Abstract
Objective
Acute kidney injury (AKI) occurs commonly in children following congenital cardiac surgery with cardiopulmonary bypass (CPB) and has been associated with increased morbidity and mortality. Aminophylline, a methylxanthine nonselective adenosine receptor antagonist, has been effective in the management of AKI in certain populations. This study sought to determine if post-operative administration of aminophylline attenuates AKI in children undergoing congenital cardiac surgery with CPB.
Design
Single-center, double-blinded, placebo-controlled, randomized clinical trial (RCT).
Setting
Tertiary center, pediatric cardiovascular intensive care unit.
Patients
144 children after congenital heart surgery with CPB.
Interventions
Seventy-two patients were randomized to receive aminophylline and 72 patients received placebo. Study drug was administered every six hours for 72 hours.
Measurements and Main Results
The primary outcome variable was development of any AKI, defined by the serum creatinine criteria of the Kidney Diseases: Improving Global Outcomes (KDIGO) criteria. Secondary outcomes included the development of severe AKI, time between CVICU admission and first successful extubation, percent fluid overload, total fluid balance, urine output, bioelectrical impedance, and serum neutrophil gelatinase-associated lipocalin (NGAL).
The unadjusted rate and severity of AKI were not different between groups; 43/72 (60%) of the treatment group and 36/72 (50%) of the placebo group developed AKI (p=0.32). Stage 2/3 AKI occurred in 23/72 (32%) of the treatment group and 15/72 (21%) of the placebo group (p=0.18). Secondary outcome measures also demonstrated no significant difference between treatment and placebo groups. Aminophylline administration was safe; no deaths occurred in either group, and rates of adverse events were similar (14% in the treatment group versus 18% in the placebo group, p =0.30).
Conclusions
In this placebo-controlled RCT, we found no effect of aminophylline to prevent AKI in children recovering from cardiac surgery performed with CPB. Future study of pre-operative aminophylline administration to prevent AKI may be warranted.
Keywords: Aminophylline, Acute Kidney Injury, Congenital Heart Defect, Cardiopulmonary Bypass, Randomized Controlled Trial, Intensive Care Unit, Pediatric
Introduction
Acute kidney injury (AKI) occurs commonly in hospitalized children and has been associated with increased morbidity and mortality (1–6). AKI is especially common in children with congenital heart defects following cardiac surgery and cardiopulmonary bypass (CPB), with the incidence ranging from 28–51% (2, 4–7). Specifically in this population, the presence of AKI has been associated with increased hospital length of stay (LOS), prolonged need for mechanical ventilation, greater hospital cost, and increased mortality (2, 4, 7–9). Importantly, even a small rise in serum creatinine adversely affects outcomes in children and adults after cardiac surgery with CPB (2, 8, 9).
AKI following cardiac surgery is due to CPB-induced ischemia-reperfusion injury (IRI), inflammation induced by exposure to the bypass circuit, hypotension, hemolysis, and exposure to nephrotoxic medications (10, 11). Recent data suggest that the systemic inflammatory response syndrome after CPB may result, in part, from adenosine subtype receptor hyper-expression (12).
Aminophylline and theophylline, methylxanthine nonselective adenosine receptor antagonists, have been effective in the management of AKI in certain clinical scenarios including heart failure, calcineurin inhibitor toxicity, and perinatal asphyxia (13–22). In the kidney, adenosine constricts the afferent arteriole and decreases glomerular blood flow; adenosine receptor blockade mitigates this vasoconstriction. Aminophylline also inhibits phosphodiesterase (PDE) at higher concentrations, which leads to increased urine output. Animal studies of aminophylline suggest a beneficial redistribution of myocardial blood flow leading to increased ventricular contractility and cardiac output (23, 24).
We hypothesized that aminophylline could provide adenosine receptor blockade and improve glomerular blood flow, therefore preventing the development of AKI associated with CPB. This single-center, double-blinded, placebo-controlled RCT was designed to determine if post-operative administration of aminophylline would attenuate AKI in children with congenital heart defects undergoing CPB and cardiac surgery.
Materials and Methods
Design
This is a double-blinded, placebo-controlled RCT performed at a single center, Lucile Packard Children’s Hospital Stanford.
Patients
Eligible subjects included all patients < 18 years of age with congenital heart defects undergoing cardiac surgery with CPB. To ensure the safest oversight for the duration of the study drug infusion, we only approached patients for consent if their anticipated cardiovascular intensive care unit (CVICU) stay would likely be at least 72 hours (based on locally-derived length of stay information). Patients were recruited in the pre-operative clinic or in the inpatient ward/ICU; the nature of the consent process for this interventional drug trial necessitated that all procedures were elective or scheduled. Because aminophylline has been associated with tachycardia and seizures at high serum levels, and its metabolism may be affected by liver or thyroid dysfunction and sepsis, we selected the following exclusion criteria: history of tachyarrhythmias, seizures, aspartate aminotransferase or alanine aminotransferase > 3 times normal, coagulopathy (International Normalized Ratio > 1.5 while not taking warfarin), sepsis, fever (>102 degrees F), or hypothyroidism. We also excluded cardiac transplant recipients (due to drug interaction with calcineurin inhibitors), neonates < 36 weeks corrected gestational age (due to immature organ development and glomerular filtration), those receiving aminophylline or theophylline, and those requiring pre-operative renal replacement therapy (RRT) or extracorporeal membrane oxygenation (ECMO) because the volume of distribution is altered by these extracorporeal systems. Study investigators or research nurses recruited participants; written, informed consent was signed by each subject’s parent or guardian. This study was approved by the Stanford University Institutional Review Board and registered with clincialtrials.gov (identifier NCT01245595).
Intervention
In both groups, the study drug was ordered by a physician upon post-operative admission to the CVICU. Study drug was initiated when hemodynamics were stable and the bedside nurse had completed the CVICU admission documentation (typically within four hours).
The pharmacist used a permuted block randomization schedule for the intervention (“aminophylline”) or placebo (“normal saline”) group. A stratified randomization was performed for subjects < three months of age to insure that equal numbers of these subjects were assigned to each study group.
The treatment group received aminophylline 5 milligrams per kilogram intravenous (IV) load over 30 minutes, followed by 1.8 milligrams per kilogram IV every six hours, for 72 hours (total 13 doses).
The control group received placebo bolus followed by IV infusions of normal saline (0.9% NS) every six hours (matched by volume and appearance to the treatment group), for 72 hours.
Aminophylline is a clear, colorless solution with a concentration of 25 milligrams per milliliter. Since aminophylline is a compound of theophylline with ethylenediamine (to improve solubility), the clinical laboratory measures trough theophylline levels. Daily theophylline trough levels were assessed for the duration of study drug administration (total 3 levels, for both groups) and securely faxed directly to the pharmacist, without entry into the electronic medical record. The pharmacist performed dose adjustments based on a sliding scale (Table 1). The pharmacist provided syringes of aminophylline or placebo of equal volume and adjusted the concentration (not volume) of aminophylline syringes to maintain a trough theophylline level of 5–7 mcg/mL. Because the concentration of the solution was altered and the volume remained unchanged, providers and parents were unable to determine which patients had dose adjustments performed (only the pharmacist was aware if the concentration was adjusted). Patients, parents, physicians, and nurses were blinded to study-group assignment.
Table 1.
Aminophylline dose sliding scale. All changes were made by the pharmacist; aminophylline concentration was adjusted and study drug volume remained constant.
| Theophylline Level (mcg/mL) | Dose Adjustment |
|---|---|
| <2 | Increase subsequent doses by 50% |
| 2–2.9 | Increase subsequent doses by 33% |
| 3–3.9 | Increase subsequent doses by 25% |
| 4–4.9 | Increase subsequent doses by 15% |
| 5–7 (Goal) | Target Level; No dose adjustment |
| 7.1–8.4 | Decrease subsequent doses by 10% |
| 8.5–9.9 | Decrease subsequent doses by 15% |
| 10–12.4 | Decrease subsequent doses by 25% |
| 12.5–14.9 | Decrease subsequent doses by 50% |
| 15–19.9 | Decrease subsequent doses by 67% |
| >19.9 | Discontinue all aminophylline doses. Contact Medical Monitor. |
Outcomes and Measurements
The primary outcome variable was the development of AKI in the first five postoperative days. AKI was diagnosed and staged by the Kidney Diseases: Improving Global Outcomes (KDIGO) AKI criteria (25); only serum creatinine criteria were used. Stage 1 AKI was defined as an elevation in serum creatinine by >/= 0.3 mg/dl or 1.5–1.9 times above pre-operative baseline, Stage 2 AKI as an increase in serum creatinine by 2–2.9 times above baseline, and Stage 3 AKI as an increase in serum creatinine by 3 times above baseline, a decrease in eGFR to <35 ml/min per 1.73 m2, or initiation of (RRT). The decision to initiate RRT was made by the pediatric cardiac intensivists and consulting pediatric nephrologist, per local standard of care. Serum creatinine was assessed per local standard of care; we recorded creatinine from the first five postoperative days. Estimated glomerular filtration rate was calculated using the Schwartz formula (k x height in centimeters/serum creatinine; k= 0.45 for age <1 year, 0.55 for females >1 year and males 1–12 years, and 0.7 for males > 12 years) (26).
Secondary outcomes included the development of severe AKI (KDIGO Stage ≥ 2), median time between post-operative CVICU admission and first successful endotracheal extubation, mean percent fluid overload [(total intake – total output/weight x 100], total fluid balance, urine output, and inotropic support (modified vasoactive inotropes score, evaluated at 0700 and 1900 on each post-operative day). We also assessed bioelectrical impedance as a noninvasive proxy for total body water which has been previously validated in this population (27, 28). Bioelectrical impedance (RJL Systems, Clinton Township, MI) was measured before CPB and daily in the CVICU.
Finally, we measured serum NGAL every 12 hours following admission to the CVICU (total six levels). NGAL was measured with the Alere Triage™ (San Diego, CA) point of care test, per guidelines from the product insert.
Patient Safety
The trial was monitored by a data safety monitoring board (DSMB) comprised of a pediatric cardiologist, biostatistician, pediatric cardiothoracic surgeon, and pediatric intensivist from outside institutions. The DSMB had access to un-blinded data.
Two physicians at Lucile Packard Children’s Hospital Stanford managed toxic theophylline trough levels (> 20 mcg/mL).
Statistical Analysis
Sample size calculations were based on the primary outcome measure of KDIGO-defined AKI. We estimated a 35% incidence of AKI in this population based on the existing literature (2, 4, 10, 11). We determined that an absolute effect size of 20% (i.e. decreasing AKI incidence from 35% to 15%) would represent a clinically-meaningful reduction in the incidence of AKI in our population. Using an alpha (two sided) of 0.05 and beta of 0.2, 72 patients in each group (144 patients total) were required to demonstrate a decrease in AKI rate from 35% to 15%. Using an intention-to-treat analysis, the final analysis included all subjects based on their randomization groups. A subgroup analysis was performed on patients less than three months old, however, the study was not powered to evaluate this group individually.
Linear mixed-effects regression analyses were performed to estimate changes in select outcomes over time. Comparisons between treatment groups and baseline categorical variables were assessed using chi-square tests. If baseline continuous data were not normally distributed, comparisons between treatment groups and the continuous variable were analyzed using non-parametric tests, and data were presented as median (IQR, intra-quartile range). Unless otherwise indicated, data are presented as mean +/− standard deviation (SD). Comparisons of unadjusted proportions and severity of AKI between treatment groups were calculated using chi-square tests, although all adjusted models of AKI were analyzed using logistic regression and adjusted for the stratification factor of age < 3 months. Analyses were performed using R statistical software (R Development Core Team, Vienna, Austria).
Results
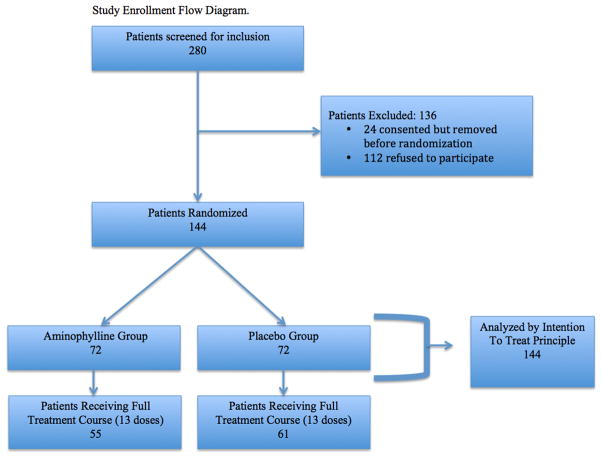
Between December 2010 and April 2014, 280 subjects were screened for inclusion and 168 (62%) consented. Twenty-four (14%) of the 168 consented subjects had complications during surgery and were withdrawn. Indications for withdrawal included: intra-operative tachyarrhythmias (n=5), hemodynamic instability (n=8), requirement of ECMO (n=1), intra-operative decision not to use CPB as planned (n=2), or surgeon preference (n=8). The remaining 144 subjects (72 placebo and 72 treatment) comprise the basis for the analysis (Figure 1). The average age was 2.3 years and median weight 5.8 (IQR 4.1–12.8) kilograms; 81 males (56%) were enrolled. The majority of cardiac procedures (102/144) were RACHS-1 category 3 or 4. Baseline pre- and postoperative characteristics were similar, with the exception of prior cardiac surgeries, which were more prevalent in the treatment group (Table 2). Fifteen patients in the treatment group and 17 in the placebo group had a cardiac catheterization an average of 2.9 days (for both groups) before cardiac surgery, with an average of 4.9 and 5.1 milliliters per kilogram of contrast administered to the treatment and placebo groups, respectively.
Figure 1.
Study enrollment flow diagram
Table 2.
Demographics
| Placebo N=72 |
Treatment N=72 |
Total N=144 |
p value | |
|---|---|---|---|---|
|
| ||||
| Gender | ||||
| M | 42 | 39 | 81 | Reference |
| F | 30 | 33 | 63 | 0.61 |
|
| ||||
| Weight (kilograms) | ||||
| Median (IQR) | 5.7 (4.2–14.0) | 5.8 (4.0–10.7) | 0.46a | |
|
| ||||
| Race | ||||
| Latino | 20 | 26 | 46 | Reference |
| Asian | 11 | 7 | 18 | 0.21 |
| Black | 2 | 2 | 4 | 0.80 |
| White | 37 | 35 | 72 | 0.40 |
| Other/Unknown | 2 | 2 | 4 | 0.86 |
|
| ||||
| Preop Inpatient | 24 | 27 | 51 | 0.6 |
|
| ||||
| Preop Inotrope Use | 2 | 5 | 7 | 0.23 |
|
| ||||
| Preop Intubation | 7 | 7 | 14 | 1.0 |
|
| ||||
| Age at surgery (days) | ||||
| Median (IQR) | 165 (58–1332) | 154 (64–656) | 0.59a | |
|
| ||||
| Prior Cardiac Surgery | ||||
| Yes | 18 | 32 | 50 | Reference |
| No | 54 | 40 | 94 | 0.02 |
|
| ||||
| RACHS-1 score | ||||
| 1 | 0 | 1 | 1 | |
| 2 | 17 | 20 | 37 | Reference |
| 3 | 29 | 25 | 54 | 0.40 |
| 4 | 23 | 25 | 48 | 0.77 |
| 5 | 3 | 1 | 4 | 0.28 |
|
| ||||
| Single Ventricle (n) | 3 | 6 | 9 | 0.3 |
|
| ||||
| CPB time (min) | 153 (93–220) | 133 (84–201) | 0.22a | |
|
| ||||
| Cross-clamp time (min) | 45 (30–96) | 52 (29–83) | 0.44a | |
|
| ||||
| Age at CVICU admit (days) Median (IQR) | 165 (58–1333) | 154 (50–656) | 0.57a | |
|
| ||||
| Chest Open | ||||
| No | 62 | 59 | 121 | Reference |
| Yes | 10 | 13 | 23 | 0.50 |
Mann-Whitney-Wilcoxon Test
IQR: interquartile range; RACHS-1: Risk Adjusted Congenital Heart Surgery-1; CPB: cardiopulmonary bypass; CVICU: cardiovascular intensive care unit.
Of the 144 subjects, 116 (81%) received a full treatment course of study drug (loading dose followed by 12 maintenance doses over 72 hours). Study drug was discontinued in 17/72 (24%) subjects in the treatment group for the following reasons: catheters removed preventing assessment of trough levels (n=8), clinical team decided to remove patient from the study (n=6), physician decision to initiate aminophylline outside of the study protocol (n=2), and cardiopulmonary arrest (n=1). Study drug was discontinued in 11/72 (15%) subjects in the placebo group for the following reasons: clinical team decided to remove patient from the study (n=3), catheters removed preventing assessment of trough levels (n=3), parent preference (n=2), cardiopulmonary arrest requiring ECMO (n=1), and surgeon preference (n=2). There was no difference in the rate of study discontinuation between the two groups (24% versus 15%, p =0.29), and patients with study drug interruptions were analyzed by intention to treat analysis for all primary and secondary endpoints. The patient demographics were not significantly different between patients with study drug interruption versus patients with complete study drug courses, with the exception of CPB time (125 minutes versus 166 minutes, respectively; p=0.005). Patients with study drug interruptions were analyzed separately, and no differences were found in AKI rates between the treatment and placebo groups. When only patients who completed a full study drug treatment course were analyzed, our findings were unchanged. The average theophylline trough for the treatment group was 6.8 ± 2.3 mcg/mL; all patients in the placebo group had undetectable theophylline levels.
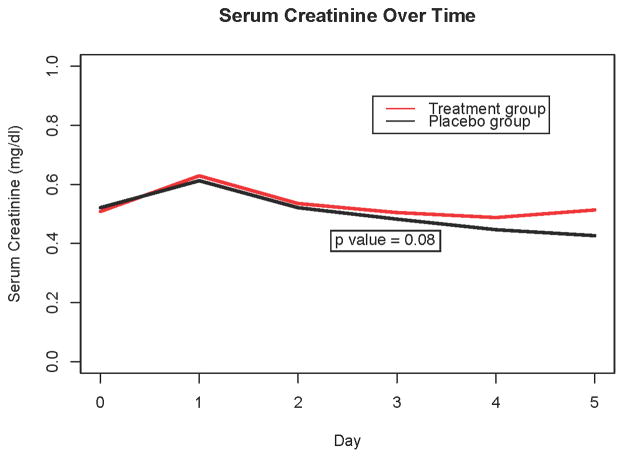
Overall, 79/144 (55%) had AKI by KDIGO serum creatinine criteria. AKI incidence was not different between groups, and there was no significant difference in any AKI after adjusting for age or in the development of severe AKI between groups (Figure 2). Both groups had similar daily estimated glomerular filtration rates and daily creatinine values (Figure 3). There was no difference in the change in creatinine from baseline to 72 hours between groups (p=0.36). There was no difference in the use of RRT between groups (11 in the placebo group versus 6 in the treatment group, p=0.30). Serum NGAL levels revealed no significant difference between groups; ten patients in each group (14%) had NGAL levels >150 ng/mL.
Figure 2. Acute Kidney Injury rates by treatment group.
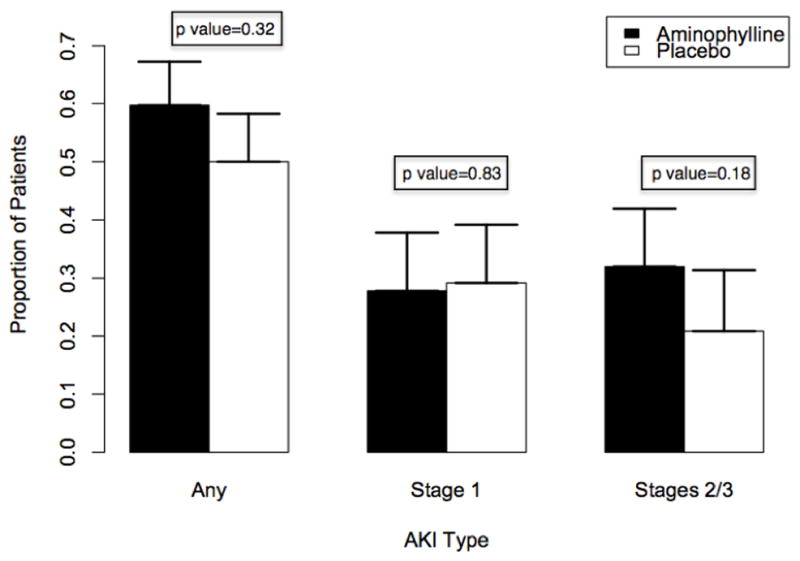
P values reflect comparison of each group with no AKI using a chi-square test. Error bars indicate standard error.
Figure 3. Serum creatinine over time.
Comparison of serum creatinine values in aminophylline versus placebo groups over time, using a mixed-effects regression analysis. Data reflect longitudinal trends rather than individual time-point differences.
For the population less than 3 months old, we found no difference in AKI rates between groups; the treatment group had 15 patients with AKI (7 Stage 1, 8 Stage 2/3) and the 9 patients with no AKI, while the placebo group had 15 patients with AKI (11 Stage 1, 4 Stage 2/3) and 10 patients with no AKI. Statistically, there was no difference in rates of “Any AKI” (p=1.0), “Stage 1 AKI” (p=0.85), or “Stage 2/3 AKI” (p=0.5).
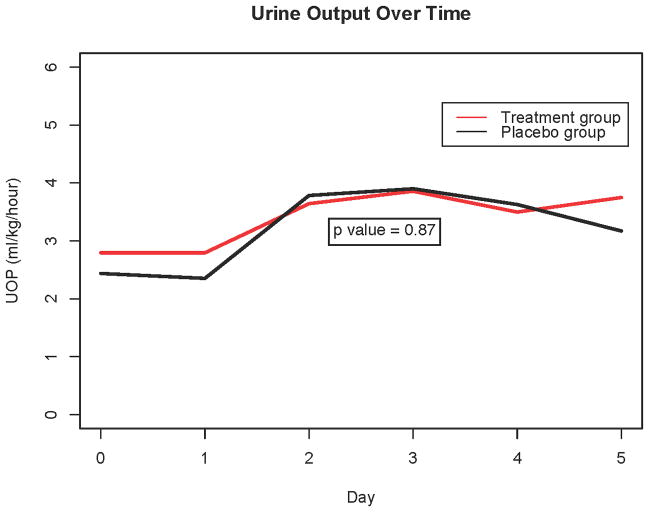
Both groups exhibited a similar increase in urine output in the first five postoperative days; for each post-operative day, there was a 0.2 mL/kg/hour increase in urine output in the placebo group, and a 0.18 mL/kg/hour increase in the treatment group (p=0.87, Figure 4). Percent fluid overload (FO) was not different between groups, as the average FO for each additional post-operative day for the treatment group was −14% and −13% for the placebo group (p = 0.88). Bioimpedance was similar between groups, as the treatment group had an increase of 27 resistance units (RU) per day and the placebo group had an increase of 14 RU (p=0.06). Diuretic dosing was not different between the two groups (comparison between groups for furosemide dosing over time: p=0.26; for chlorothiazide dosing over time: p=0.12).
Figure 4. Urine output over time.
Comparison of urine output (UOP) in aminophylline versus placebo groups over time, using a mixed-effects regression analysis. Data reflect longitudinal trends rather than individual time-point differences.
Secondary outcome measures of CVICU illness severity indicated no significant impact of aminophylline treatment. Specifically, there was no difference in time from post-operative CVICU admission to first successful extubation between the treatment and placebo groups (median 3, IQR 1–9 days versus median 3, IQR 1–5 days, p=0.42), and the modified vasoactive-inotrope score was not different between groups (treatment group median 5, IQR 0–8.5 versus placebo group median 5.5, IQR 0–10, p = 0.36).
No deaths occurred during the study period. Eleven adverse events occurred in 10 patients in the treatment group (14%) and 13 adverse events occurred in 13 patients in the placebo group (18%) (p = 0.30; Table 3).
Table 3.
Adverse events.
| Placebo (n) | Treatment (n) | Total (n) | |
|---|---|---|---|
| Adverse Event | |||
| Accelerated junctional rhythm# | 0 | 2 | 2 |
| Junctional ectopic tachycardia# | 2 | 4 | 6 |
| Ventricular tachycardia | 0 | 1 | 1 |
| Ectopic atrial tachycardia | 2 | 0 | 2 |
| Sinus tachycardia | 1 | 1 | 2 |
| Unspecified tachycardia | 1 | 1 | 2 |
| Low cardiac output requiring CPR or ECMO | 1 | 1 | 2 |
| Cardiac arrhythmia leading to CPR | 1 | 0 | 1 |
| Low cardiac output with pulmonary hypertension | 1 | 0 | 1 |
| AKI* | 1 | 1 | 2 |
| Bleeding | 1 | 0 | 1 |
| Sepsis | 1 | 0 | 1 |
| Arm and jaw pain | 1 | 0 | 1 |
| Total (any event) | 13^ | 11^ | 24 |
We differentiated accelerated junctional rhythm (hemodynamically insignificant, not requiring medication) from junctional ectopic tachycardia (hemodynamically significant or requiring medication)
AKI refers to acute kidney injury for which the managing intensivist initiated aminophylline outside of the study protocol.
p = 0.3
CPR: cardiopulmonary resuscitation; ECMO: extracorporeal membrane oxygenation
Discussion
In our study, AKI occurred in 55% of children undergoing congenital cardiac surgery with CPB; this is a rate consistent with prior studies using updated, relative creatinine change-based definitions. However, post-operative administration of aminophylline did not reduce the incidence of AKI in children who required CPB during cardiac surgery. This was true even when AKI was stratified by severity stage. Furthermore, aminophylline did not have an impact on urine output, fluid balance, or time from post-operative CVICU admission to extubation. Aminophylline was safe in this medically-complex population, as rates of serious adverse events were similar between the treatment and placebo groups, and no significant complications were directly attributable to aminophylline administration.
The majority of studies showing improved renal function with adenosine receptor blockade were performed in neonates. Three RCTs of term neonates with birth asphyxia found that theophylline improved glomerular function and creatinine clearance (14, 18, 29); additionally, Jenik et al. found aminophylline use was associated with reduced fluid overload (18). Mazkereth et al. studied the tubular effects of aminophylline in the developing kidney of premature neonates. The loading dose resulted in increased diuresis, and analysis of urinary electrolytes suggested that tubular reabsorption is targeted (21). Cattarelli et al. randomized 50 preterm neonates with respiratory distress to receive three days of theophylline therapy versus placebo; patients receiving theophylline had higher urine output, a lower incidence of oligo-anuria, and lower serum creatinine at 24 hours (19).
Similarly, adenosine receptor blockade has shown benefit in older children with diverse causes of kidney injury. In a prospective, observational study of ten oliguric children in an ICU, theophylline increased urine output from 1.58 to 3.75 mL/kg/hour (13). Similar to our study, theophylline levels were maintained at 5 mcg/mL. Another study of a pediatric ICU population demonstrated that a single dose of aminophylline resulted in increased urine output with no significant change in heart rate or blood pressure (22). In a study of 24 infants receiving ECMO support, theophylline in combination with furosemide resulted in higher urine output but no significant improvement in renal function or creatinine clearance (30). A retrospective study of ten patients receiving tacrolimus demonstrated that aminophylline administration was associated with increased urine output, presumably due to prevention of tacrolimus-induced renal vasoconstriction (20). A subsequent prospective trial of 18 children with tacrolimus-induced nephrotoxicity resulted in improved urine output, but it was not powered to detect an increase in creatinine clearance (31).
In heart failure, studies of the adenosine A1-receptor antagonist, rolofylline, have been less favorable. The PROTECT trial was a RCT of rolofylline in adults with acute heart failure and renal dysfunction. In this pilot, dose-finding study, patients receiving rolofylline had trends toward improved dyspnea and lower rates of worsening heart failure and renal dysfunction (32). However, rolofylline did not prevent worsening renal dysfunction or volume overload in a subsequent phase III clinical trial of 2000 adult patients (33). While rolofylline provides selective adenosine A1-receptor blockade, aminophylline is a nonselective adenosine receptor antagonist; whether differential receptor blockade will impact renal dysfunction is speculative (34).
Our prior retrospective study detailed aminophylline use in children with AKI in the CVICU. We found that aminophylline augmented urine output and improved creatinine clearance (17). In that study, children developed AKI at various times in the CVICU, and therefore the causes of their AKI were likely more heterogeneous than the current study. These patients experienced ongoing renal injury with AKI related to sepsis, hypotension, acute tubular necrosis (ATN), and nephrotoxin exposure. In contrast, all AKI events in the current study were related to the single, episodic injury of IRI or CPB. Thus, the patients in our prospective trial were quite different than those in previously published, positive studies.
There are other factors contributing to the neutral results of our study. First, for safety reasons, we did not administer aminophylline until subjects returned to the CVICU after surgery. Therefore, the renal injury (from cardiopulmonary bypass and cardiac surgery) occurred before we initiated therapy to prevent development of AKI (as defined by the KDIGO guidelines). All subjects were administered study drug within 4 hours of CVICU admission, but up to ten hours after the initiation of CPB. A prior biomarker study of AKI demonstrated renal injury and elevation of NGAL levels within two hours after the initiation of CPB (35); it is possible that administration of aminophylline prior to CPB would produce a protective effect. Similar to current practice with N-acetyl cysteine protocols for contrast-induced nephropathy, pre-treatment of AKI-prone children before their anesthetic induction and initiation of CPB may mitigate AKI in the post-operative period. However, due to the constraints of coordinating an interventional drug trial with a medication not yet shown to be safe in this fragile population, we did not think it was prudent at the time the study was planned to treat subjects prophylactically before the initiation of CPB. At a minimum, our study demonstrates that aminophylline is safe, and that pre-operative administration would be a reasonable treatment strategy for future clinical trials.
We also excluded patients that were tachycardic with arterial hypotension upon arrival to the CVICU or whom the attending surgeon anticipated hemodynamic instability (defined as receiving intervention designed to raise systemic blood pressure); this decision was to ensure the safety of our patients while receiving a medication previously unstudied in this population. However, this exclusion may have withdrawn patients who were likely to experience significant AKI. Furthermore, it is possible that a specific study of neonates may show a benefit of aminophylline administration, since neonatal renal perfusion is a fraction of that in older children due to higher intra-renal vascular resistances and reduced cortical blood flow (36).
Additionally, creatinine is a less sensitive and late marker of injury, and it is possible that a renal injury biomarker panel would better identify patients who developed true injury. Our study was not powered to detect a difference in NGAL levels, the biomarker we used to assess renal injury.
Importantly, aminophylline administration was safe and did not result in an increased incidence of serious adverse events. While we excluded subjects with a history of seizures or tachyarrhythmias, patients receiving aminophylline did not experience more frequent side effects. A pharmacodynamic study of aminophylline suggested that nausea, tachycardia, and seizures are more likely to occur when serum theophylline levels reach > 20 mcg/mL (37); our target level of 5–7 mcg/mL appears safe in the pediatric cardiac population after CPB.
Several factors limited our study. In defining AKI, we used only KDIGO serum creatinine criteria; urine output criteria were not available in the six-hour timeframe used for KDIGO urine output criteria. While our subjects were randomized in a blocked and evenly distributed fashion, baseline differences between the two groups did exist. Subjects receiving aminophylline had a greater number of prior cardiac surgeries, presumably by chance, since the blocked randomization patter was strictly followed and no alterations in patient randomization occurred based on preoperative status. Because repeated renal insults may contribute to AKI, this difference may have resulted in a higher than expected rate of AKI in the aminophylline group. Thus, the fact that there were more patients with prior cardiac surgery in the treatment group could bias our results towards the null. Similarly, we screened subjects who were anticipated to be in the CVICU for three days or more; generalizability to a less acute patient population cannot be assumed. All subjects who were randomized and received study drug were analyzed with an intention-to-treat analysis, and 81% of patients received a full-treatment course. Although these premature discontinuations may affect the incidence of AKI in the two groups, the research protocol approximates the behavior of clinicians in a complex CVICU population. Finally, the subjects in our study were enrolled over four years, which may expose our findings to an era effect.
Conclusions
In summary, this is the first double-blinded, placebo-controlled RCT of aminophylline to prevent AKI in children recovering from cardiac surgery with CPB. Our study does not support the early post-operative use of aminophylline to prevent AKI. However, aminophylline is safe in this population and may have a role as a pre-operative therapy. Future study of pre-operative aminophylline administration to prevent AKI may be warranted.
Acknowledgments
The project described in this manuscript was supported by the Stanford Child Health Research Institute and the NIH-NCATS-CTSA grant # UL1 TR001085. Dr. David Axelrod had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank: Sara Sherman-Levine CPNP and Aihua Zhu MD for recruiting patients and collecting data; Steven Chinn PharmD for coordinating randomization and study drug preparation; Howard Rosenfeld MD (Chair), Ana-Lia Graciano MD, Teimour Nasirov MD, and Ginny Gildengorin PhD for serving on the DSMB; Carol Conrad MD and Felice Su MD for serving as independent medical monitors; the nurses and physicians who supported this study; and the patients and families who participated in the research protocol.
Footnotes
This trial was performed at Lucile Packard Children’s Hospital Stanford, Stanford University Medical Center.
The authors report no relevant conflicts of interest.
References
- 1.Lafrance J-P, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol JASN. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zappitelli M, Bernier P-L, Saczkowski RS, et al. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76:885–892. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care Lond Engl. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care Lond Engl. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baskin E, Saygili A, Harmanci K, et al. Acute renal failure and mortality after open-heart surgery in infants. Ren Fail. 2005;27:557–560. doi: 10.1080/08860220500199035. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol CJASN. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 8.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol JASN. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 9.Lassnigg A, Schmid ER, Hiesmayr M, et al. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36:1129–1137. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 10.Blinder JJ, Goldstein SL, Lee V-V, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143:368–374. doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Krawczeski CD, Zappitelli M, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerbaul F, Bénard F, Giorgi R, et al. Adenosine A2A receptor hyperexpression in patients with severe SIRS after cardiopulmonary bypass. J Investig Med Off Publ Am Fed Clin Res. 2008;56:864–871. doi: 10.2310/JIM.0b013e3181788d02. [DOI] [PubMed] [Google Scholar]
- 13.Bell M, Jackson E, Mi Z, et al. Low-dose theophylline increases urine output in diuretic-dependent critically ill children. Intensive Care Med. 1998;24:1099–1105. doi: 10.1007/s001340050723. [DOI] [PubMed] [Google Scholar]
- 14.Bhat MA, Shah ZA, Makhdoomi MS, et al. Theophylline for renal function in term neonates with perinatal asphyxia: a randomized, placebo-controlled trial. J Pediatr. 2006;149:180–184. doi: 10.1016/j.jpeds.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 15.Press NJ, Gessi S, Borea PA, et al. Therapeutic potential of adenosine receptor antagonists and agonists. Expert Opin Ther Pat. 2007;17:979–991. doi: 10.1517/13543776.17.8.979. [DOI] [PubMed] [Google Scholar]
- 16.Ng GYT, Baker EH, Farrer KFM. Aminophylline as an adjunct diuretic for neonates? a case series. Pediatr Nephrol. 2004;20:220–222. doi: 10.1007/s00467-004-1692-9. [DOI] [PubMed] [Google Scholar]
- 17.Axelrod DM, Anglemyer AT, Sherman-Levine SF, et al. Initial experience using aminophylline to improve renal dysfunction in the pediatric cardiovascular ICU. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2014;15:21–27. doi: 10.1097/01.pcc.0000436473.12082.2f. [DOI] [PubMed] [Google Scholar]
- 18.Jenik AG, Ceriani Cernadas JM, Gorenstein A, et al. A randomized, double-blind, placebo-controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics. 2000;105:E45. doi: 10.1542/peds.105.4.e45. [DOI] [PubMed] [Google Scholar]
- 19.Cattarelli D, Spandrio M, Gasparoni A, et al. A randomised, double blind, placebo controlled trial of the effect of theophylline in prevention of vasomotor nephropathy in very preterm neonates with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 2006;91:F80–84. doi: 10.1136/adc.2005.073650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaughlin GE, Land MP, Rossique-Gonzalez M. Effect of aminophylline on urine flow in children with tacrolimus-induced renal insufficiency. Transplant Proc. 2000;32:817–820. doi: 10.1016/s0041-1345(00)00993-3. [DOI] [PubMed] [Google Scholar]
- 21.Mazkereth R, Laufer J, Jordan S, et al. Effects of theophylline on renal function in premature infants. Am J Perinatol. 1997;14:45–49. doi: 10.1055/s-2007-994095. [DOI] [PubMed] [Google Scholar]
- 22.Pretzlaff RK, Vardis RJ, Pollack MM. Aminophylline in the treatment of fluid overload. Crit Care Med. 1999;27:2782–2785. doi: 10.1097/00003246-199912000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Crea F, Gaspardone A, Araujo L, et al. Effects of aminophylline on cardiac function and regional myocardial perfusion: Implications regarding its antiischemic action. Am Heart J. 1994;127:817–824. doi: 10.1016/0002-8703(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 24.Rutherford JD, Vatner SF, Braunwald E. Effects and mechanism of action of aminophylline on cardiac function and regional blood flow distribution in conscious dogs. Circulation. 1981;63:378–387. doi: 10.1161/01.cir.63.2.378. [DOI] [PubMed] [Google Scholar]
- 25.Kellum JA, Lameire N KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care Lond Engl. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol CJASN. 2009;4:1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 27.Novak I, Davies PS, Elliott MJ. Noninvasive estimation of total body water in critically ill children after cardiac operations. Validation of a bioelectric impedance method. J Thorac Cardiovasc Surg. 1992;104:585–589. [PubMed] [Google Scholar]
- 28.Yamaguchi H, Yamauchi H, Hazama S, et al. Evaluation of body fluid status after cardiac surgery using bioelectrical impedance analysis. J Cardiovasc Surg (Torino) 2000;41:559–566. [PubMed] [Google Scholar]
- 29.Bakr AF. Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia--a study in a developing country. Pediatr Nephrol Berl Ger. 2005;20:1249–1252. doi: 10.1007/s00467-005-1980-z. [DOI] [PubMed] [Google Scholar]
- 30.Lochan SR, Adeniyi-Jones S, Assadi FK, et al. Coadministration of theophylline enhances diuretic response to furosemide in infants during extracorporeal membrane oxygenation: a randomized controlled pilot study. J Pediatr. 1998;133:86–89. doi: 10.1016/s0022-3476(98)70183-0. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin GE, Abitbol CL. Reversal of oliguric tacrolimus nephrotoxicity in children. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2005;20:1471–1475. doi: 10.1093/ndt/gfh785. [DOI] [PubMed] [Google Scholar]
- 32.Cotter G, Dittrich HC, Weatherley BD, et al. The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J Card Fail. 2008;14:631–640. doi: 10.1016/j.cardfail.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Voors AA, Dittrich HC, Massie BM, et al. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) J Am Coll Cardiol. 2011;57:1899–1907. doi: 10.1016/j.jacc.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 34.Yap SC, Lee HT. Adenosine and protection from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21:24–32. doi: 10.1097/MNH.0b013e32834d2ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–2309. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jose PA, Fildes RD, Gomez RA, et al. Neonatal renal function and physiology. Curr Opin Pediatr. 1994;6:172–177. doi: 10.1097/00008480-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Drug summary - MICROMEDEX® 2.0 [Internet] [cited 2013 Jan 22] Available from: http://www.thomsonhc.com.laneproxy.stanford.edu/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/1B7DA5/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/83194D/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/evidencexpert.IntermediateToDocumentLink?docId=025250&contentSetId=100&title=Aminophylline&servicesTitle=Aminophylline.