Abstract
Emotional abnormalities are prominent across the schizophrenia spectrum. To better define these abnormalities, we examined state emotional functions across opposing ends of the spectrum, notably in chronic outpatients with schizophrenia (Study 1) and college students with psychometrically defined schizotypy (Study 2). In line with existing studies, we predicted that individuals with schizophrenia would show unusually co-activated positive and negative emotions while college students with schizotypy would show abnormally low positive and abnormally high negative emotions. Drawing from the affective science literature, we employed continuous emotion ratings in response to a dynamic and evocatively “bittersweet” stimulus. Participants included 27 individuals with schizophrenia, 39 individuals with psychometrically defined schizotypy and 26 community and 35 college control participants. Participants continuously rated their state happiness and sadness throughout a six-minute clip from a tragicomic film (i.e., Life is Beautiful). In contrast to expectations as well as the extant literature, there were no state emotional abnormalities noted from either schizophrenia-spectrum group. Of particular note, neither individuals with schizophrenia nor individuals with schizotypy were abnormal in their experience of state negative, positive or coactivated emotions. Conversely, abnormalities in trait emotion were observed in both groups relative to their respective control groups. These results help confirm that the schizophrenia-spectrum is not characterized by deficits in state emotional experience and suggest that sadness is not abnormally co-activated with pleasant emotions. These results are critical for clarifying the “chronometry” of emotional dysfunctions across the schizophrenia-spectrum.
Keywords: schizophrenia, schizotypy, anhedonia, emotion, affect, negative, affective chronometry
1. INTRODUCTION
Emotional dysfunctions manifest across the schizophrenia-spectrum. At one extreme of the spectrum, individuals with chronic schizophrenia show high self-reported trait negative affectivity (tNA – disposition involving proclivity to experience unpleasant emotions), low self-reported trait positive affectivity (tPA – disposition involving proclivity to experience pleasant emotions), clinically-rated anhedonia (i.e., diminished pleasant emotional experience), and high levels of state negative emotion during laboratory and mobile assessment studies (Cohen and Minor, 2010; Horan et al., 2008; Kring and Moran, 2008, Myin-Germeys et al., 2009). These emotional abnormalities are by no means benign, as they are associated with poor functioning and illness course (Blanchard et al., 1998; Horan et al., 2008); reflect potentially heritable biomarkers of illness risk (Keshavan et al., 2005, Lenzenweger 2006; Tarbox and Pogue-Guile, 2011) and represent potentially important treatment targets (Gard et al., 2007). In support of the notion that emotional dysfunctions reflect a vulnerability marker, college students with schizotypy – defined in terms of high levels of magical ideation, ideas of reference, social anhedonia and other subclinical schizophrenia-like phenomena, also show a host of emotional abnormalities – many of which are similar in both quality and magnitude to individuals with chronic schizophrenia. For example, individuals with schizotypy report levels of tNA and tPA that are similar to individuals with schizophrenia (Cohen et al., 2012a; Horan et al., 2008) and show anhedonia via both self-report and clinician-rated measures (Blanchard et al., 2011). When directly compared with each other, college students with self-reported schizotypal traits, but not individuals with schizophrenia, report experiencing low levels of state positive emotion in reaction to evocative laboratory stimuli (Cohen et al., 2012a). These findings are counterintuitive in that college individuals with schizotypy should be healthier than outpatients with chronic schizophrenia in virtually every conceivable way (e.g., Cohen et al., 2014; Lenzenweger, 2006). The present study explored dysfunctions in emotional experience in schizophrenia (Study 1) and schizotypy (Study 2) using highly sensitive laboratory methods.
Despite a fairly large literature exploring emotional experience across the schizophrenia-spectrum, there are two major knowledge gaps that have yet to be addressed. First concerns the emotional valence of the dysfunction(s). Most prior studies have focused on positive and negative valences; conceptualized as either distinct constructs, or opposing ends of a linear dimension (Russell, 1980). The “evaluative space model” of emotion posits that positive and negative emotions are separable, not antagonistic, and in some case, are co-activated (Cacioppo et al., 1997). Consider “bittersweet” emotions, for example, elicited when one moves from friends and family to improve occupational circumstances, during which both happiness and sadness are experienced simultaneously (Larsen and McGraw, 2014; see also "emotional complexity" as discussed by Lindquist and Barrett, 2008). Emotional ambivalence – a term coined by Eugene Bleuler to highlight the existence of opposing emotional experience to a common object is relevant here (Bleuler, 1950). Importantly, ambivalence does not necessarily refer to the co-activation of opposing emotions, and could alternatively reflect the rapid oscillation between opposing emotions; for example as “affective oscillating” emotions as described by Norris et al., (2010). Definitional and conceptual ambiguities aside, there is evidence that individuals across the schizophrenia-spectrum experience positive and negative emotions that are temporally proximate in an abnormal way. Individuals with schizophrenia have reported abnormally co-activated positive and negative emotions when processing unambiguously neutral and positively valenced laboratory stimuli across a number of studies (see Cohen and Minor, 2010 for a meta-analysis; Tremeau et al., 2009b). In schizotypy, abnormal co-activation of self-reported “trait-like” positive and negative emotions (vis a vis ambivalence) are seen on standardized questionnaires (MacAulay et al., 2014), and, per a recent experience sampling study, are associated with abnormal social motivation and emotion regulation (Burgin et al., 2014). On the other hand, laboratory evidence of co-activated positive and negative systems in schizotypy is limited (e.g., Cohen et al., 2011). Importantly, no study to our knowledge has directly explored co-activated emotions in the schizophrenia-spectrum using stimuli meant to simultaneously activate positive and negative valence systems – an important endeavor for understanding how these emotional abnormalities manifest. This was the primary goal of the present study.
A second major knowledge gap involves how temporal features of emotion (e.g., “affective chronometry”; Davidson, 1998) may be abnormal across the schizophrenia-spectrum. Laboratory studies of emotional experience typically involve processing static stimuli for relatively brief periods of time and quantifying experience at one time point, often immediately following stimulus offset. This is a limitation because emotional experience is dynamic over time. Moreover, quantifying emotional experience, particularly in the absence of the evocative stimuli as is often done in laboratory studies, requires the involvement of episodic and semantic processing systems that are distinct from those involved in “on-line” current reports (Strauss and Gold, 2012). Illustrative of this point, studies have found that individuals with schizophrenia are not abnormal in state (e.g., “on-line” or “consummatory”) experience of pleasure but have found low positive experience when anticipating emotional experience (Kring et al., 2011) or when recalling past emotional experiences (Herbener et al., 2007). To our knowledge, no published studies have explored emotional chronometry using dynamic laboratory stimuli. The second primary goal of this study was to evaluate emotional experience using continuous assessment of emotional experience to dynamic stimuli over an extended period of time. As an adjunct to the primary analyses of this study, we examined how emotional experience varies as a function of positive, negative and disorganization symptoms and traits in schizophrenia and schizotypy; important in light of evidence that the schizophrenias-spectrum is at least phenotypically heterogeneous (and likely mechanistically heterogeneous) (e.g., Kwapil et al., 2012).
2. STUDY 1 METHODS
2.1 Patient participants
The patient group included 27 adults with Diagnostic & Statistical Manual of Mental Disorders 4th edition (DSM-IV; American Psychiatric Association [APA], 1994) diagnosed schizophrenia, recruited from an outpatient clinic. Individuals were clinically stable at the time of testing and were receiving pharmacotherapy under the supervision of a multi-disciplinary team. A nonpatient control group of 26 individuals from the community was also recruited. Exclusion criteria included: a) Global Assessment of Functioning rating (GAF; APA, 1994) below 30, b) documented evidence of intellectual disability from medical records, c) current or historical DSM-IV diagnosis of alcohol or drug abuse suggestive of severe physiological symptoms (e.g., delirium tremens, repeated loss of consciousness) assessed from clinical interview, and d) history of significant head trauma (requiring overnight hospitalization) from clinical interview or medical records. Exclusion criteria for the controls also included the presence of a DSM-IV diagnosis of schizophrenia, depression, mania or endorsement of clinically significant psychosis symptoms (present or by history), assessed from clinical interview.
2.2 Diagnostic and symptom ratings
Diagnoses were made based on information obtained from medical records and from The Structured Clinical Interview for DSM-IV (SCID-IV; First et al., 1996). The Scales for the Assessment of Positive and Negative Symptoms (SAPS & SANS respectively; Andreasen, 1984a, 1984b) were used to measure positive and negative symptoms in individuals with schizophrenia. Summary scores reflecting positive (i.e., global delusion and hallucination scores), disorganization (i.e., global thought disorder and bizarre behavior scores), and negative, composed of alogia-blunted affect (i.e., global alogia and blunted affect scores) and anhedonia-avolition (i.e., global anhedonia and avolition scores) were used. Preliminary diagnoses and symptom ratings were made by one of four doctoral level students who were trained to criterion (intra-class correlation coefficient values > .70). Diagnoses and ratings were videotaped and reviewed during a monthly case conference meeting that was led by a licensed clinical psychologist with considerable diagnostic experience (Alex S. Cohen).
2.3 Self-reported symptoms and trait affect
Trait affectivity was measured using the Positive And Negative Affect Schedule (PANAS; Watson and Clark, 1999) with instructions to indicate how they “generally feel”. A scale from 1 to 5 was employed. tPA and tNa scores showed acceptable reliability across each group (α’s > .75).
2.4 Continuous emotion assessment task
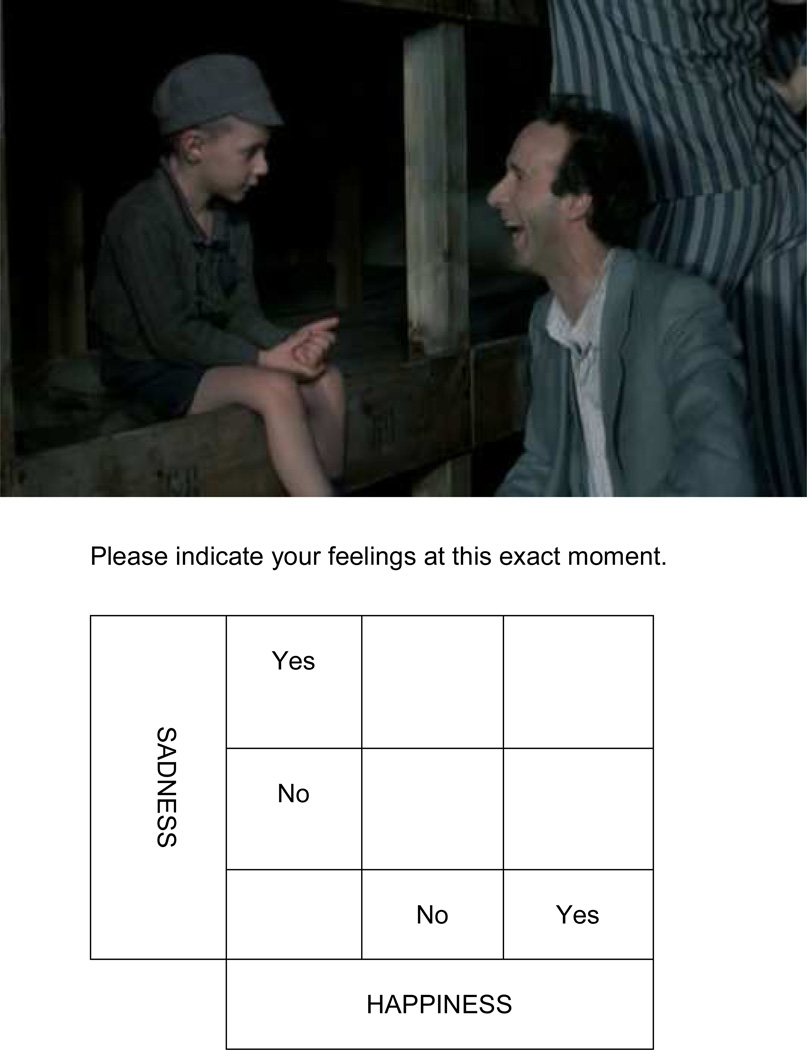
Participants were seated in front of a computer monitor and completed a brief tutorial on using an “evaluative space grid” (Larsen et al., 2009) to continuously assess their emotional experience (See Figure 1). Briefly, participants were asked to indicate their emotional experience “at that moment” on a grid where the x-axis reflects happiness and the y-axis reflects sadness. Both the evaluative space grid and the current experience were displayed to the participant throughout the task. The cursor initially appeared in the cell representing neutrality (i.e., no happiness or sadness), after which participants used the mouse to move the cursor throughout the space. The cell location of the mouse cursor was recorded every 500 ms. Standardized instructions, adapted from those of Larsen et al. (2009) were used. A film clip approximately six minutes long from the end of the movie Life is Beautiful, involving a father entertaining his son while awaiting execution in a Nazi concentration camp, was played. This clip has been used to elicit “ambivalent” and mixed emotions (Larsen and Green, 2013; Larsen et al., 2001; Larsen and McGraw, 2011).
Figure 1.
An example stimulus and response system for the continuous emotion task used in Study 1.
Pilot testing of the 5 × 5 evaluative space grid revealed that individuals with schizophrenia struggled with the abstract nature of positive and negative emotions being orthogonal, so the community sample used a simplified 2 × 2 grid featuring dichotomous (i.e., “yes” or “no”) measures of happiness and sadness (rather than the expanded 5 × 5 grid used in (Larsen and McGraw, 2011; Larsen et al., 2001). Percent time experiencing emotion (i.e., defined separately as reporting no emotion, only positive emotion, only negative emotion, or “mixed” emotions) was computed for each participant. Additionally, data were computed on the percent time experiencing each valence, number of times participants changed valences (i.e., mixed, null, positive, negative) and the average amount of time spent for each emotional “episode”.
2.5 Analyses
The analyses were conducted in four steps. First, we computed and compared descriptive and clinical variables between groups. Second, we conducted a “manipulation check” to determine whether our laboratory procedure evoked positive, negative and co-activated states as intended. We plotted emotional response as a function of time course for visual inspection. Third, we compared the groups in trait and state emotion summary variables. Finally, we evaluated whether clinical variables, notably in terms of positive, negative or disorganized schizophrenia symptoms, were associated with emotional response. Generally, speaking, we hypothesized that the schizophrenia group would show increased negative emotion and similar positive emotion as controls on the emotion-induction task. Unless otherwise noted, all variables were relatively normal in distribution (i.e., skew < 1.5). Given the exploratory nature of this project, correction for multiple analyses was not conducted.
3. RESULTS
3.1 Demographic and descriptive variables (Table 1)
Table 1.
Descriptive and clinical data for participant groups in Study 1 and 2.
| STUDY 1 | STUDY 2 | |||
|---|---|---|---|---|
| Community Controls |
SZ | College Controls |
Stypy | |
| % Women | 57% | 16% | 61% | 62% |
| % Caucasian | 43% | 63% | 81% | 77% |
| % African American | 57% | 37% | 11% | 5% |
| % SZ Family History | - | - | 0% | 19% |
| Age | 39.07 (13.32) | 40.00 (12.25) | 18.78 (1.97) | 18.67 (1.22) |
| Education | 14.46 (2.25) | 11.07 (1.67) | 12.46 (.80) | 12.49 (.76) |
| SYMPTOM RATINGS | ||||
| Hallucinations | 1.67 (1.59) | |||
| Delusions | 1.78 (1.65) | |||
| Bizarre Behavior | 0.63 (0.88) | |||
| Thought Disorder | 2.52 (1.58) | |||
| Affective Flattening | 1.33 (1.54) | |||
| Alogia | 2.30 (1.56) | |||
| Avolition | 1.48 (1.28) | |||
| Anhedonia | 0.30 (0.78) | |||
| SCHIZOTYPY TRAITS | ||||
| Positive | 8.25 (4.94) | 30.95 (9.12) | ||
| Negative | 2.92 (2.18) | 12.08 (5.89) | ||
| Disorganization | 8.06 (4.83) | 25.54 (4.77) | ||
| Trait Positive Affectivity | 3.78 (0.48)a | 3.75 (0.84) a | 6.92 (1.41) b | 5.95 (1.39) b |
| Trait Negative Affectivity | 1.61 (0.44) a | 2.18 (0.87) a | 2.94 (1.13) b | 3.96 (1.59) b |
Scale from 1 to 5;
Scale from 1 to 10.
Note – SZ = Schizophrenia, Stypy = Schizotypy
The schizophrenia and control group did not differ in ethnicity or age (p’s > .10), but the schizophrenia group had significantly fewer women than the community control group (χ2 [1, 53] = 8.15, p = .004). To compensate for potential demographic issues, we controlled for potential confounding effects of ethnicity, age or sex in subsequent analyses – though the results did not change whether demographic variables were controlled for or not.
3.2 Manipulation Check
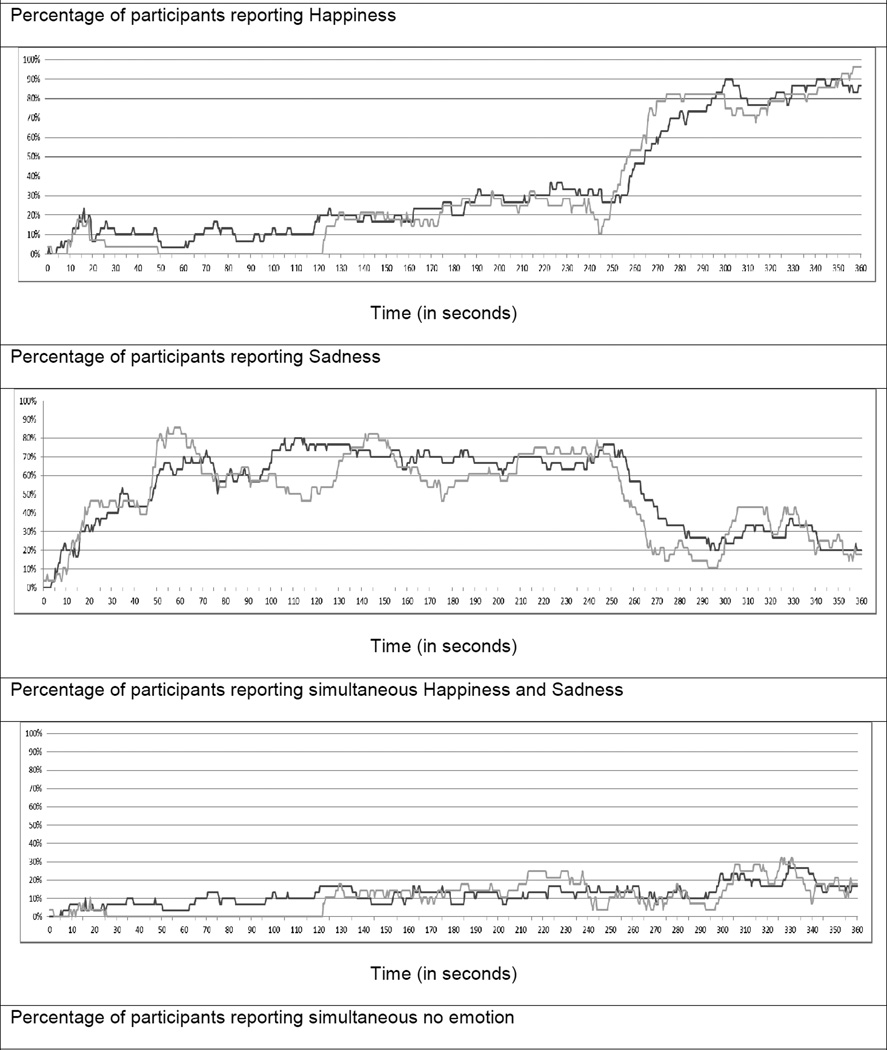
Only one participant (from the schizophrenia group) indicated experiencing no emotion for the duration of the task. Excluding this individual did not affect the pattern of results in this study. On average, participants reported experiencing negative emotion during much of the emotion-induction procedure (Mean ± standard deviation = 41% ± 23), positive emotion (21% ± 14) and “no emotion” (28% ± 25) for similar amounts of time, and co-activated emotion for approximately 10% of the procedure (10% ± 13). The time course figures for emotional experience are plotted in Figure 2.
Figure 2.
Affective chronometry of Schizophrenia (Dark Grey) and Control (Light Grey) groups.
Trait affect (Table 1)
Group differences for tNA (t[52] = 3.34, p = .002, d = .87) but not tPA (t[52] = 1.33, p = .19, d = .05) affect were observed.
3.3 Emotional experience (Table 2)
Table 2.
Descriptive statistics for continuous emotional experience task.
| STUDY 1 | STUDY 2 | |||
|---|---|---|---|---|
| Community Controls | Schizophrenia | College Controls | College Schizotypy | |
| Percent Time Episodes | ||||
| Null Emotion | 0.27 (0.22) | 0.27 (0.26) | 0.33 (0.24) | 0.29 (0.24) |
| Positive Emotion | 0.21 (0.14) | 0.22 (0.16) | 0.29 (0.23) | 0.31 (0.27) |
| Negative Emotion | 0.41 (0.24) | 0.41 (0.21) | 0.20 (0.21) | 0.21 (0.20) |
| Mixed Emotion | 0.10 (0.10) | 0.10 (0.17) | 0.18 (0.23) | 0.19 (0.19) |
| N Emotion Episodes | ||||
| Null Emotion | 3.44 (2.14) | 3.56 (2.42) | 2.15 (1.40) | 1.95 (1.26) |
| Positive Emotion | 2.72 (2.41) | 2.30 (1.90) | 2.15 (1.46) | 2.33 (2.32) |
| Negative Emotion | 4.04 (4.27) | 3.19 (2.53) | 1.21 (1.30) | 1.49 (1.23) |
| Mixed Emotion | 3.20 (4.46) | 2.04 (2.08) | 1.53 (1.74) | 2.13 (2.71) |
| Mean Length Emotion Episodes (In seconds) | ||||
| Null Emotion | 41.88 (67.84) | 44.67 (70.53) | 64.24 (47.50) | 55.83 (41.08) |
| Positive Emotion | 36.02 (31.12) | 45.47 (45.20) | 54.28 (46.45) | 52.20 (50.78) |
| Negative Emotion | 82.69 (100.89) | 79.99 (80.95) | 45.53 (45.01) | 45.55 (47.55) |
| Mixed Emotion | 14.66 (17.96) | 16.91 (23.51) | 28.17 (32.64) | 34.13 (41.33) |
| Mean Ratings | ||||
| Null Emotion | - | - | 2.08 (0.92) | 2.16 (0.92) |
| Positive Emotion | - | - | 1.72 (0.59) | 1.74 (0.54) |
| Negative Emotion | - | - | 0.87 (0.46) | 0.93 (0.41) |
| Mixed Emotion | - | - | 0.79 (0.49) | 0.79 (0.47) |
In contrast to expectations, there were no statistically significant group differences in percentage of time spent reporting null, positive, negative or mixed emotions using t-tests (p’s > .10). Consequent power analysis using the observed effect sizes (d’s < .35) suggested that 130 patients and 130 controls would be needed for any statistical significance to be observed (α = .05, β = .80).
3.4 Symptom Correlates
Increasing clinically-rated anhedonia was associated with more time reporting no emotion (r[25] = .59, p = .001) and less time reporting negative emotion (r[25] = −.55, p = .003); but was not significantly associated with positive or mixed (p’s < .72) emotions. Longer positive emotion episodes were associated with greater positive symptoms, including delusions (r[25] = .41, p = .02), bizarre behavior (r[25] = .41, p = .04), thought disorder (r[25] = .41, p = .04), and were also associated with alogia (r[25] = .43, p = .03).
4. STUDY 2 METHODS
4.1 College participants
Schizotypy and nonpatient control groups
Participants from the college groups were undergraduate freshman and sophomores (N = 10,258) approached by email to participate in an on-line survey and offered a chance to win monetary prizes. Embedded within this survey were a consent form, basic demographic questions, a modified version of the Schizotypal Personality Questionnaire – Brief, which featured an expanded response format (Cohen et al., 2010), the Brief Symptom Inventory (Derogatis and Melisaratos, 1983) and infrequency items (Chapman and Chapman, 1983). The final screening sample included 2,303 complete, valid responses (defined as an infrequency score < 3). Based on theory that schizotypy is a categorical construct with a population incidence approaching ten percent (Lenzenweger, 2006), we adopted a conservative strategy where the top five percent of scorers (computed using ethnicity- and gender-determined means) on the positive/disorganization and/or negative subscales were invited to participate in the laboratory phase of the study. To address concerns that depressive symptoms can give “false positives” on negative schizotypy scales, individuals scoring high on the negative scale were only considered eligible if they a) also showed elevation on the positive or disorganization scales, or b) had a depression scale score from the Brief Symptom Inventory (Derogatis and Melisaratos, 1983) below their gender and ethnicity determined mean (final n = 39). Control subjects (n = 35) were identified based on scores below the ethnicity and gender-determined means for each of the positive, negative, and disorganization SPQ factors. Trait affectivity was measured using the Positive And Negative Affect Schedule (PANAS; Watson and Clark, 1999) using a scale from 1 to 10 (α’s > .75).
4.2 Continuous emotion assessment task
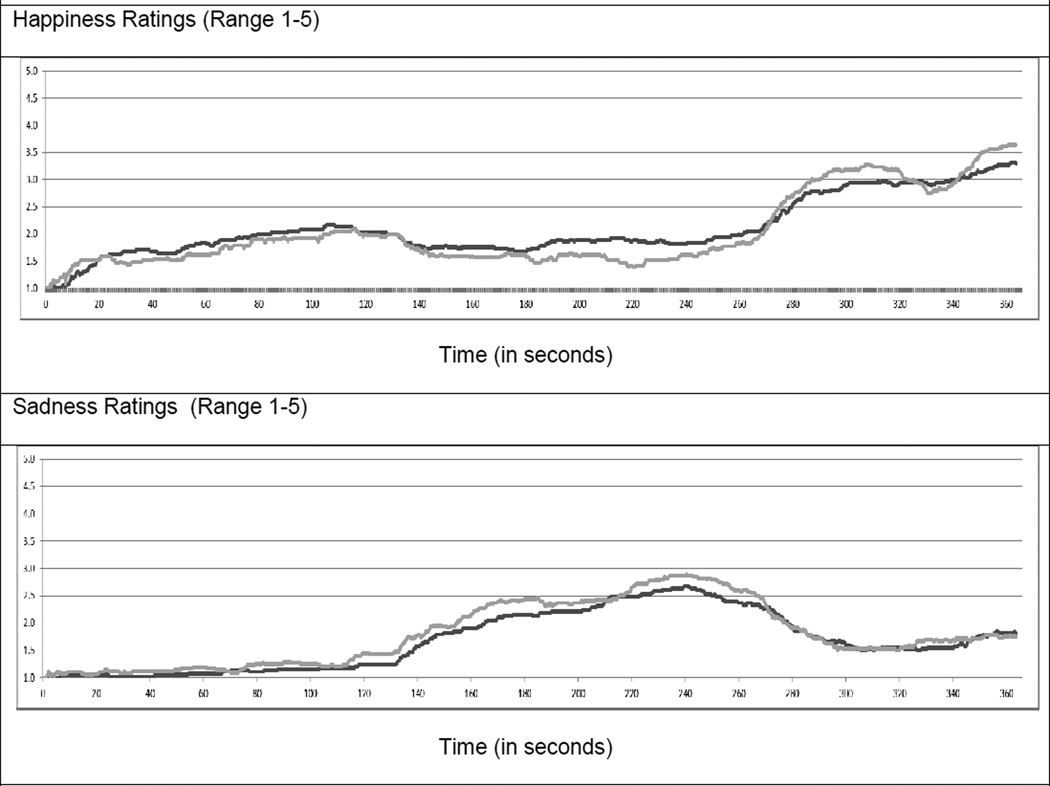
A similar measure was used as in Study 1 with two key differences. First, the cell location of the mouse cursor was recorded every 100 milliseconds (ms) for college students instead of 500 ms for community samples (difference due to using computers with greater processing capability). Second, following Larsen et al. (2009), the college sample provided separate ratings of their happiness and sadness with a 5 × 5 version of the evaluative space grid. Hence, we were able to report mean ratings for the college groups.
4.3 Analyses
The analyses were conducted in identical fashion as Study 1, though schizotypal traits, as opposed to schizophrenia symptoms, were examined in the fourth step (correlational analyses). We hypothesized that the schizotypy group would show increased negative and decreased positive emotion compared to controls on the emotion-induction task. Unless otherwise noted, all variables were relatively normal in distribution (i.e., skew < 1.5).
5. RESULTS
5.1 Demographic and descriptive variables (Table 1)
The college groups were equivalent in descriptive and demographic variables.
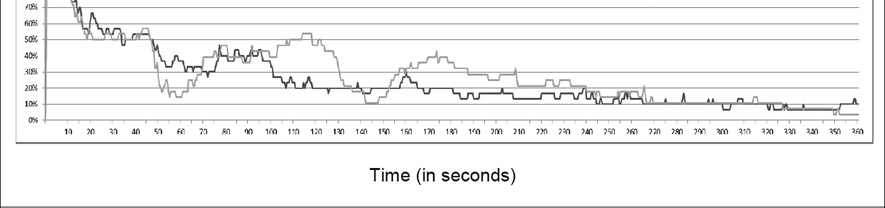
5.2 Manipulation Check (Figure 3)
Figure 3.
Affective chronometry of schizotypy (Dark Grey) and control (Light Grey) groups.
The college participants reported experiencing similar periods of time experiencing positive (30% ± 25; defined as positive rating greater than 1 and negative rating = 1) and “no emotion” (31% ± 24; defined as both positive and negative ratings = 1), and similar time with co-activated (19% ± 21; defined as both positive and negative ratings > 1) and negative (21% ± 20; defined as negative ratings > 1 and positive ratings = 1) emotion states. The average positive (2.12 ± .92) ratings for the task were slightly higher than the average negative ratings (1.73 ± .56).
5.3 Trait affect (Table 1)
Individuals with schizotypy showed significantly lower tPA (t[72] = 3.09, p = .003, d = .69) and higher tNA (t[72] = 3.16, p = .002, d = .75) relative to controls.
5.4 Emotional experience (Table 2)
As in study 1, t-tests indicated that there were no statistically significant group differences in any of the emotion variables. Consequent power analysis using the observed effect sizes (d’s < .27) suggested that 217 individuals with schizotypy and 217 controls would be needed for any statistical significance to be observed (α = .05, β = .80).
5.5 Symptom Correlates
Within the schizotypy group, negative schizotypy traits were associated with fewer positive experiences at a trend level (r[37] = −.31, p = .06), but there were no significant correlations between schizotypy traits and emotion variables (p’s > .10).
6. GENERAL DISCUSSION
These studies evaluated the experience of positive and negative emotion and their coactivation using continuous temporal ratings from dynamic (and evocatively positive and negative) stimuli. We examined this phenomenon in relatively extreme “ends” of the schizophrenia-spectrum; “middle aged” outpatients with chronic schizophrenia and college students with psychometrically defined schizotypy. There were three critical findings. First, replicating prior research, college students with schizotypy reported trait affect (Cohen et al., 2012a; Horan et al., 2008) that was abnormal, and in the case of positive affect, more pathological than outpatients with chronic schizophrenia (relative to their respective control groups). Interestingly, the present study found that individuals with schizophrenia were no different than controls in tPA. Second, both the schizophrenia and schizotypy groups were similar to their respective control groups in emotional states. Third, there was variability in both the schizophrenia and schizotypy groups in emotional experience as a function of symptoms and traits. These points are explicated below.
With respect to state emotions, individuals with both schizotypy and schizophrenia were unremarkable. This was unexpected, as several dozen published studies have demonstrated that schizophrenia is associated with a relatively robust abnormal state negative emotional response to neutral and pleasant stimuli (see Cohen and Minor, 2010 for a meta-analysis) and that schizotypy is associated with abnormal state positive emotion (e.g., Cohen, et al., 2012a) using laboratory methods. It is possible that, in fact, these abnormalities demonstrated in prior studies are an artifact of methodologies in that assessment of “on-line’ state emotion was contaminated by other abilities (Strauss and Gold, 2012). Appraisal of one’s emotional experience following offset of a static stimulus – particularly one that involves static stimuli that may have limited engagement or appeal, may require working memory, episodic memory and other abilities that may be abnormal across the schizophrenia-spectrum. Likewise, static stimuli may somehow promote disengagement that contributes to reduced resources being available or allocated to down-regulate negative affect. Hence, engagement of emotion and/or cognitive regulation abilities may be implicated in state emotion abnormalities in the schizophrenia-spectrum. Whereas the present study involved fictional “others” (i.e., film characters) experiencing hypothetical events, individuals with schizophrenia may experience more abnormal mixed emotions in response to bittersweet events that affect the self (e.g., winning one amount of money but missing out on an opportunity to win an even larger amount; Larsen et al., 2004). An alternate explanation is that the stimuli used in the present study did not evoke the appropriate categories of emotion to show abnormal activation and coactivation. In particularly, sadness, which is but one of many negatively valenced emotional states, may not be abnormal in the schizophrenia-spectrum. Some evidence suggests that schizophrenia-related emotional abnormalities may be most pronounced for fear (Tremeau et al., 2009a).
With respect to trait emotions, the present findings are consistent with those elsewhere finding that individuals with schizotypy show similar or more severe emotional abnormalities than patients with chronic schizophrenia (Cohen et al., 2012a). Importantly, pathological autobiographical self-report in schizotypy is by no means isolated to emotional experience, as college schizotypy samples have reported, for example, cognitive complaints on the order of nearly two SDs (Chun et al., 2013) and over one SD in emotional expression abnormalities (Leung et al., 2010) and subjective quality of life (Cohen et al., 2014), compared to their peers. However, these self-report abnormalities must be understood within the context of “objective” abnormalities, as behavioral data often fail to support the magnitude of these deficits. For example, functioning on standardized tests of cognitive functioning are largely unremarkable (see Chun et al., 2013 for a meta-analysis) as are comparisons employing objective computerized analysis of facial (Cohen et al., 2013) and vocal expression (Cohen, et al., 2012b). These discrepancies across subjective and objective domains of functioning raise questions about whether there is a dysfunction or bias with how schizotypal individuals process, evaluate and/or report autobiographical information. While it has yet to be determined whether “objective” abnormalities of emotion exist within schizotypy (and how these might be defined; though see Cohen et al., 2012a, Cohen et al., 2015 for discussion of emotional abnormalities in schizotypy), subjective biases are a central to cognitive conceptualizations of schizotypy. Beck et al., (2006) note a characteristic and distorted core belief of schizotypal personality disorder involves being “different, worthless and abnormal”. This highlights a potential intervention target. Clearly, more research on this topic is needed before any conclusions can be drawn, but the pathological report of emotional experience in schizotypy may involve cognitive and self-appraisal abnormalities.
Findings that negative symptoms and traits were associated with emotional abnormalities in both schizophrenia and schizotypy groups (at a trend level or greater) highlight phenotypic heterogeneity across both. In the schizophrenia group, clinical ratings of anhedonia were associated with more time spent experiencing no emotion, while in schizotypy, self-reported negative traits were associated with fewer positive experiences overall. These findings are consistent with some studies in the literature (e.g., Earnst and Kring, 1999), but overall, our understanding of how emotional abnormalities vary as a function of phenotypic heterogeneity is poorly understood. It bears mention that abnormal positive experience, or “state anhedonia” has been observed in depression (Bylsma et al., 2008) and it may be the case that depression symptoms were responsible for these relationships. Adopting a “symptom specific” research approach, such as involved in the Research Domain Criteria (RDoC), may help clarify state abnormalities across psychopathological conditions and states.
The present findings reflect an important entry in the quest to understand emotional dysfunctions across the schizophrenia-spectrum, despite limitations, notably that: a) medication and other treatment effects were unaccounted for, b) the community groups were not well demographically matched in some variables, c) the sample generally had modest levels of negative symptoms (notably clinically rated anhedonia), d) different response scales for the emotion task were used in the community versus college groups, e) schizophrenia and schizotypal symptoms/traits were measured using different approaches across the two studies; thus potentially introducing method variance, f) we did not assess whether subjects were naïve to the dynamic stimuli used in this study, and g) only one stimulus was employed. Further research exploring the affective chronometry of experience across the schizophrenia-spectrum, and their functional and neural sequelae, is warranted. The present findings support the notion that emotional abnormalities across the schizophrenia-spectrum do not reflect impairments in online, consummatory or state emotional systems (Strauss and Gold, 2014; Kring and Moran, 2000).
Acknowledgments
We would like to thank the people who participated in the study, as well as the National Institute of Mental Health (R03MH092622).
Funding: Funding for this study was provided by grant R03MH092622 from the National Institute of Mental Health; the funding agency had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors. Alex S Cohen was the primary investigator for this project and designed the study and wrote the bulk of the manuscript. Jeff Larsen, Gregory Strauss, Dallas Callaway and Kyle Mitchell helped manage the literature searches, the analyses and provided conceptual material to the planning and presentation of this project. All authors contributed to and have approved the final manuscript.
Conflicts of Interest: There are no conflicts of interest to report.
Contributor Information
Alex S. Cohen, Louisiana State University, Department of Psychology
Dallas A. Callaway, Louisiana State University, Department of Psychology
Kyle R. Mitchell, Louisiana State University, Department of Psychology
Jeff T. Larsen, University of Tennessee, Department of Psychology
Gregory P. Strauss, Binghamton University, Department of Psychology
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. fourth. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) Iowa City, IA: The University of Iowa; 1984a. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984b. [Google Scholar]
- Beck AT, Freeman A, Davis DD. Cognitive Therapy for Personality Disorders. second. New York: The Guilford Press; 2006. [Google Scholar]
- Blanchard JJ, Collins LM, Aghevli M, Leung WW, Cohen AS. Social anhedonia and schizotypy in a community sample: The Maryland Longitudinal Study of Schizotypy. Schizophr. Bull. 2011;37(3):587–602. doi: 10.1093/schbul/sbp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr. Bull. 1998;24(3):413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox or the group of schizophrenias. New York: International Universities Press; 1950. [Google Scholar]
- Burgin CJ, Chun CA, Horton LE, Barrantes-Vidal N, Kwapil TR. Splitting of associative threads: the expression of schizotypal ambivalence in daily life. J. Psychopathol. Behav. 2014;37(2):349–357. [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clin. Psychol. Rev. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. Beyond bipolar conceptualizations and measures: the case of attitudes and evaluative space. Pers. Soc. Psychol. Rev. 1997;1(1):3–25. doi: 10.1207/s15327957pspr0101_2. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Infrequency Scale. Madison, WI: Unpublished test; 1983. [Google Scholar]
- Chun CA, Minor KS, Cohen AS. Neurocognition in psychometrically defined college Schizotypy samples: we are not measuring the "right stuff". J. Int. Neuropsychol. Soc. 2013;19(3):324–337. doi: 10.1017/S135561771200152X. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Auster T, MaCaulay R, McGovern J. The paradox of schizotypy: resemblance to prolonged severe mental illness in subjective but not objective quality of life. Psychiat. Res. 2014;217(3):185–190. doi: 10.1016/j.psychres.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Beck MR, Najolia GM, Brown LA. Affective disturbances in psychometrically defined schizotypy across direct, but not indirect assessment modes. Schizophr. Res. 2011;128(1–3):136–142. doi: 10.1016/j.schres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Callaway DA, Najolia GM, Larsen JT, Strauss GP. On 'risk' and reward: Investigating state anhedonia in psychometrically defined schizotypy and schizophrenia. J. Abnorm. Psychol. 2012a;121(2):407–415. doi: 10.1037/a0026155. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Matthews RA, Najolia GM, Brown LA. Toward a more psychometrically sound brief measure of schizotypal traits: introducing the SPQ-Brief Revised. J. Pers. Disord. 2010;24(4):516–537. doi: 10.1521/pedi.2010.24.4.516. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Mohr C, Ettinger U, Chan RC, Park S. Schizotypy as an organizing framework for social and affective sciences. Schizophr. Bull. 2015;41(suppl 2):S427–S435. doi: 10.1093/schbul/sbu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Morrison SC, Brown LA, Minor KS. Towards a cognitive resource limitations model of diminished expression in schizotypy. J. Abnorm. Psychol. 2012b;121(1):109–118. doi: 10.1037/a0023599. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Morrison SC, Callaway DA. Computerized facial analysis for understanding constricted/blunted affect: initial feasibility, reliability, and validity data. Schizophr. Res. 2013;148(1–3):111–116. doi: 10.1016/j.schres.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition Emotion. 1998;12(3):307–330. [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol. Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- Earnst KS, Kring AM. Emotional responding in deficit and non-deficit schizophrenia. Psychiat. Res. 1999;88(3):191–207. doi: 10.1016/s0165-1781(99)00083-9. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient. New York: Biometrics Research; 1996. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res. 2007;93(1):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbener ES, Rosen C, Khine T, Sweeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. J. Abnorm. Psychol. 2007;116(1):43–55. doi: 10.1037/0021-843X.116.1.43. [DOI] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophr. Bull. 2008;34(5):856–874. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA. Premorbid indicators and risk for schizophrenia: a selective review and update. Schizophr. Res. 2005;79(1):45–57. doi: 10.1016/j.schres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Kring AM, Germans Gard M, Gard DE. Emotion deficits in schizophrenia: timing matters. J Abnorm. Psychol. 2011;120(1):79–87. doi: 10.1037/a0021402. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr. Bull. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Brown LH, Silvia PJ, Myin-Germeys I, Barrantes-Vidal N. The expression of positive and negative schizotypy in daily life: an experience sampling study. Psychol. Med. 2012;42(12):2555–2566. doi: 10.1017/S0033291712000827. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Green JD. Evidence for mixed feelings of happiness and sadness from brief moments in time. Cognition Emotion. 2013;27:1469–1477. doi: 10.1080/02699931.2013.790782. [DOI] [PubMed] [Google Scholar]
- Larsen JT, McGraw AP. Further evidence for mixed emotions. J. Pers. Soc. Psychol. 2011;100:1095–1110. doi: 10.1037/a0021846. [DOI] [PubMed] [Google Scholar]
- Larsen JT, McGraw AP. The case for mixed emotions. Soc. Personal. Psychol. Compass. 2014;8(6):263–274. [Google Scholar]
- Larsen JT, McGraw AP, Cacioppo JT. Can people feel happy and sad at the same time? J. Pers. Soc. Psychol. 2001;81(4):684–696. [PubMed] [Google Scholar]
- Larsen JT, McGraw AP, Mellers BA, Cacioppo JT. The agony of victory and thrill of defeat: Mixed emotional reactions to disappointing wins and relieving losses. Psychol. Sci. 2004;15:325–330. doi: 10.1111/j.0956-7976.2004.00677.x. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, McGraw AP, Hawkley LC, Cacioppo JT. The evaluative space grid: a single-item measure of positivity and negativity. Cognition Emotion. 2009;23:453–480. [Google Scholar]
- Lenzenweger MF. Schizotaxia, schizotypy, and schizophrenia: Paul E. Meehl's blueprint for the experimental psychopathology and genetics of schizophrenia. J. Abnorm. Psychol. 2006;115(2):195–200. doi: 10.1037/0021-843X.115.2.195. [DOI] [PubMed] [Google Scholar]
- Leung WW, Couture SM, Blanchard JJ, Lin S, Llerena K. Is social anhedonia related to emotional responsivity and expressivity? A laboratory study in women. Schizophr. Res. 2010;124(1–3):66–73. doi: 10.1016/j.schres.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Barrett LF. Emotional complexity. In: Lewis M, Haviland-Jones JM, Feldman Barrett L, editors. Handbook of Emotions. New York: Guilford Press; 2008. pp. 513–530. [Google Scholar]
- MacAulay RK, Brown LS, Minor KS, Cohen AS. Conceptualizing schizotypal ambivalence: factor structure and its relationships. J. Nerv. Ment. Dis. 2014;202(11):793–801. doi: 10.1097/NMD.0000000000000196. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Oorschot M, Collip D, Lataster J, Delespaul P, van Os J. Experience sampling research in psychopathology: opening the black box of daily life. Psychol. Med. 2009;39(09):1533–1547. doi: 10.1017/S0033291708004947. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson GG, Cacioppo JT. The current status of research on the structure of evaluative space. Biol. Psychol. 2010;84(3):422–436. doi: 10.1016/j.biopsycho.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. J. Pers. Soc. Psychol. 1980;39(6):1161–1178. [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am. J. Psychiat. 2012;169:364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbox SI, Pogue-Geile MF. A multivariate perspective on schizotypy and familial association with schizophrenia: a review. Clin. Psychol. Rev. 2011;31(7):1169–1182. doi: 10.1016/j.cpr.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau F, Antonius D, Cacioppo JT, Ziwich R, Jalbrzikowski M, Saccente E, Silipo G, Butler P, Javitt D. In support of Bleuler: objective evidence for increased affective ambivalence in schizophrenia based upon evocative testing. Schizophr. Res. 2009a;107(2–3):223–231. doi: 10.1016/j.schres.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Tremeau F, Antonius D, Goggin M, Czobor P, Butler P, Malaspina D, Gorman JM. Emotion antecedents in schizophrenia. Psychiat. Res. 2009b;169:43–50. doi: 10.1016/j.psychres.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. PANAS-X: Manual for the Positive and Negative Affect Schedule - Expanded Form. The University of Iowa; 1999. [Google Scholar]