrate (HMB)-free acid attenuates circulating TNF-α and TNFR1 expression postresistance exercise. J Appl Physiol (1985) 2013;115(8):1173–1182. doi: 10.1152/japplphysiol.00738.2013. doi: 10.1152/japplphysiol.00738.2013. [DOI] [PubMed] [Google Scholar]
- 7.Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R701–715. doi: 10.1152/ajpregu.00840.2010. doi: 10.1152/ajpregu.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eley H, Russell S, Tisdale M. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295(6):E1409–1416. doi: 10.1152/ajpendo.90530.2008. doi: 90530.2008 [pii] 10.1152/ajpendo.90530.2008. [DOI] [PubMed] [Google Scholar]
- 9.Eley H, Russell S, Tisdale M. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-alpha and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295(6):E1417–1426. doi: 10.1152/ajpendo.90567.2008. doi: 90567.2008 [pii] 10.1152/ajpendo.90567.2008. [DOI] [PubMed] [Google Scholar]
- 10.Russell ST, Tisdale MJ. Mechanism of attenuation by beta-hydroxy-beta-methylbutyrate of muscle protein degradation induced by lipopolysaccharide. Mol Cell Biochem. 2009;330(1-2):171–179. doi: 10.1007/s11010-009-0130-5. doi: 10.1007/s11010-009-0130-5. [DOI] [PubMed] [Google Scholar]
- 11.Nissen S, Sharp R, Panton L, Vukovich M, Trappe S, Fuller JJ. beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr. 2000;130(8):1937–1945. doi: 10.1093/jn/130.8.1937. [DOI] [PubMed] [Google Scholar]
- 12.Melis G, ter Wengel N, Boelens P, van Leeuwen P. Glutamine: recent developments in research on the clinical significance of glutamine. Curr Opin Clin Nutr Metab Care. 2004;7(1):59–70. doi: 10.1097/00075197-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Appleton J. Arginine: Clinical potential of a semi-essential amino. Altern Med Rev. 2002;7(6):512–522. [PubMed] [Google Scholar]
- 14.Tong B, Barbul A. Cellular and physiological effects of arginine. Mini Rev Med Chem. 2004;4(8):823–832. doi: 10.2174/1389557043403305. [DOI] [PubMed] [Google Scholar]
- 15.Williams J, Abumrad N, Barbul A. Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg. 2002;236(3):369–374. doi: 10.1097/00000658-200209000-00013. discussion 374-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berk L, James J, Schwartz A, Hug E, Mahadevan A, Samuels M, et al. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122) Support Care Cancer. 2008;16(10):1179–1188. doi: 10.1007/s00520-008-0403-7. [DOI] [PubMed] [Google Scholar]
- 17.May P, Barber A, D'Olimpio J, Hourihane A, Abumrad N. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183(4):471–479. doi: 10.1016/s0002-9610(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 18.Marcora S, Lemmey A, Maddison P. Dietary treatment of rheumatoid cachexia with beta-hydroxy-beta-methylbutyrate, glutamine and arginine: a randomised controlled trial. Clin Nutr. 2005;24(3):442–454. doi: 10.1016/j.clnu.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Clark R, Feleke G, Din M, Yasmin T, Singh G, Khan F, et al. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. JPEN J Parenter Enteral Nutr. 24(3):133–139. doi: 10.1177/0148607100024003133. [DOI] [PubMed] [Google Scholar]
- 20.2008. A computer-based approach for assessing dietary supplement use in conjunction with dietary recalls.
- 21.Bianchini E, Faita F, Gemignani V, Giannoni M, Demi M. The Assessment of Flow-Mediated Dilation (FMD) of the Brachial Artery. Computers in Cardiology. 2006;33:509–512. e-pub ahead of print. [Google Scholar]
- 22.Corretti M, Anderson T, Benjamin E, Celermajer D, Charbonneau F, Creager M, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 23.Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You're only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 2014;29(4):250–264. doi: 10.1152/physiol.00059.2013. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329. doi: 10.1016/j.arr.2010.11.002. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth E. Nonnutritive effects of glutamine. J Nutr. 2008;138(10):2025S–2031S. doi: 10.1093/jn/138.10.2025S. [DOI] [PubMed] [Google Scholar]
- 26.Ligthart-Melis GC, van de Poll MC, Boelens PG, Dejong CH, Deutz NE, van Leeuwen PA. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am J Clin Nutr. 2008;87(5):1282–1289. doi: 10.1093/ajcn/87.5.1282. [DOI] [PubMed] [Google Scholar]
- 27.Potenza M, Nacci C, Mitolo-Chieppa D. Immunoregulatory effects of L-arginine and therapeutical implications. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1(1):67–77. doi: 10.2174/1568008013341811. [DOI] [PubMed] [Google Scholar]
- 28.Preli RB, Klein KP, Herrington DM. Vascular effects of dietary L-arginine supplementation. Atherosclerosis. 2002;162(1):1–15. doi: 10.1016/s0021-9150(01)00717-1. [DOI] [PubMed] [Google Scholar]
- 29.Bode-Böger S, Muke J, Surdacki A, Brabant G, Böger R, Frölich J. Oral L-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8(2):77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 30.Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, et al. L-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism. 2013;62(2):255–264. doi: 10.1016/j.metabol.2012.08.004. doi: 10.1016/j.metabol.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Jahangir E, Vita J, Handy D, Holbrook M, Palmisano J, Beal R, et al. The effect of L-arginine and creatine on vascular function and homocysteine metabolism. Vasc Med. 2009;14(3):239–248. doi: 10.1177/1358863X08100834. doi: 14/3/239 [pii] 10.1177/1358863X08100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, et al. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297(1):H425–432. doi: 10.1152/ajpheart.00689.2008. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5(3):243–255. doi: 10.1007/s12265-012-9359-6. doi: 10.1007/s12265-012-9359-6. [DOI] [PubMed] [Google Scholar]
- 34.Barodka VM, Joshi BL, Berkowitz DE, Hogue CW, Nyhan D. Review article: implications of vascular aging. Anesth Analg. 2011;112(5):1048–1060. doi: 10.1213/ANE.0b013e3182147e3c. doi: 10.1213/ANE.0b013e3182147e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.