Abstract
Rationale
Low high-density lipoprotein cholesterol (HDL-C) in coronary heart disease (CHD) patients may be due to rate-limiting amounts of lecithin:cholesterol acyltransferase (LCAT). Raising LCAT may be beneficial for CHD, as well as for Familial LCAT Deficiency (FLD), a rare disorder of low HDL-C.
Objective
To determine safety and tolerability of recombinant human LCAT (rhLCAT) infusion in subjects with stable CHD and low HDL-C and its effect on plasma lipoproteins.
Methods and Results
A phase 1b, open-label, single-dose escalation study was conducted to evaluate safety, tolerability, pharmacokinetics, and pharmacodynamics of rhLCAT (ACP-501). Four cohorts with stable CHD and low HDL-C were dosed (0.9, 3.0, 9.0, and 13.5 mg/kg, single 1-hour infusions) and followed for 28 days. ACP-501 was well-tolerated and there were no serious adverse events. Plasma LCAT concentrations were dose-proportional, increased rapidly and declined with an apparent terminal half-life of 42 hours. The 0.9 mg/kg dose did not significantly change HDL-C; however, 6 hours following doses of 3.0, 9.0, and 13.5 mg/kg, HDL-C was elevated by 6%, 36%, and 42%, respectively, and remained above baseline up to 4 days. Plasma cholesteryl esters followed a similar time-course as HDL-C. ACP-501 infusion rapidly decreased small and intermediate-sized HDL, whereas large HDL increased. Preβ-HDL also rapidly decreased and was undetectable up to 12 hours post ACP-501 infusion.
Conclusions
ACP-501 has an acceptable safety profile after a single IV infusion. Lipid and lipoprotein changes indicate that rhLCAT favorably alters HDL metabolism and support rhLCAT use in future clinical trials in CHD and FLD patients.
ClinicalTrials.gov Identifier
NCT01554800 https://www.clinicaltrials.gov/ct2/show/NCT01554800?term=ACP-501&rank=1
Keywords: Cholesterol, high density lipoprotein, lecithin:cholesterol acyltransferase, cardiovascular disease, recombinant enzyme replacement, lipids and lipoprotein metabolism, acute coronary syndrome, LCAT, LCAT deficiency, first-in-human clinical trial
Subject Terms: Acute Coronary Syndromes, Lipids and Cholesterol, Pharmacology, Pathophysiology
INTRODUCTION
Lecithin:cholesterol acyltransferase (LCAT) is a plasma enzyme secreted by the liver.1–3 It catalyzes the production of cholesteryl ester (CE) from free cholesterol and phosphatidylcholine (lecithin). In humans, about 90% of CE in plasma is formed by LCAT, and the reaction mostly occurs on high-density lipoproteins (HDL) (so called α-LCAT activity), and to a lesser extent on apoB-containing particles (β-LCAT activity). The esterification of cholesterol by LCAT helps maintain HDL levels by promoting the maturation of small discoidal forms of HDL (preβ-HDL and α4-HDL) into larger spherical forms of HDL (α1-3-HDL), which have a longer half-life.4 In humans, most HDL-CEs are eventually transferred in exchange for triglycerides to very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), and low-density lipoproteins (LDL) by cholesteryl ester transfer protein (CETP).5
Mutations in the human LCAT gene are rare and result in either Familial LCAT Deficiency (FLD) or Fish Eye Disease (FED).6 The former is characterized by extremely low HDL-C, corneal opacities, anemia, and progressive renal disease, which eventually requires either dialysis or renal transplantation. Despite their low HDL-C levels, FLD patients do not appear to have a marked increased risk of coronary heart disease (CHD), most likely because they typically also have low LDL-C. There is no effective therapy for FLD, but in LCAT-KO mice, it has been shown that intravenous (IV) administration of recombinant human LCAT (rhLCAT) can rapidly correct the lipid abnormalities in this disorder.7
Besides its role in the pathogenesis of FLD, LCAT has long been proposed to play a role in reverse cholesterol transport (RCT),8 the pathway for removal of excess cellular cholesterol, and hence may also be important in protecting against the development of CHD.9 In the first step of RCT, free cholesterol effluxes from foam cells in plaque by the ABCA1 transporter to plasma acceptors, such as preβ-HDL and other small forms of HDL. The conversion of free cholesterol on HDL to CE increases the capacity of HDL to remove additional cholesterol, and maintains the gradient for cholesterol efflux from cells.10 This concept is consistent with the findings of low LCAT activity and increased preβ-HDL in CHD patients,5, 11 but there are also contradictory data on the relationship between HDL, LCAT and CHD1, 12 particularly from recent large scale GWAS studies.13
It has been postulated that a therapy that could rapidly remove plaque cholesterol would stabilize vulnerable plaques in acute coronary syndrome (ACS) patients and reduce the likelihood of additional ischemic events.14 One such promising approach has been the IV infusion of reconstituted high-density lipoprotein (rHDL) particles containing phospholipid and either the apolipoprotein A-I (apoA-I) variant, apoA-IMilano,15 or native apoA-I.16 The intent is to provide additional preβ-HDL-like particles to rapidly promote cholesterol efflux from atherosclerotic lesions. A possible limitation of this approach may be that preβ-HDL levels are often already elevated in CHD patients, together with low levels of large, cholesteryl ester-rich HDL (α1-HDL),17, 18 and therefore, additional pharmacologic elevation of preβ-HDL particles without optimizing the other steps of the RCT pathway, such as the esterification of cholesterol by LCAT, may be less effective. In fact, in pre-clinical animal models, transient increased expression of LCAT by either infusion of the recombinant protein7 or by adenoviral expression19 can in itself substantially raise HDL-C and may therefore increase RCT. Although adenoviral overexpression of LCAT in mice expressing human apoA-I and CETP was not found to increase the fecal excretion of cholesterol,20 transgenic overexpression of hLCAT in rabbits that express endogenous CETP has nonetheless been shown to protect against diet-induced atherosclerosis.21 Similarly, IV administration of rhLCAT to wild type rabbits has also been shown to protect against atherosclerosis.22
Overall, the above combined clinical observations and preclinical animal data created the impetus for us to undertake development of rhLCAT as a potential therapy for CHD and FLD. We report here a first-in-human clinical trial in which we assessed the safety and tolerability of a single infusion of rhLCAT (designated ACP-501) in subjects with stable CHD and low HDL-C. The pharmacokinetics (PK) of ACP-501 and its effect on the pharmacodynamics (PD) of HDL-C and HDL subpopulations were assessed, as well as its impact on HDL function.
METHODS
Study subjects
Between May and August of 2012, 16 subjects were enrolled in this study (ClinicalTrials.gov identifier: NCT01554800) at the National Institutes of Health (NIH). Non-smoking men and women with a BMI between 18–35 kg/m2 and a total body weight ≥ 50 kg (110 lbs) were eligible, if they were between the ages of 30 and 85 years and had a history of stable (>60 days) CHD, as determined by either prior myocardial infarction, prior revascularization (e.g., percutaneous coronary intervention, coronary artery bypass graft), angiographically proven coronary atherosclerosis, or non-invasive (e.g., MRI, CT) evidence of CAD. Female subjects of child-bearing potential (neither pregnant nor breast-feeding) were required to use birth control during study conduct. Chronic concomitant medications must have been stable for at least 2 months prior to screening. Inclusion criteria also required that HDL-C was <50 mg/dL for males and <55 mg/dL for females to select for patients that may potentially show a greater effect from the ACP-501 treatment. Exclusion criteria are shown in Online Table I. The study protocol, amendments and subject informed consent documents were approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute.
Clinical study design
A Phase 1b, open-label, single-dose escalation design was used to evaluate the safety, tolerability, PK and PD of 4 doses of ACP-501 (0.9, 3.0, 9.0, and 13.5 mg/kg) in each of the 4 dosing cohorts (N=4). A total of 23 subjects consented to participate, of whom 16 subjects were treated with ACP-501. Subjects remained on their standard medical care for the duration of the trial. Subjects were admitted (Day 0) to the NIH Clinical Center approximately 12 hours prior to the infusion start time to allow for the collection and assessment of entry criteria. Study subjects then received a single-dose intravenous (IV) infusion (Day 1) and remained an inpatient for at least 24 hours following the infusion. During the follow-up phase, subjects were seen at an outpatient clinic on the mornings of Day 3, Day 4, Day 5, Day 7, and Day 28. Blood samples for safety, PK and PD measurements were obtained at hours 0, 1, 6, 12, 24, and 48 (morning of Day 3), 72 (morning of Day 4), 96 (morning of Day 5), 168 (morning of Day 8) and on Day 28 following the start of the ACP-501 infusion (Day 1, hour 0). Only 1 subject was initially treated in each cohort to determine the safety and tolerability of ACP-501 prior to exposing additional subjects. After a review of vital signs, electrocardiograms (ECGs), clinical laboratory tests, and adverse event (AE) data from each cohort by a Safety Review Group, subjects in the following cohort received the next higher dose of ACP-501.
Clinical study endpoints
The primary endpoint was safety and tolerability of a single dose of ACP-501, as determined by treatment emergent AE reporting, which reflected clinically significant changes in clinical chemistries, hematology, vital signs, physical examination, and ECGs, as well as infusion site reactions and toxicities. Secondary endpoints included the PK profile of ACP-501 after a single dose, and the PD effects of ACP-501, as assessed by plasma biomarkers, such as changes in HDL-C and CE, as well as other lipid and lipoprotein assays (HDL subfractions, preβ-HDL, non-HDL-C, LDL-C, total cholesterol (TC), triglycerides, apolipoprotein B (apoB), apoA-I, apoA-II, TC/HDL-C, and cholesterol efflux).
Additional methods
ACP-501 formulation and administration, detailed analytical methods, cholesterol efflux and ex vivo LCAT activity methods, and statistical analysis are included in the Online Methods section.
RESULTS
Subject baseline characteristics
Subject demographic and other baseline characteristics are summarized in Table 1. Overall, the majority of subjects were male (87.5%) and Caucasian (75.0%), with a median age of 67 years (range, 60 to 77 years). At baseline, all subjects had a normal or slightly elevated LCAT mass, with a median value of 8.02 μg/mL (ref. range, 5–11 μg/mL). The mean baseline HDL-C was 37 mg/dL. Because it is one of the potential target groups for ACP-501 therapy, all patients were selected for a positive history of CHD, and the majority also had at least 3 of the following concurrent medical conditions: hypertension, diabetes mellitus, arthritis and/or dyslipidemia. Subjects were on an average of 7 concomitant medications (not including herbal and vitamin supplements), with all but 1 subject receiving one or more lipid altering medications (13/16 on statins, 7/16 on fish oil, and 5/16 on niacin).
Table 1.
Demographic and Other Baseline Characteristics
| Characteristic | ACP-501 Dose (mg/kg) | ||||
|---|---|---|---|---|---|
| 0.9 (n = 4) | 3.0 (n = 4) | 9.0 (n = 4) | 13.5 (n = 4) | Total (N = 16) | |
| Age, years | |||||
| Mean (SD) | 66.3 (2.4) | 68.3 (7.9) | 70.5 (6.8) | 65.3 (2.9) | 67.6 (5.4) |
| Median | 67.0 | 68.5 | 72.0 | 66.5 | 67.0 |
| Min – max | 63–68 | 60–76 | 61–77 | 61–67 | 60–77 |
| Sex, n (%) | |||||
| Male | 3 (75.0) | 4 (100) | 3 (75.0) | 4 (100) | 14 (87.5) |
| Female | 1 (25.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 2 (12.5) |
| Race, n (%) | |||||
| Caucasian | 3 (75.0) | 4 (100) | 2 (50.0) | 3 (75.0) | 12 (75.0) |
| Asian | 1 (25.0) | 0 (0.0) | 1 (25.0) | 1 (25.0) | 3 (18.8) |
| Black/African American | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (6.3) |
| Ethnicity, n (%) | |||||
| Not Hispanic or Latino | 4 (100) | 4 (100) | 4 (100) | 4 (100) | 16 (100) |
| Height, cm | |||||
| Median | 166.4 | 178.3 | 172.4 | 177.6 | 175.2 |
| Min – max | 162.0–175.9 | 166.5–181.9 | 167.5–177.4 | 174.5–184.8 | 162.0–184.8 |
| Weight, kg | |||||
| Median | 81.2 | 93.0 | 82.4 | 79.1 | 83.9 |
| Min – max | 75.6–85.1 | 76.9–95.3 | 78.5–94.2 | 66.8–96.2 | 66.8–96.2 |
| BMI, kg/m2 | |||||
| Median | 28.8 | 28.8 | 28.8 | 25.4 | 28.1 |
| Min – max | 27.1–30.2 | 27.7–29.6 | 25.2–30.8 | 21.4–28.2 | 21.4–30.8 |
| LCAT mass, μg/mL | |||||
| Median | 7.9 | 7.5 | 8.4 | 8.2 | 8.0 |
| Min – max | 6.5–11.9 | 6.9–8.6 | 6.3–13.5 | 6.2–8.9 | 6.2–13.5 |
| HDL-C, mg/dL | |||||
| Mean (SD) | 40.5 (6.6) | 37.8 (4.3) | 35.3 (4.6) | 34.8 (3.4) | 37.0 |
| Median | 38.0 | 38.5 | 35.0 | 34.0 | |
| Min – max | 36–50 | 32–42 | 30–41 | 32–39 | 32–50 |
| LDL-C, mg/dL | |||||
| Mean (SD) | 72.5 (50.0) | 54.3 (5.4) | 71.8 (14.1) | 97.0 (44.2) | 73.9 |
| Median (SD) | 51.5 | 55 | 74 | 99 | |
| Min – max | 41–146 | 47–60 | 54–85 | 41–149 | 41–149 |
| TG, mg/dL | |||||
| Mean (SD) | 130.8 (60.3) | 95.3 (25.5) | 147.3 (71.3) | 182.0(139.7) | 138.9 |
| Median | 135 | 98 | 130 | 151 | |
| Min – max | 60–194 | 66–119 | 87–242 | 49–378 | 49–378 |
| VLDL-C, mg/dL | |||||
| Mean (SD) | 26.0 (12.2) | 19.0 (5.3) | 29.3 (14.1) | 36.5 (28.1) | 27.7 |
| Median (SD) | 26.5 | 19.5 | 26.0 | 30.0 | |
| Min – max | 12–39 | 13–24 | 17–48 | 10–76 | 10–76 |
| TC, mg/dL | |||||
| Mean (SD) | 138.0 (52.3) | 111.0 (9.0) | 138.8 (16.0) | 166.3 (53.9) | 138.5 |
| Median | 119 | 108 | 135 | 182 | |
| Min – max | 101–214 | 104–124 | 124–161 | 92–209 | 92–214 |
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LCAT, lecithin:cholesterol acyltransferase; SD, standard deviation
Safety profile and tolerability of ACP-501
All 16 subjects were able to receive their full dose of ACP-501, diluted with 270 mL of normal saline, by intravenous infusion over a 1-hour period. During 28 days of follow up, there were no serious adverse events (SAE), or severe AEs, or any infusion site reactions, such as localized redness, pain, swelling in the area, or increased warmth. All clinically significant changes noted in the physical examination, ECGs, vital signs, urinalysis, clinical chemistries and hematology labs were reported as AEs. A total of 8 AEs (Online Table II) occurred in 7 subjects. Two AEs, both mild rashes, were considered to be possibly related to the ACP-501 treatment. In both cases, the rash spontaneously resolved without treatment. The other 6 AEs, which included dehydration during a heat wave, hypertriglyceridemia following alcohol ingestion, and hyperglycemia, were considered to be clinically insignificant and unrelated to the drug treatment. No subject withdrew from the study due to an AE.
ACP-501 pharmacokinetics
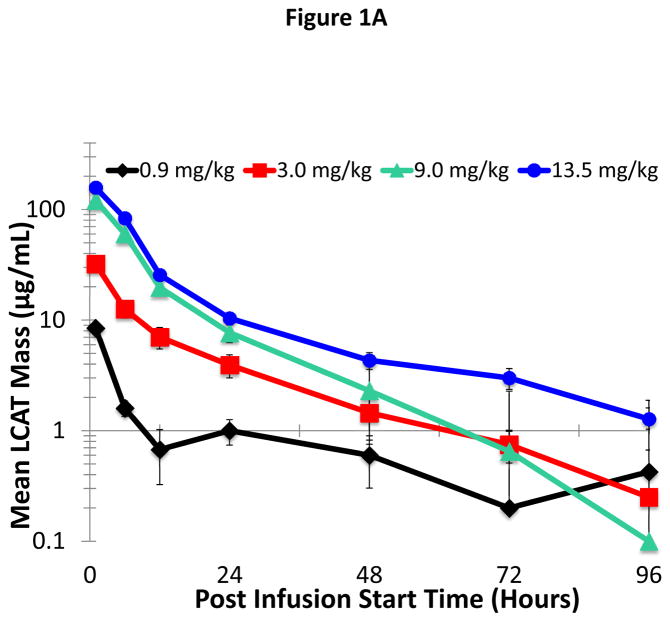
PK parameters for ACP-501 were calculated following a single IV infusion of ACP-501 at doses of 0.9, 3.0, 9.0, or 13.5 mg/kg administered over 1 hour. The upper dose was based on the no observed adverse effect level (NOAEL) of 60 mg/kg for ACP-501 as determined in a nonhuman primate toxicology study. Peak concentrations of ACP-501 were detected 1 hour following the start of the infusion and showed multi-exponential decay (Figure 1A, Online Table III). The assay used to measure ACP-501 mass (rhLCAT) also measures endogenous LCAT, but the individual subject baseline LCAT levels, which was on average approximately 8 μg/mL (Table 1), was subtracted from the result shown in Figure 1A. Peak ACP-501 levels were significantly higher than baseline LCAT levels, even for the lowest dose. The vast majority of ACP-501 was cleared from the circulation by 2 days and was estimated to have a terminal half-life of approximately 42 hours, based on analysis of the 9.0 and 13.5 mg/kg cohort data. Peak exposure (Cmax) and systemic plasma exposure (area under the curve [AUC]0-48, AUC0-t, and AUC0-∞) generally increased in proportion to the dose and to the measured Cmax at each dose (Median: 8.6, 32.2, 107, and 163 μg/mL for 0.9, 3.0, 9.0, and 13.5 mg/kg, respectively) (Online Tables III and IV).
Figure 1. Plasma kinetic decay curve of LCAT.
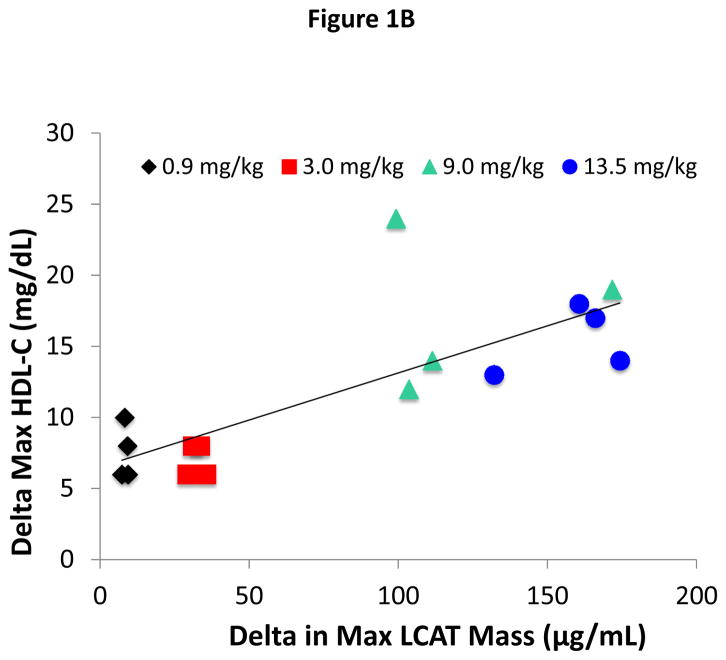
(A) ACP-501 was intravenously infused over a 1 hour time point and total LCAT mass was measured at the indicated time points by an ELISA that measures both rhLCAT (ACP-501) and endogenous LCAT. Results represent the mean (N=4) ±1 SEM for each dose cohort. The baseline LCAT mass for each patient at time 0 was subtracted from subsequent time points. (B) Dose-response relationship between ACP-501 dose and change in HDL-C. The maximum HDL-C concentration change from baseline that occurred any time after the infusion was plotted against the plasma level of total LCAT mass after completion of infusion. Results are shown for individual subjects for the indicated dose cohorts.
Effect of ACP-501 on HDL-C levels
The effect of ACP-501 on lipoproteins was first assessed by examining its effect on HDL-C. A dose-response relationship was observed between the dose of ACP-501 and the peak HDL-C levels, which typically occurred between 12 and 24 hours after the start of the infusion. When expressed as maximum % change from baseline, ACP-501 at the 0.9, 3.0, 9.0 or 13.5 mg/kg dose increased HDL-C by 21, 19, 48, and 44% over the initial 4 days post-infusion, respectively. The mean % change from baseline in the 9.0 and 13.5 mg/kg ACP-501 dose groups were similar and statistically greater than that in the 0.9 mg/kg low-dose group (p = 0.0020 and p = 0.0061, respectively). An overall positive linear relationship was observed (Figure 1B) between maximum change from baseline in HDL-C in absolute units and maximum change from baseline in ACP-501 mass, with a Pearson correlation coefficient of 0.7909 (p = 0.0003). Overlap, however, was observed for some patients in the 9.0 and 13.5 mg/kg doses for both the maximum change from baseline in HDL-C and in ACP-501 mass post infusion.
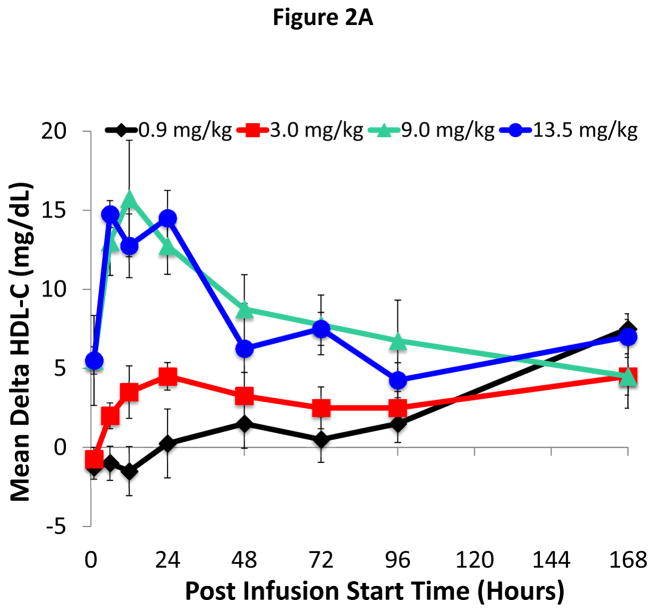
The mean absolute change from baseline in HDL-C versus study time through the Day 7 visit is illustrated in Figure 2A. HDL-C began to increase 1 hour post infusion in both the 9.0 and 13.5 mg/kg dose groups, and 6 hours post infusion in the 3.0 mg/kg group. In contrast, only a relatively small change in HDL-C occurred for the 0.9 mg/kg dose group at the later time points. For the 9.0 and 13.5 mg/kg dose groups, the increase in HDL-C peaked between 6 and 24 hours post-infusion. A dose response for increase in AUCs (AUC0-48, AUC0-96, and AUC0-168) of HDL-C was also observed across the 0.9, 3.0, and 9.0 mg/kg groups, with the AUCs of the 13.5 mg/kg dose similar to those of the 9.0 mg/kg dose (OnlineTable IV).
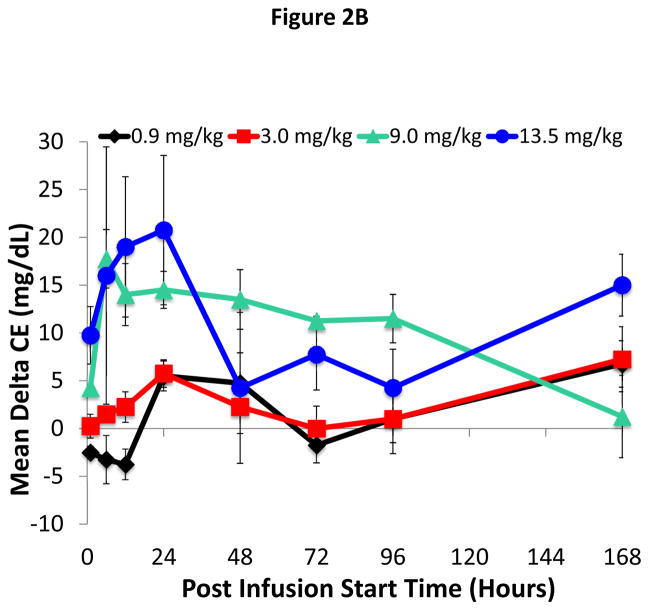
Figure 2. Time course for changes in HDL-C and CE after ACP-501 infusion.
(A) Mean absolute change from baseline in plasma HDL-C through day 7. (B) Mean absolute change from baseline in plasma CE through day 7. Results represent mean (N=4) ±1 SEM for each dose cohort.
Effect of ACP-501 on plasma cholesteryl ester
Similar to HDL-C, statistically significant mean % changes in maximum plasma CE of 10, 11, 19, and 22% were observed at doses of 0.9, 3.0, 9.0, and 13.5 mg/kg, respectively. The mean % changes in the 9.0 and 13.5 mg/kg ACP-501 groups were statistically significant versus the low-dose group (p = 0.0273 and p = 0.0077, respectively). As shown in Figure 2B, the mean absolute change from baseline in plasma CE started to increase by 1 hour post-infusion in both the 9.0 and 13.5 mg/kg ACP-501 dose groups, peaked between 6–24 hours and then gradually decreased over several days. Smaller changes in CE were observed at the lower doses, but during the initial 48 hours post-infusion, the AUCs for plasma CE increased proportional to the dose (Online Table V).
Effect of ACP-501 on other lipid and lipoprotein parameters
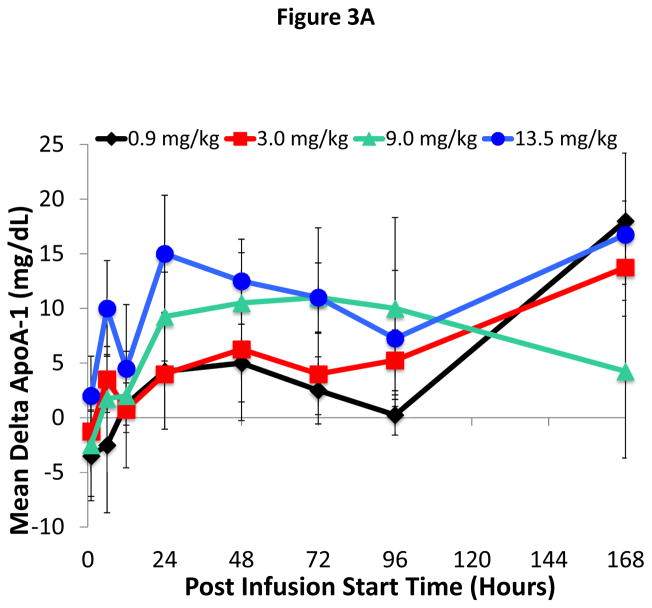
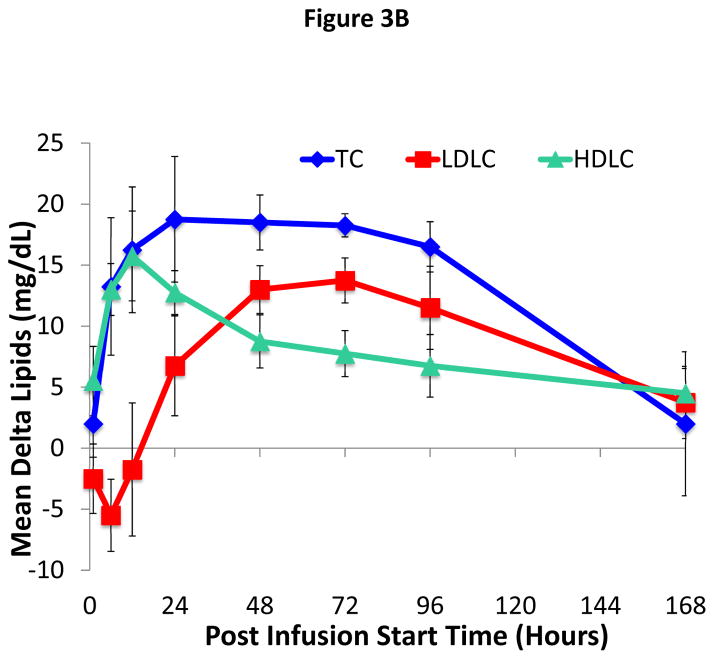
A full lipid profile (TC, TG, LDL-C, apoB, apoA-I, apoA-II, and TC/HDL-C) was also assessed at time points up to and including Day 7. There was an approximately 10–15 mg/dL increase in apoA-I in the 9.0 and 13.5 mg/kg dose groups, which remained partially elevated compared to baseline up through day 8 (Figure 3A). As shown for the 9.0 mg/kg dose cohort (Figure 3B), changes in TC for the first 24 hours after infusion closely paralleled changes observed in HDL-C. Later when HDL-C started to decrease, however, TC still remained elevated. LDL-C showed a slight transient decrease immediately after ACP-501 infusion and then later increased by day 3 by approximately 10–15 mg/dL before decreasing again back to baseline along with TC. All other dose cohorts (both higher and lower) also showed a slight drop in LDL-C immediately after the infusion and then a later increase in LDL-C but only back to baseline or only slightly above baseline by day 3 (Online Figure I). There was no apparent ACP-501 infusion effect on TG, apoB, and apoA-II throughout the 7-days post-infusion for each dose group. Finally, the atherogenic profile of subjects, as defined by TC/HDL-C ratio, was decreased throughout the 7-days post-infusion with a maximum decrease of approximately 22% within the initial 24 hours post infusion for both the 9.0 and 13.5 mg/kg dose groups.
Figure 3. Time course for changes in apoA-I and cholesterol levels after ACP-501 infusion.
(A) Mean absolute change from baseline in apoA-I levels over time through day 7. Results represent mean (N=4) ±1 SEM for each dose cohort. (B) Mean absolute change from baseline for indicated lipids is shown through day 7. Results represent mean (N=4) ±1 SEM for the 9.0 mg/kg dose cohort.
In Online Figure II, we also measured the effect of ACP-501 treatment on HDL and LDL particle counts, as determined by NMR. In contrast to HDL-C, ACP-501 appeared to have only a minimal effect on HDL-P. A small increase of about 10–15% was observed in HDL-P for time points after 48 hours, for the two highest dose cohorts, but this change did not reach statistical significance. No major changes were also observed in LDL-P following ACP-501 treatment.
Effect of ACP-501 on HDL subfraction distribution
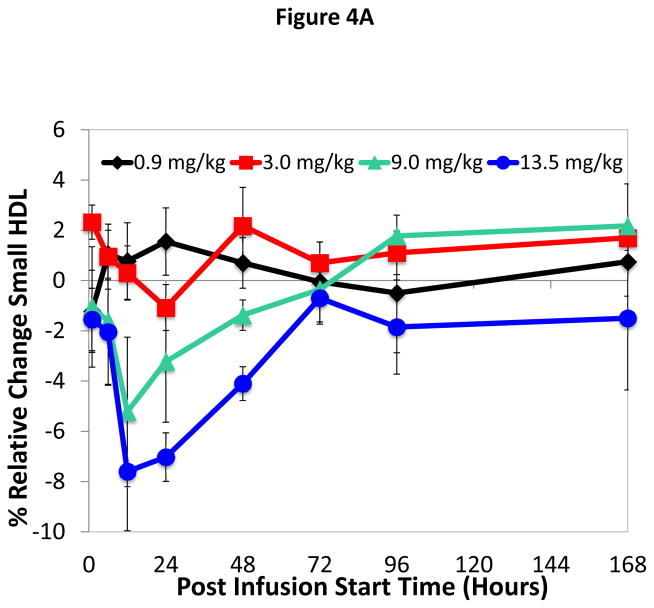
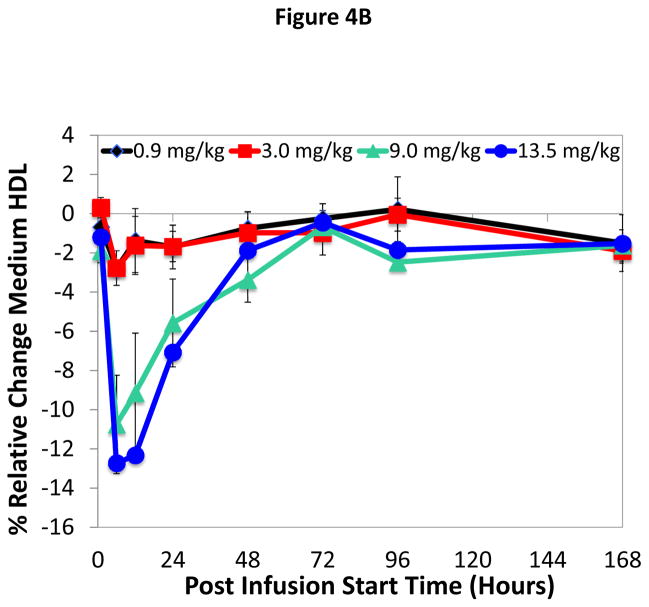
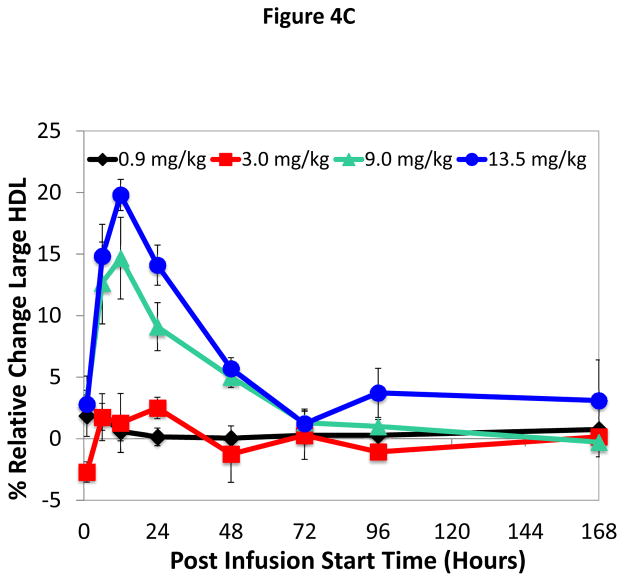
Infusion of ACP-501 caused a rapid conversion of small HDL subfractions to larger HDL subfractions (Figure 4A–C), which is consistent with the known effect of LCAT on HDL maturation. Within 12 hours of infusion for the 9.0 and 13.5 mg/kg dose 1) small and intermediate size HDL subfractions decreased by approximately 8 and 13% for the 9.0 and 13.5 mg/kg dose, respectively, and 2) a corresponding 10 and 15% increase was observed in large size HDL subfractions. HDL subfraction distribution returned to baseline by 168 hours. Similar changes in the small, medium and large HDL subfraction distribution after ACP-501 treatment (9.0 and 13.5 mg/kg dose) were detected by NMR analysis (Online Table VI).
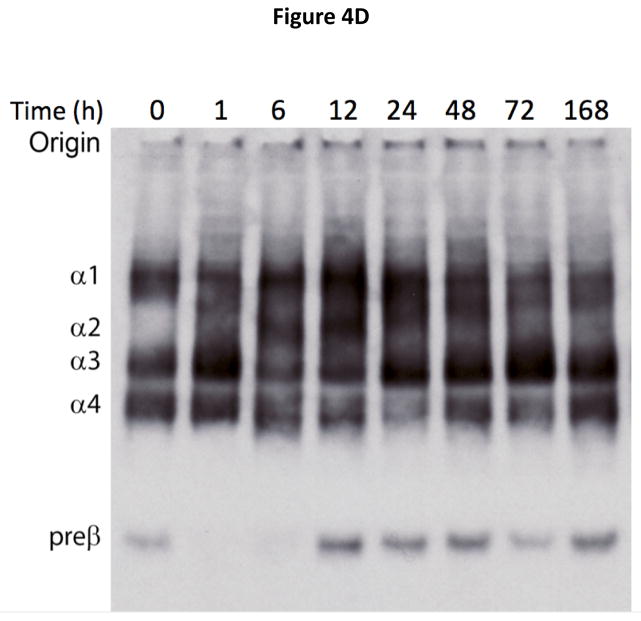
Figure 4. Time course for changes in HDL subfraction distribution after ACP-501 infusion.
Mean % change from baseline in small (A), medium (B), and large (C) HDL subfractions over time, determined by Lipoprint electrophoresis followed by staining with Sudan Black. Results represent mean (N=4) ±1 SEM for each dose cohort. (D) Non-denaturing gel electrophoresis of serum from a patient receiving 9.0 mg/kg ACP-501 collected at the indicated time points and immunoblotted for apoA-I.
Analysis of HDL subfractions by non-denaturing gel electrophoresis showed a similar pattern (Figure 4D). Preβ-HDL, which is not detectable by the Lipoprint® tube gel or NMR methods used above, disappeared 1 hour after the infusion, and then reappeared at 12 hours at slightly higher levels. An increase in the larger spherical HDL subfractions (α1-3-HDL) occurred by 6 to 12 hours and a decrease in the discoidal α4-HDL subspecies was evident during this time. By 168 hours the HDL subfraction distribution was similar to baseline.
Effect of ACP-501 on ex vivo cholesterol efflux and LCAT activity
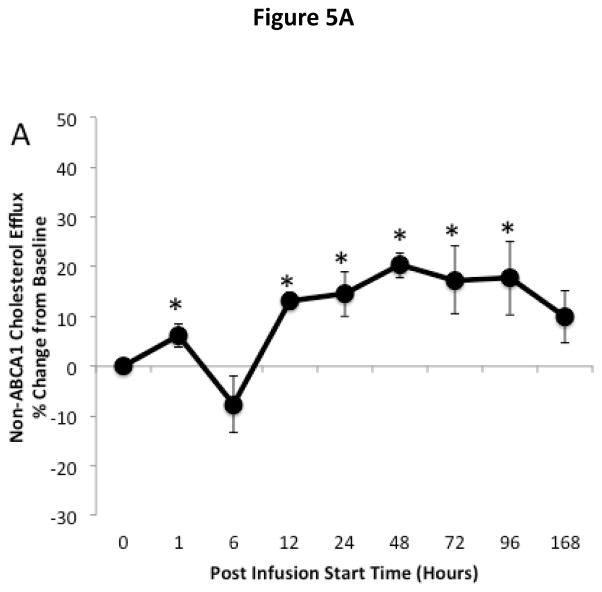
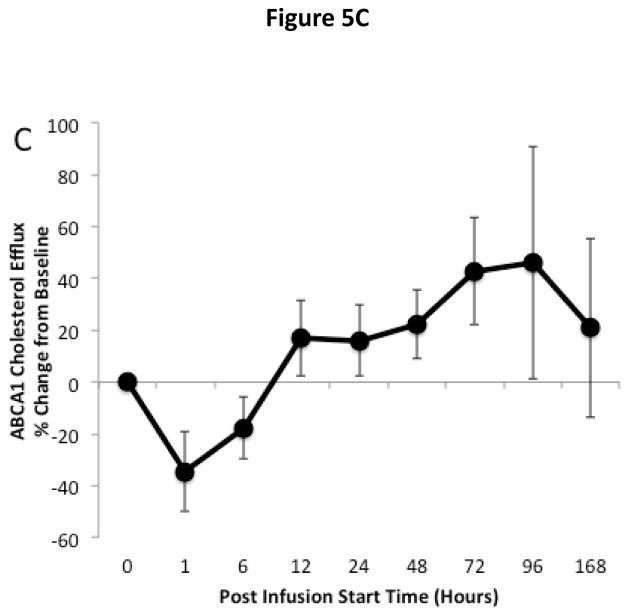
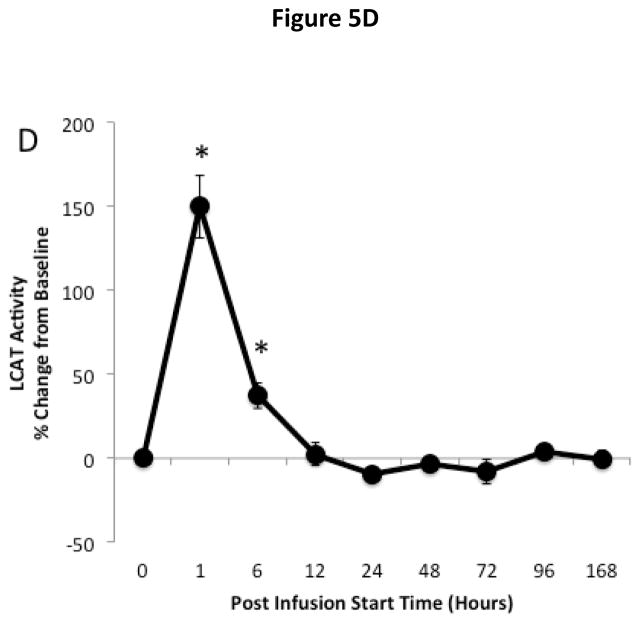
The effect of ACP-501 infusion on ex vivo HDL-mediated cholesterol efflux is shown for the 9 mg/kg dose cohort in Figure 5. Serum was treated with PEG to remove non-HDL lipoproteins and incubated with J774 macrophages not stimulated with cAMP to measure non-ABCA1 dependent cholesterol efflux (Fig. 5A) and with J774 macrophages stimulated with cAMP to induce ABCA1 for the measurement of global cholesterol efflux (Fig. 5B)23. The ABCA1-dependent component of cholesterol efflux (Fig. 5C) was calculated by subtracting the non-ABCA1 dependent cholesterol efflux from the global efflux results. Non-ABCA1 dependent cholesterol efflux increased by about 20% compared to baseline following ACP-501 treatment and remained elevated for several days. Similar results were seen in global cholesterol efflux, but the increase was slightly larger and at peak levels it was about 30% higher than baseline. This is consistent with an increase in ABCA1-dependent cholesterol efflux from the ACP-501 treatment, but the results for ABCA1-dependent cholesterol efflux were highly variable and the change was not statistically significant. We also consistently observed a small and transient (<12 h) decrease in ABCA1-dependent cholesterol efflux (p=0.06) after ACP-501 treatment, which corresponded to the time points when preβ-HDL levels were low following the treatment (Figure 4D). The percent of cholesterol esterified during the efflux assay was also monitored from the cells not treated with cAMP as a measure of LCAT activity. Samples collected within the first 6 hours after the infusion of ACP-501 showed a marked increase in the percent of effluxed cholesterol in the media that was esterified, but this parameter returned to baseline thereafter (Fig. 5D).
Figure 5. Time course for changes in HDL-mediated cholesterol efflux and LCAT activity after ACP501 infusion.
(A) Mean % change from baseline in non-ABCA1 dependent cholesterol efflux from J774 cells not stimulated with cAMP. (B) Mean % change from baseline in global cholesterol efflux from J774 cells stimulated with cAMP. (C) Mean % change from baseline in ABCA1 dependent cholesterol efflux. (D) Mean % change from baseline in cholesterol esterification during the ex vivo cholesterol efflux study from J774 cells not stimulated with cAMP. Results represent mean (n=4) ±1 SEM for the 9 mg/kg dose cohort. Mean absolute values at baseline ±1 SEM were the following: Non-ABCA1 cholesterol efflux (4.35 ± 0.44 %/4h), Global cholesterol efflux (8.14 ± 1.79 %/4h), ABCA1 cholesterol efflux (3.79 ± 1.71 %/4h), LCAT activity (15.6 ± 3.0 %/4h). (*) Indicates values with P<0.05 compared to baseline.
DISCUSSION
Because this is the first study investigating the infusion of rhLCAT (ACP-501) in human subjects, the finding that ACP-501 had an acceptable safety profile was the most important outcome of this study. All patients were able to complete the study, and no SAEs were observed. In addition, a dose-proportional increase in HDL-C was found after infusion of ACP-501, consistent with the known effect of LCAT on raising HDL-C by catalyzing the esterification of cholesterol. Overall, these two findings support the future development of ACP-501.
In terms of PK and PD, most of the findings were expected. Peak levels of LCAT mass occurred shortly after the ACP-501 infusion and began to return to baseline by 96 hours and eventually reached basal levels at 168 hours consistent with the known half-life of LCAT. ACP-501 infusion produced a dose related increase in HDL-C AUC up through the 9.0 mg/kg dose. Depending on baseline values, doses of ACP-501 higher than 9.0 mg/kg increased HDL-C on average between 36–42% and as much as 67% in one patient. Similarly, the total amount of plasma CE was also increased. It was of interest that the PD response (HDL-C and plasma CE) persisted beyond the PK (ACP-501 levels), suggesting that changes related to the remodeling of HDL by the infused rhLCAT persisted for several days. The formation of larger HDL with a longer half-life could potentially explain these observations.4 There was also a modest rise of apoA-I in the 3.0, 9.0, and 13.5 mg/kg dose groups that lasted over the course of 7 days. For the 9.0 mg/kg dose of ACP-501, a delayed and transient increase in LDL-C was also observed after HDL-C peaked and then started to decline. One possible interpretation of these findings is that CE in HDL was transferred to LDL via CETP.24 This change, however, was variable, and was not seen in either the lower or highest dose cohorts (Online Figure I). No significant or consistent changes with ACP-501 treatment were also observed in any of the dose cohorts for either apoB or LDL-P. Both of these alternative markers of LDL have been shown in several studies to be better than LDL-C as a CVD risk marker,25 but the possible effect of ACP-501 in changing LDL-C and the role of CETP will have to be monitored carefully in future studies.
Based on the known effect of LCAT on HDL metabolism,8, 26–29 the infusion of ACP-501 most likely raised HDL-C by enhancing its ability to act as a cholesterol “sink”. Assuming a 70 kg adult, the mean increase of 15 mg/dL for HDL-C with the 9.0 and 13.5 mg/kg dose of ACP-501 (Figure 2) should correspond to approximately 470 mg of cholesterol being mobilized by the treatment. Although the tissue source of this cholesterol was not investigated, RBCs are likely to be an important source of free cholesterol as they contain an abundant pool of free cholesterol30 that is accessible to LCAT. In FLD patients, free cholesterol is significantly enriched on RBCs, likely contributing to their anemia; future studies to determine the effect of ACP-501 treatment on anemia and RBC half-life will help to clarify this issue. The overall change in cholesterol mobilization seen in this study is comparable to what has been observed in clinical trials involving the infusion of rHDL, which have shown promise in early stage clinical trials.14–16 Larger HDL particles were also formed after ACP-501 infusion presumably as a consequence of CE enrichment at the expense of the preβ-HDL, which again is consistent with the known effects of LCAT on HDL maturation. Radiotracer studies in humans have shown that the vast majority of esterified cholesterol is eventually delivered to the liver in one of the last steps in RCT.24
In addition to modulating the lipid content of HDL, ACP-501 also appeared to affect its ability to promote cholesterol efflux from cells, using an in vitro cell based assay (Figure 5). Using LDL-depleted serum, ACP-501 treatment caused a modest increase compared to baseline in non-ABCA1 cholesterol efflux, which persisted for several days and is consistent with previous studies.20 An even larger increase of about 30% compared to baseline was observed in global cholesterol efflux, which also persisted for several days. This is consistent with an increase in ABCA1-dependent cholesterol efflux from the ACP-501 treatment, but the results were more variable and did not reach statistical significance. We also observed a slight transient decrease in ABCA1-dependent cholesterol efflux from macrophages following ACP-501 treatment. The acute drop in ABCA1-dependent cholesterol efflux corresponded to the time period when preβ-HDL was depleted from the serum (Figure 4D). Because the macrophage cells in the in vitro assay were stimulated to induce the expression of ABCA1, this is consistent with a mechanism whereby ACP-501 enhanced cholesterol efflux and the maturation of HDL in vivo, thus depleting preβ-HDL and producing large HDL with lower ability to remove cholesterol by ABCA1 during the in vitro cholesterol efflux assay31. This result is also consistent with previous studies showing that serum from LCAT deficient patients with high preβ-HDL levels had an increase in the in vitro cholesterol efflux from cells expressing ABCA1.32 We also observed that more of the effluxed cholesterol, during the in vitro cholesterol efflux study, was esterified after the ACP-501 treatment, which would also be predicted to enhance net cholesterol efflux from cells.10, 24 Additional studies, however, will be needed to fully understand how these in vitro findings relate to in vivo cholesterol efflux and the overall effect of LCAT on the RCT pathway.
Most of the evidence from prior animal models supports the rationale for stimulating RCT and reducing atherosclerosis by increasing the amount of LCAT. LCAT transgenic rabbits have very high HDL-C levels and are protected from diet-induced atherosclerosis.21 Furthermore, wild type rabbits treated with rhLCAT are also protected against diet-induced atherosclerosis.22 Studies using adenoviral gene transfer have demonstrated increased HDL-C in monkeys,19 enhanced biliary cholesterol excretion in hamsters33 and cholesterol “unloading” from pre-established atherosclerotic lesions in rabbits.34 Importantly, these are all animal species that, like humans, transport most of their plasma cholesterol in LDL and express CETP. Without CETP there would be predicted to be a block in the transport of CE to the liver, thus causing the accumulation of CE in HDL (“dysfunctional HDL”), as occurs in C57Bl/6 mice over-expressing LCAT7, 35 and possibly in humans treated with CETP inhibitors. Co-expression of CETP in LCAT transgenic mice corrects dysfunctional HDL and reduces atherosclerosis.36 In another mouse study, adeno-associated viral LCAT expression increased fecal cholesterol excretion from radiolabeled macrophages implanted into the peritoneal cavity of LCAT-KO mice but did not do so from WT mice that also expressed CETP.20 Transgenic rabbits lacking the LDL-receptor were also not protected against atherosclerosis when they overexpressed LCAT, which suggests that the delivery of cholesterol transferred by CETP to LDL and its subsequent uptake by hepatic LDL-receptors may be a critical step in this pathway. ACP-501 may perhaps work synergistically with statins, and other therapies, such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors that up-regulate the LDL-receptor.
Recent data from human studies also suggest that LCAT may be rate-limiting in CHD patients, although some contradictory studies have also been described. Participants in the current study had normal LCAT mass and this criteria, along with LCAT activity and preβ-HDL, were not used to select patients, but it has been previously shown that low LCAT activity and high preβ-HDL are associated with CHD.5 For example, LCAT activity was lower in subjects with CHD in the Copenhagen City Heart Study.11 In this study, preβ-HDL levels as measured by ELISA were also markedly higher in the CHD group, even in those without dyslipidemia, compared to a control group matched for traditional lipid markers. These data confirmed earlier work on subjects with CHD, using electrophoresis to measure preβ-HDL.17 Moreover, in earlier studies, the severity of CHD (i.e. number of diseased coronary vessels) was correlated with LCAT activity.37 The increase in preβ-HDL levels in CHD has been ascribed to reduced levels of LCAT, resulting in delayed conversion of preβ-HDL and α4-HDL to larger spherical forms of HDL.35 Others, however, have reported increased LCAT mass in patients with metabolic syndrome,38 but this could perhaps be a compensatory response. A recent GWAS study examining polymorphisms in LCAT associated with low HDL-C also failed to show an association with cardiovascular outcomes.13 It is important to note, however, that the acute infusion of a large dose of rhLCAT is a “non-physiologic” intervention, and thus it may be difficult to discern the effect of this type of treatment on atherosclerosis from any type of observational study in humans.
In summary, the current study supports the future investigation of rhLCAT in Phase 2a multiple-dose trials at weekly doses. In addition to CHD, future clinical trials may also be aimed at treating FLD patients, based on the previous demonstration that rhLCAT can reverse the lipid and lipoprotein abnormalities in LCAT-KO mice.7 rhLCAT may ameliorate the anemia and, even more importantly, the life-threatening renal complications that develop in this disorder.3, 6 Key outstanding questions for rhLCAT therapy include the following: 1) the immunogenic potential of this protein, 2) the optimal dosing regimen, and 3) the optimal biomarkers for efficacy. In regard to CHD, it will be important to determine in future studies whether rhLCAT in humans can enhance RCT and/or whether it can also positively affect one of the many other proposed anti-atherogenic functions of HDL.9 It will also be important to identify biomarkers that may predict a good response to the therapy, such as low LCAT activity, low HDL-C levels and/or high preβ-HDL.
Supplementary Material
Novelty and Significance.
What Is Known?
Decreased high-density lipoprotein cholesterol (HDL-C) levels are associated with increased risk for atherosclerosis.
Lecithin:cholesterol acyltransferase (LCAT) catalyzes plasma free cholesterol (FC) to cholesteryl ester (CE), converting small HDL to large HDL in the proposed second step of reverse cholesterol transport (RCT).
In patients with coronary heart disease (CHD), the levels of small HDL enriched in FC are elevated and HDL-C is decreased, suggesting that LCAT availability may be rate-limiting for RCT.
LCAT is also defective in Familial LCAT Deficiency and Fish Eye Disease and accounts for the clinical manifestations of these two genetic disorders.
What New Information Does This Article Contribute?
Recombinant human LCAT (rhLCAT) was tested as a novel therapeutic agent and was found to be safe and well-tolerated in patients with CHD and low HDL-C, with no clinically meaningful changes in safety labs or serious adverse events.
Plasma concentrations of rhLCAT, HDL-C and CE increased and preβ-HDL rapidly decreased after rhLCAT infusion.
Small HDL was converted to antiatherogenic larger HDL.
rhLCAT is the first-in-class therapy that stimulates the rate-limiting second step in HDL reverse cholesterol transport, thus offering a new mechanism to increase HDL.
rhLCAT was developed as a new treatment to raise HDL-C in patients with CHD and low HDL-C and for patients with Familial LCAT deficiency, a rare orphan disorder of extremely low HDL-C.
We conducted a first-in-human phase 1b, open-label, single IV infusion, dose-escalation of 4 doses of rhLCAT in patients with stable CHD and low HDL-C to evaluate safety, tolerability, pharmacokinetics and pharmacodynamics. rhLCAT was safe and well-tolerated with no infusion site reactions. HDL-C was unchanged at the lowest no-effect dose but was elevated by 6%, 36% and 42% by 6 hours after increasing doses and remained above baseline for at least 4 days. FC (the substrate of LCAT) was reduced after rhLCAT infusion but later reappeared while CE (the product) paralleled the rapid appearance of HDL-C. Following infusion of rhLCAT, small and intermediate-sized HDL particles decreased, while the larger more atheroprotective HDLs were formed. LCAT appears to be rate-limiting step in the pathway of HDL maturation in CHD patients with low HDL-C. The lipid and lipoprotein changes indicate that rhLCAT favorably alters HDL metabolism and support rhLCAT use in future clinical trials in CHD and Familial LCAT Deficiency patients.
Acknowledgments
The authors thank the 7th floor outpatient clinic and the 5th floor inpatient unit personnel at the NIH Clinical Center for their assistance in the study.
SOURCES OF FUNDING
Research was supported by research funds from the intramural NHLBI program and a NIH-CRADA with AlphaCore Pharma.
Nonstandard Abbreviations and Acronyms
- LCAT
lecithin:cholesterol acyltransferase
- RCT
reverse cholesterol transport
- ACP-501
recombinant human LCAT
- rHDL
reconstituted high-density lipoprotein
- rhLCAT
recombinant human LCAT
- CETP
cholesteryl ester transfer protein
- FLD
Familial LCAT Deficiency
- CE
cholesteryl esters
- TC
total cholesterol
- ApoB
apolipoprotein B
- ApoA-I
apolipoprotein A-I
Footnotes
DISCLOSURES
R. Shamburek, A. Shamburek, L. Freeman, M. Amar, M. Sampson, A. Wolska, A. Remaley: NIH-CRADA Research funding from AlphaCore Pharma, LLC; Significant; R. Bakker-Arkema: Employment; Significant; AlphaCore Pharma, LLC. Ownership Interest; Modest; AlphaCore Pharma, LLC., B. Auerbach: Employment; Significant; AlphaCore Pharma, LLC. Ownership Interest; Significant; AlphaCore Pharma, LLC. R. Homan: Employment; Significant; AlphaCore Pharma, LLC. Ownership Interest; Significant; AlphaCore Pharma, LLC. B. Krause: Employment; Significant; AlphaCore Pharma, LLC. Ownership Interest; Significant; AlphaCore Pharma: S. Adelman, H. Collins: Employment; Significant; Vascular Strategies LLC.
References
- 1.Rousset X, Shamburek R, Vaisman B, Amar M, Remaley AT. Lecithin cholesterol acyltransferase: An anti- or pro-atherogenic factor? Curr Atheroscler Rep. 2011;13:249–256. doi: 10.1007/s11883-011-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calabresi L, Franceschini G. Lecithin:Cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc Med. 2010;20:50–53. doi: 10.1016/j.tcm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. Lecithin: Cholesterol acyltransferase--from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2009;16:163–171. doi: 10.1097/med.0b013e328329233b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rashid S, Patterson BW, Lewis GF. Thematic review series: Patient-oriented research. What have we learned about hdl metabolism from kinetics studies in humans? J Lipid Res. 2006;47:1631–1642. doi: 10.1194/jlr.R600008-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Kane JP, Malloy MJ. Prebeta-1 hdl and coronary heart disease. Curr Opin Lipidol. 2012;23:367–371. doi: 10.1097/MOL.0b013e328353eef1. [DOI] [PubMed] [Google Scholar]
- 6.Calabresi L, Simonelli S, Gomaraschi M, Franceschini G. Genetic lecithin:Cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis. 2012;222:299–306. doi: 10.1016/j.atherosclerosis.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Rousset X, Vaisman B, Auerbach B, Krause BR, Homan R, Stonik J, Csako G, Shamburek R, Remaley AT. Effect of recombinant human lecithin cholesterol acyltransferase infusion on lipoprotein metabolism in mice. J Pharmacol Exp Ther. 2010;335:140–148. doi: 10.1124/jpet.110.169540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glomset JA. The plasma lecithins:Cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 9.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czarnecka H, Yokoyama S. Regulation of cellular cholesterol efflux by lecithin:Cholesterol acyltransferase reaction through nonspecific lipid exchange. J Biol Chem. 1996;271:2023–2028. doi: 10.1074/jbc.271.4.2023. [DOI] [PubMed] [Google Scholar]
- 11.Sethi AA, Sampson M, Warnick R, Muniz N, Vaisman B, Nordestgaard BG, Tybjaerg-Hansen A, Remaley AT. High pre-beta1 hdl concentrations and low lecithin: Cholesterol acyltransferase activities are strong positive risk markers for ischemic heart disease and independent of hdl-cholesterol. Clin Chem. 2010;56:1128–1137. doi: 10.1373/clinchem.2009.139931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunnen S, Van Eck M. Lecithin:Cholesterol acyltransferase: Old friend or foe in atherosclerosis? J Lipid Res. 2012;53:1783–1799. doi: 10.1194/jlr.R024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haase CL, Tybjaerg-Hansen A, Qayyum AA, Schou J, Nordestgaard BG, Frikke-Schmidt R. Lcat, hdl cholesterol and ischemic cardiovascular disease: A mendelian randomization study of hdl cholesterol in 54,500 individuals. J Clin Endocrinol Metab. 2012;97:E248–256. doi: 10.1210/jc.2011-1846. [DOI] [PubMed] [Google Scholar]
- 14.Krause BR, Remaley AT. Reconstituted hdl for the acute treatment of acute coronary syndrome. Curr Opin Lipidol. 2013;24:480–486. doi: 10.1097/MOL.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 15.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant apoa-i milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 16.Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 17.Asztalos BF, Roheim PS, Milani RL, Lefevre M, McNamara JR, Horvath KV, Schaefer EJ. Distribution of apoa-i-containing hdl subpopulations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:2670–2676. doi: 10.1161/01.atv.20.12.2670. [DOI] [PubMed] [Google Scholar]
- 18.Asztalos BF, Collins D, Cupples LA, Demissie S, Horvath KV, Bloomfield HE, Robins SJ, Schaefer EJ. Value of high-density lipoprotein (hdl) subpopulations in predicting recurrent cardiovascular events in the veterans affairs hdl intervention trial. Arterioscler Thromb Vasc Biol. 2005;25:2185–2191. doi: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 19.Amar MJ, Shamburek RD, Vaisman B, Knapper CL, Foger B, Hoyt RF, Jr, Santamarina-Fojo S, Brewer HB, Jr, Remaley AT. Adenoviral expression of human lecithin-cholesterol acyltransferase in nonhuman primates leads to an antiatherogenic lipoprotein phenotype by increasing high-density lipoprotein and lowering low-density lipoprotein. Metabolism. 2009;58:568–575. doi: 10.1016/j.metabol.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanigawa H, Billheimer JT, Tohyama J, Fuki IV, Ng DS, Rothblat GH, Rader DJ. Lecithin: Cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation. 2009;120:160–169. doi: 10.1161/CIRCULATIONAHA.108.825109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeg JM, Santamarina-Fojo S, Berard AM, Cornhill JF, Herderick EE, Feldman SH, Haudenschild CC, Vaisman BL, Hoyt RF, Jr, Demosky SJ, Jr, Kauffman RD, Hazel CM, Marcovina SM, Brewer HB., Jr Overexpression of lecithin:Cholesterol acyltransferase in transgenic rabbits prevents diet-induced atherosclerosis. Proc Natl Acad Sci U S A. 1996;93:11448–11453. doi: 10.1073/pnas.93.21.11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou MSJ, Kelley K, Fordstrom P, Chan J, Tonn G, Carlson T, Retter M, Meininger D, Cheng J, Gates A, Woodward A, Delaney J, Zhang R, Tang J, Liu Q, Cao P, Luchoomun J, Voogt J, Turner S, ]Shan B, Boone T, Rudel L, Schwarz M. Lecithin cholesterol acyltransferase promotes reverse cholesterol transport and attenuates atherosclerosis progression in new zealand white rabbits. Circulation. 2009;120:S1175. [Google Scholar]
- 23.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via abca1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J Lipid Res. 2004;45:1594–1607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Lipoproteins A, Cole TG, Contois JH, Csako G, McConnell JP, Remaley AT, Devaraj S, Hoefner DM, Mallory T, Sethi AA, Warnick GR Vascular Diseases Division Working Group on Best P. Association of apolipoprotein b and nuclear magnetic resonance spectroscopy-derived ldl particle number with outcomes in 25 clinical studies: Assessment by the aacc lipoprotein and vascular diseases division working group on best practices. Clin Chem. 2013;59:752–770. doi: 10.1373/clinchem.2012.196733. [DOI] [PubMed] [Google Scholar]
- 26.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho KH, Shin DG, Baek SH, Kim JR. Myocardial infarction patients show altered lipoprotein properties and functions when compared with stable angina pectoris patients. Exp Mol Med. 2009;41:67–76. doi: 10.3858/emm.2009.41.2.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahsan L, Ossoli AF, Freeman LA, Vaisman BL, Amar MJ, Shamburek RD, Remaley AT. Role of lecithin: Cholesterol acyltransferase in hdl metabolism and atherosclerosis. In: Komoda T, editor. The hdl handbook: Biological functions and clinical implications. New York: Elsevier; 2014. pp. 159–194. [Google Scholar]
- 31.Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L, Jessup W. Hdl particle size is a critical determinant of abca1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116:1133–1142. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- 32.Duivenvoorden R, Holleboom AG, van den Bogaard B, Nederveen AJ, de Groot E, Hutten BA, Schimmel AW, Hovingh GK, Kastelein JJ, Kuivenhoven JA, Stroes ES. Carriers of lecithin cholesterol acyltransferase gene mutations have accelerated atherogenesis as assessed by carotid 3.0-t magnetic resonance imaging [corrected] J Am Coll Cardiol. 2011;58:2481–2487. doi: 10.1016/j.jacc.2010.11.092. [DOI] [PubMed] [Google Scholar]
- 33.Zhang AH, Gao S, Fan JL, Huang W, Zhao TQ, Liu G. Increased plasma hdl cholesterol levels and biliary cholesterol excretion in hamster by lcat overexpression. FEBS Lett. 2004;570:25–29. doi: 10.1016/j.febslet.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Van Craeyveld E, Lievens J, Jacobs F, Feng Y, Snoeys J, De Geest B. Apolipoprotein a-i and lecithin:Cholesterol acyltransferase transfer induce cholesterol unloading in complex atherosclerotic lesions. Gene Ther. 2009;16:757–765. doi: 10.1038/gt.2009.8. [DOI] [PubMed] [Google Scholar]
- 35.Miida T, Obayashi K, Seino U, Zhu Y, Ito T, Kosuge K, Hirayama S, Hanyu O, Nakamura Y, Yamaguchi T, Tsuda T, Saito Y, Miyazaki O, Okada M. Lcat-dependent conversion rate is a determinant of plasma prebeta1-hdl concentration in healthy japanese. Clin Chim Acta. 2004;350:107–114. doi: 10.1016/j.cccn.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Foger B, Chase M, Amar MJ, Vaisman BL, Shamburek RD, Paigen B, Fruchart-Najib J, Paiz JA, Koch CA, Hoyt RF, Brewer HB, Jr, Santamarina-Fojo S. Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. The Journal of Biological Chemistry. 1999;274:36912–36920. doi: 10.1074/jbc.274.52.36912. [DOI] [PubMed] [Google Scholar]
- 37.Solajic-Bozicevic N, Stavljenic-Rukavina A, Sesto M. Lecithin-cholesterol acryltransferase activity in patients with coronary artery disease examined by coronary angiography. Clin Investig. 1994;72:951–956. doi: 10.1007/BF00577734. [DOI] [PubMed] [Google Scholar]
- 38.Dullaart RP, Perton F, Sluiter WJ, de Vries R, van Tol A. Plasma lecithin: Cholesterol acyltransferase activity is elevated in metabolic syndrome and is an independent marker of increased carotid artery intima media thickness. J Clin Endocrinol Metab. 2008;93:4860–4866. doi: 10.1210/jc.2008-1213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.