Abstract
Purpose of the review
To discuss whether vascular dysfunction and autonomic dysfunction are related to primary open-angle glaucoma stratified by the intraocular pressure (IOP) level (the high tension glaucoma and normal tension glaucoma subtypes).
Recent findings
Patients with POAG across the spectrum of IOP exhibit a variety of ocular and non-ocular vascular abnormalities. Interestingly common genetic variation in NOS3 and the CAV1/CAV2 genomic regions, which code for proteins involved in setting vascular tone, are associated with POAG. These markers seem to stratify with POAG subtypes by sex or pattern of initial visual field loss. Overall it is clear that there is also cardiovascular autonomic dysfunction in HTG and NTG but it is unclear if this dysfunction is more common in NTG compared to HTG.
Summary
Overall POAG is likely a heterogeneous disease but stratifying cases by IOP level associated with initial optic nerve damage may be less useful than using other endophenotype approaches. Embracing the evidence suggesting systemic endothelial and autonomic dysfunction are operative in POAG will help move beyond an IOP-centric view of the disease and facilitate “tearing down the wall” that divides treating physicians and a better understanding of POAG pathogenesis.
Keywords: Primary open-angle glaucoma, high-tension glaucoma, normal, tension glaucoma, vascular dysfunction, autonomic dysfunction
Introduction
In primary open angle glaucoma (POAG), there are no obvious anterior segment abnormalities and the filtration angle is physically open while the optic head is excavated and the neuroretinal rim is eroded. There are no obvious clinical clues regarding why the intraocular pressure (IOP) is elevated in the POAG subset referred to as high-tension glaucoma (HTG) cases. Furthermore, it is unclear why optic nerve pathology develops in patients in the POAG subset referred to as normal-tension glaucoma (NTG) cases. The ultimate goal in POAG is to define the disease in terms of biochemical pathways as opposed to describing it as an IOP-related optic neuropathy without obvious secondary cause. It is likely that the term POAG encompasses several diseases produced by distinct biochemical mechanisms. On an interim basis, it is reasonable to stratify POAG into HTG and NTG subtypes and ask if candidate disease mechanisms are operative at higher or lower IOP at presentation. In fact, from an experimental perspective, it makes sense to compare NTG patients to controls with similar IOP in order to explore whether any putative mechanism makes the optic nerve vulnerable to degeneration in POAG. Here we will discuss whether two pathophysiologic processes – vascular dysfunction and autonomic dysfunction – are related to POAG stratified by the IOP level associated with optic nerve degeneration.
Text of review
Vascular dysfunction in Primary Open-Angle Glaucoma
Broadly speaking, the endothelial monolayer functions at the interface between the blood and underlying interstitial tissue to modulate vascular tone, thrombus formation, cell adhesion, smooth muscle proliferation and directed sequestration of inflammatory mediators. One hypothesis is that POAG is categorized by impaired chemical endothelial signaling between both: a) the inner wall Schlemm’s canal endothelial cells as well as endothelial cells located in the ciliary body and the posterior longitudinal muscle that helps to set outflow resistance and b) the ocular vascular endothelial cell and underlying luminal smooth muscle for vessels supplying the retinal ganglion cells (RGCs). This hypothesis could explain why POAG occurs across a spectrum of IOP but it does not consider the role systemic endothelial cell dysfunction may play in the disease.
What is the evidence for endothelial cell dysfunction in POAG and what are biological mediators of this impairment? A pharmacologic intervention study in untreated NTG patients found that forearm blood vessels demonstrated impaired vasodilation in response to intravenous acetylcholine.1 This finding suggests there is a generalized abnormality of vascular endothelium in POAG. Various researchers studied flow-mediated vasodilation in the brachial artery and found that both NTG and HTG patients had impaired responses compared to controls.2–4 One group provided evidence that impaired flow mediated vasodilation was related to reduced circulating endothelial progenitor cells, which are derived from the bone marrow and serve to replace and repair the endothelial cell lining.3 Evans et al. compared the changes in retrobulbar ocular blood flow in POAG patients with normal subjects during supine and upright posture.5 They concluded that posture change exposes a vascular autoregulatory abnormality in the vessels distal to the central retinal artery. Feke et al. confirmed these results in HTG and NTG cases.6,7 In open-angle glaucoma (OAG), there is evidence for vascular dysregulation in the choroid,8,9 optic nerve head,10,11 central retinal artery,12 and perifoveal macular capillaries.13 In fact there is evidence that this vascular dysregulation extends to the cerebral vasculature.14
Since compromised endothelial cell signaling plays a role in POAG, it is important to discover the biological mediators of this impairment. This information could translate into more rational treatments for POAG. There is compelling evidence that impaired nitric oxide (NO) signaling plays an important role in the endothelial cell dysfunction associated with POAG and this evidence comes from human genetics and laboratory studies. One study found no difference in two functional NOS3 gene variants (the gene coding for the enzyme responsible for NO generated by vascular endothelium) between HTG and NTG cases.15 On the other hand, several other research group16–23 have found associations between functional NOS3 gene variants and POAG cases versus controls. In fact, three studies find that the -786C/T NOS3 locus is associated with HTG, particularly in women (Table 1).16,22,23 Interestingly, the -786 C/T NOS3 polymorphism is a function variant that results in reduced nitric oxide levels in cells because of alterations in NOS3 transcription rates.24 In addition, polymorphisms in the genomic region corresponding to the caveolin genes, which code for proteins that reciprocally control NOS3 activity in endothelial endocytotic membranes,25 are also associated with POAG.26 The association between CAV variants and POAG was particularly strong for early paracentral vision loss cases. While one study found that the maximum untreated IOP in early paracentral visual field (VF) loss cases (21.6 mm Hg) was lower than in isolated peripheral visual loss cases (28.3 mm Hg), the IOP in the paracentral cases was still above the statistical norm.27 Another study did not find more paracentral visual loss in NTG versus HTG, suggesting there is a range of IOP at presentation for this pattern of glaucomatous damage.28 When soluble guanylate cyclase (sGC), the intracellular receptor for NO, is knocked out in a murine model, IOP increases nominally (~1–2 mm Hg), there is abnormal retinal vascular reactivity to NO donators, and optic nerve degeneration ensues.29 Interestingly, a polymorphism (rs11722059) in the genomic region between GUY1A3 (codes for the alpha-1 subunit of soluble guanylate cyclase) and GUY1B3 (codes for the beta-1 subunit of soluble guanylate cyclase) was associated with POAG among women with early paracentral visual loss.29 This locus is in high linkage disequilibrium with another GUCY1A3/GUCY1B3 variant (rs13139571) associated with blood pressure in a large European consortium.30 These data suggest a biochemical pathway starting with endothelial cell acetylcholine receptor activation and concluding with smooth muscle cell relaxation plays an important role in POAG pathogenesis (Figure 1).
Table 1.
Summary of association (odds ratios and 95% confidence intervals) between -786 C/T NOS3* polymorphism and high-tension glaucoma (HTG) stratified by sex
| USA22 | |||
|---|---|---|---|
| Genotype | HTG overall | HTG (men) | HTG (women) |
| T/T (reference group) | 1.0 | 1.0 | 1.0 |
| C/T | 1.10 (0.82 – 1.48) | 1.06 (0.63 – 1.77) | 1.13 (0.79 – 1.62) |
| C/C | 1.47 (0.86 – 2.51) | 1.02 (0.48 – 2.17) | 1.80 (1.14 – 2.85) |
| # Cases/# Controls | 354/1065 | 112/334 | 252/731 |
| Brazil16 | |||
| T/T (ref) | 1.0 | 1.0 | 1.0 |
| C/T | 1.08 (1.00 – 3.25) | – | – |
| C/C | 1.28 (0.48 – 3.42) | – | – |
| C/T or C/C | – | 1.01 (0.41 – 2.49) | 2.28 (1.11 – 4.76) |
| # Cases/# Controls | 90/127 | 29/64 | 61/63 |
| Egypt23 | |||
| T/T | 1.0 | 1.0 | 1.0 |
| C/T | 1.11 (0.67 – 1.84) | 1.01 (0.49 -2.10) | 1.19 (0.59 – 2.42) |
| C/C | 2.54 (1.26 – 5.13) | 2.17 (0.84 – 5.63) | 3.06 (1.07 – 8.74) |
| # Cases/# Controls | 160/110 | 76/56 | 84/54 |
The -786 C/T NOS3 polymorphism is a function variant that results in reduced nitric oxide levels in cells because of alterations in NOS3 transcription rates.24
Figure 1.
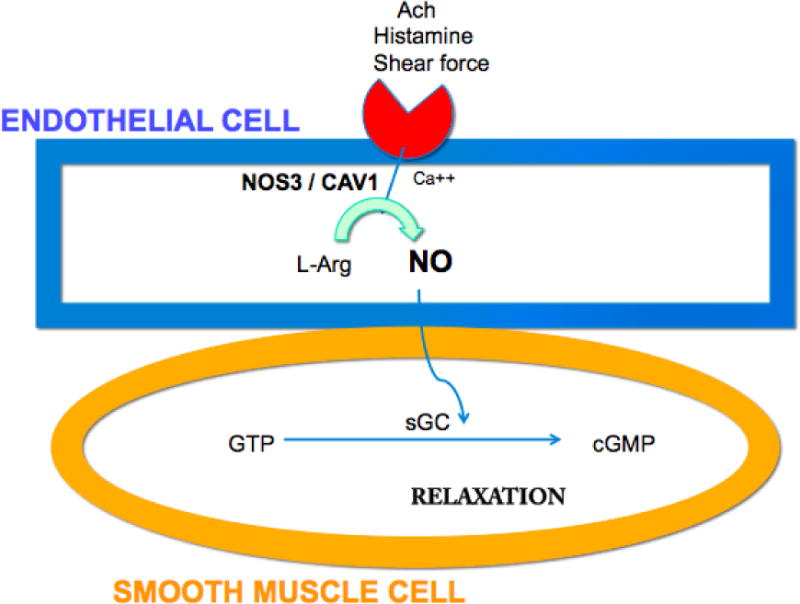
Simplified schematic of endothelial cell mediated smooth muscle cell relation mediated by nitric oxide. The vascular endothelial cell (blue) receives signals, which activate NOS3 leading to formation of nitric oxide (NO). NO permeates into the smooth muscle cell (orange) and binds to soluble guanylate cyclase (sGC) to mediate relaxation. Ach=acetylcholine; NOS3=Nitric oxide synthase 3; CAV=caveolin; Ca++= calcium ion; L-arg=L-arginine; GTP=guanosine triphosphate; sGC=soluble guanylate cyclase; cGMP=cyclic guanosine monophosphate.
Endothelin-1 (ET-1), a 21 amino acid peptide made by vascular endothelium throughout the body including tissues relevant to glaucoma (non-pigmented ciliary epithelium, ciliary body muscle and iris31), is a potent vasoconstrictor and serves to counterbalance endothelial cell NO mediated vasodilation. Considerable interest in ET-1 emerged when perineural optic nerve delivery in rabbits produced optic nerve excavation without elevated IOP.32 However ET-1 is not an NTG-specific biomarker as subsequent studies found that plasma ET-1 levels were not necessarily higher in NTG compared with HTG or chronic angle closure glaucoma.33–35 In fact not every study shows that ET-1 levels are higher in glaucoma subtypes versus controls.34,35 ET-1 is abluminally directed from the endothelial cell to the underlying smooth muscle and assessing serum levels may not be sufficiently sensitive to understand the role this peptide plays in glaucoma pathogenesis. Nonetheless, interventional studies with NTG patients clearly implicate the endothelin processing system in glaucoma pathogenesis. For example the physiological increase in ET-1 plasma levels after shifting from supine to standing was absent in NTG patients.34 After cold-pressor challenge associated with donning a cooling head vestment for 30 minutes, POAG patients experienced a 34% increase in plasma ET-1 levels compared to only a 7% increase in controls.36 Patients with progressive POAG had higher ET-1 levels compared to those with stable disease.37 Interestingly, while untreated NTG patients had a normal systemic vasoconstriction when exposed to intra-arterial ET-1, intra-arterial injection of a selective endothelin A (ETA) receptor antagonist (BQ123) produced less forearm vasodilation than in controls.38 This is a critical experiment because there are two endothelin receptors—ETA and ETB – and antagonism of ETA unmasks ETB related activation of the NO-sGC pathway that produces vasodilation. Therefore, antagonizing ETA could expose an impaired NO-mediated response via ETB activation, although other explanations must also be considered such as increased ETB- receptor mediated vascular tone or pre-existing poor ETA-receptor mediated tone. The cardiology literature indicates that ETA-receptor antagonist-mediated vasodilation is inhibited by blocking NO synthesis.39 Overall interaction with ETA and ETB receptors is intertwined with NO production, which is key for physiologically appropriate vascular regulation. It is clear that endothelin processing is abnormal in POAG, especially in NTG patients, but the problem is complex and may point more to NO than to endothelin itself. To complicate matters further the problem with NO may involve how NOS3 interacts with caveolin as retinal blood flow tends to respond passively to posture change in POAG.6,7 Available studies show no significant relation between various endothelin processing gene variants (see Figure 2 for the genes involved in ET-1 processing) and POAG after controlling for multiple comparisons,40–42 although more study is needed. This is in contrast to the evidence of replication for associations between a functional variant in the NOS3 promoter region (−786C), which is adversely associated with HTG in women.16,22,23 and between CAV1/CAV2 variants and POAG.43,44
Figure 2.
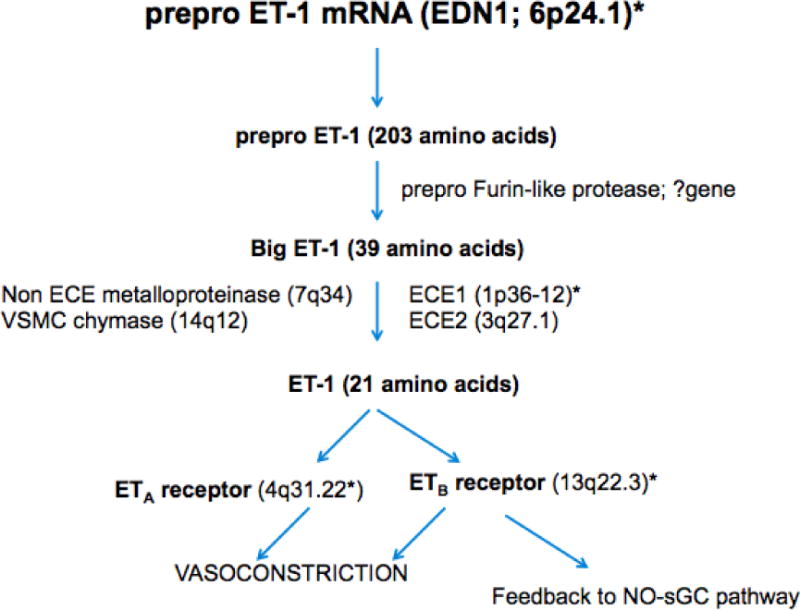
Endothelin-1 (ET-1) processing with chromosomal locations for processing enzymes. ET-1 is formed from prepro ET-1, which is cleaved by a protease to big ET-1 with subsequent conversion to the active 21 amino acid peptide that binds either the ETA or ETB receptor. Receptor binding triggers vasoconstriction but ETB receptor activation also leads to compensatory endothelial cell relaxation. *Common gene variants in ET-1, ECE1 (endothelial converting enzyme), ETA and ETB receptors have not been associated with POAG after accounting for multiple comparisons.40–42 Other abbreviations: NO=nitric oxide; sGC=soluble guanylate cyclase.
How do we reconcile the impaired NO signaling paradigm in POAG with the observed increased disc hemorrhages which represents an important biomarker of glaucoma progression? First we must revisit optic nerve anatomic features that would make it vulnerable to damage in the face of vascular dysregulation. It is important to remember that central retinal artery occlusion produces selective RGC layer dropout, a feature shared with glaucoma. Furthermore a subset of POAG patients with paracentral loss will develop fairly profound loss of retinal sensitivity in a discrete VF zone resembling vascular injury. The optic nerve can be considered a neurovascular pedicle where vessels make acute turns as they emerge onto the retinal surface. This creates opportunity for large shear forces to develop in the smaller vessels if there are significant alterations in blood flow. If shear forces exceed the loading capacity of the vessel wall, then the vessel will rupture and bleed. Second, the scleral ring of Elschnig serves to create a compartment syndrome in the pre-laminar portion of the optic nerve head. Thus optic nerve hemorrhages can act like space occupying lesions and compress RGC axons in the optic nerve head. Simple positional changes such as laying down prompt physiologic increases in ocular perfusion pressure (blood pressure minus IOP) of ~ 30 mm Hg.6 Such hemodynamic changes must be accompanied by changes in vascular tone that keep ocular blood flow relatively constant. Studies of retinal blood flow changes unmasked by positional change suggest that in POAG these vessels behave like passive sieves, with blood flow increasing as much as 100% when patients recline for 30 minutes.45 It is suspected that some complex interaction between ET-1, NO and CAV1 is responsible for this aberrant retinal hemodynamic response to posture change. Such increases in retinal blood flow could translate into large shear forces that induce hemorrhaging in lamina cribrosa capillaries. In the Ocular Hypertension Treatment Study,46 the Normal Tension Glaucoma Study47 and the Early Manifest Glaucoma Treatment Study (EMGTS),48 disc hemorrhage was associated with disease progression in multivariable models that also account for IOP. Nonetheless disc hemorrhage does occur in chronic angle closure glaucoma, suggesting that it could also be a secondary event after IOP-induced optic nerve damage.49 The reason why disc hemorrhages might occur more commonly in NTG than HTG may relate to the fact that the higher IOP tamponades the micro-bleeding that occurs in HTG cases.
If disc hemorrhages in POAG reflect the workings of an impaired NO signaling system, then interventions that improve NO signaling could stop disc hemorrhages and stabilize VF loss. Interestingly the Low Pressure Glaucoma Treatment Study50 found that brimonidine, an alpha 2 agonist that has vasomodulatory activity mediated through NO signaling,51 was effective in slowing VF loss in NTG patients. Brimonidine use was also associated with a trend toward less frequent occurrence of disc hemorrhages.52 The counter view that the study did not demonstrate the neuroprotective effect of brimonidine but rather the deleterious effect of timolol seems unlikely. First the disease progression in the timolol arm of the study (39% in 3 years) was comparable to the untreated arm of the Collaborative NTG Study (35%).53 Second, in the EMGTS, patients with OAG across the spectrum of IOP achieved neuroprotective benefited from treatment consisting of laser trabeculoplasty plus betaxolol versus observation.54
Autonomic dysfunction in primary open-angle glaucoma
The autonomic nervous system (ANS) is housed in the medulla oblongata with the hypothalamus serving as an integrator. The ANS has parasympthetic, sympathetic and enteric arms that controls many bodily functions including body temperature, heart rate, breathing rate, perspiration, digestion, salivation, swallowing, coughing, sneezing, vomiting, sexual arousal and function, pupil diameter, and accommodation. Interestingly, the parasympathetic and sympathetic arms of the ANS are therapeutic targets for glaucoma. The level of IOP may itself be partially controlled by the ANS. In fact, chemical stimulation of the dorsomedial and perifornical hypothalamus where central autonomic regulatory neurons are housed, causes marked rises in IOP.55 This is interesting because diurnal variation in IOP is more variable in POAG patients compared to controls.56 This wider fluctuation in IOP coupled with instability of blood flow regulation probably contributes to POAG, regardless of IOP level. It should be noted that the ability to regulate retinal blood flow is only partially under autonomic function as there is a paracrine control of blood flow in the retina where vessels are not innervated.57
Patients with Familial Dysautonomia (FD) who exhibit a wide array of autonomic function abnormalities uniformly exhibit an optic nerve phenotype. The optic nerve pathology seen in FD predominately involves the maculopapillary bundles, pathologic features shared by some POAG patients (as discussed above) and by patients with mitochondrial disease.58,59 Another disease with well known vasomotor and autonomic abnormalities is Nail Patella syndrome,60 a condition that represents a familial form of NTG61 caused by LMX1B.62 Interestingly common variants in LMX1B are linked to both NTG and HTG.63 At this juncture the link between LMX1B and autonomic dysfunction is not entirely clear. What is quite remarkable is that three studies show reduced low frequency heart rate variability in NTG patients versus controls and these changes are not accompanied by dramatically different blood pressure, even during the nocturnal period.64–67 However, other studies suggest features consistent with cardiac autonomic dysfunction are shared by HTG68,69 and exfoliation glaucoma patients.70 The problem of autonomic dysfunction seems inextricably linked to endothelial cell dysfunction in that affected NTG patients with low frequency heart rate variability tend to have paracentral visual defects and concomitant nail fold microvascular abnormalities.71 Furthermore these patients also tend to have higher plasma ET-1 than age matched controls.72 Aside from cardiovascular autonomic function, other bodily functions under the ANS have not been well studied in NTG, or HTG and this represents a research opportunity in the field glaucoma.
Conclusion
While one cannot claim that impaired NO and endothelin signaling represents a unifying hypothesis in our understanding of POAG, they do appear to play an important role in disease pathogenesis for both HTG and NTG cases. This discussion highlights the role of genetics in contributing to this process. It appears that sub-endothelial plaque formation (atherosclerosis with endothelial cell proliferation) is not an aspect of endothelial dysfunction in POAG.73 Furthermore, POAG patients do not have increased risk of cardiovascular-related mortality.74 While studies focused on NTG patients have helped to highlight mechanisms involved in optic nerve degeneration in POAG, these mechanisms are operative in NTG and HTG. More studies with novel alternative stratification of POAG (such stratifying disease on the basis of VF loss patterns) are needed. The current body of knowledge reviewed here clearly implicates systemic processes in POAG and these processes need to be addressed if we are going to more favorably impact this disease. More study in the fields of genetic epidemiology, immunology and cardiovascular medicine are likely to contribute to an improved understanding of POAG. Hopefully we may someday drop the word “primary” from POAG and replace it with real descriptors that speak to the multiple etiologies that exist in this condition.
Key points.
Impaired nitric oxide signaling, altered endothelin generation after physiologic perturbation and reduced circulating endothelial progenitor cells all appear to be aspects of a systemic endothelial cell dysfunction in primary open-angle glaucoma.
Vascular beds throughout the body exhibit abnormalities in primary open-angle glaucoma patients.
Beat-to-beat variability in heart rate represents a form of autonomic dysfunction documented in primary open-angle glaucoma patients who present with a variety of intraocular pressures.
Acknowledgments
None
Financial support and sponsorship: NEI R01 EY015473, NHGRI HG 004728, the Harvard Glaucoma Center of Excellence and a Harvard Medical School Distinguished Scholar Award provide overall support for this work.
The discovery of the role caveolin and soluble guanylate cyclase genes play in POAG was supported by a large collaborative effort between several institutions enrolled in the NEIGHBOR (National Eye Institute Glaucoma Human Genetics Collaboration). The NEIGHBOR data collection and analysis is supported by NIH/NEI R01EY022305 (Janey L. Wiggs). Support for collection of cases, controls and analysis for individual datasets is as follows. Genotyping services for the NEIGHBOR study were provided by the Center for Inherited Disease Research (CIDR) and were supported by the National Eye Institute through grant HG005259-01 (JL Wiggs). Additionally, CIDR is funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268200782096C. Genotyping for the Mass Eye and Ear dataset and some Nurses Health Study and Health Professionals Follow-up Study cases was completed at the Broad Institute and supported by project grant HG004728 (LR Pasquale) and U01-HG004424 (Broad Institute). Genotype data cleaning and analysis for the GLAUGEN study was supported by U01 HG004446 (Cathy Laurie). Collecting and processing samples for the NEIGHBOR dataset was supported by the National Eye Institute through ARRA grants 3R01EY015872-05S1 (JL Wiggs) and 3R01EY019126-02S1 (Michael A. Hauser). Funding for the collection of NEIGHBOR cases and controls was provided by NIH grants: EY015543 (RR Allingham), EY006827 (Douglas Gaasterland); HL73042, HL073389, EY13315 (MA Hauser); CA87969, CA49449, UM1 CA186107, UM1 CA 167552, EY009149 (Paul R Lichter), HG004608 (Cathy McCarty), EY008208 (Felipe A Medeiros), EY015473 (LR Pasquale), EY012118 (M Pericak-Vance), EY015682 (Anthony Realini), EY011671 (Julia E Richards), EY09580 (JE Richards), EY013178 (Joel S Schuman), RR015574, EY015872 (JL Wiggs), EY010886 (JL Wiggs), EY009847 (JL Wiggs), EY011008, EY144428 (Khang Zhang), EY144448 (K Zhang), EY18660 (K Zhang).
The discovery that soluble guanylate cyclase knock out mice have a phenotype resembling POAG with vascular dysfunction was also supported by several grants including a Harvard Catalyst-the Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758) with financial contributions from Harvard University and its affiliated academic health care centers (Emmanuel S Buys and Bruce R Ksander). A Shaffer Fund Grant from the Glaucoma Research Foundation (ES Busy), NEI R21 EY020987 (ES Buys, BR Ksander), NEI R01 EY022746-01 (ES Buys), an American Heart Association Fellow-to-Faculty Transition Award #11FTF7290032 (Rajeev Malhotra), NCRR 1S10RR022586 (Marielle Sscherrer-Crosbie), NIH CA87969, CA49449, EY09611, CA055075 (all to Jae Hee Kang), the Massachusetts Lions Eye Research Fund (HG, RR); and the FWO-Flanders and UGent-GOA funds (Peter Brouckaert) also supported this effort.
Footnotes
Conflict of interest: Dr. Pasquale has been a speaker for Allergan. He also served as a nonpaid consultant to Novartis and a paid consultant to Bausch + Lomb. He has received support to travel to the Think Tank Meeting in NYC sponsored by the Glaucoma Foundation. He has also received travel support to attend the Nantucket Glaucoma meeting by Aerie Pharmaceuticals and Glaukos.
Reference section
- 1.Henry E, Newby DE, Webb DJ, O’Brien Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1710–4. [PubMed] [Google Scholar]
- 2.Su WW, Cheong ST, Ho WJ, et al. Glaucoma is associated with peripheral vascular endothelial dysfunction. Ophthalmology. 2008;115:1173–78. doi: 10.1016/j.ophtha.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Fadini GP, Pagano C, Baesso I, et al. Reduced endothelial progenitor cells and brachial artery flow-mediated dilation as evidence of endothelial dysfunction in ocular hypertension and primary open angle glaucoma. Acta Ophthalmol. 2010;88:135–41. doi: 10.1111/j.1755-3768.2009.01573.x. [DOI] [PubMed] [Google Scholar]
- 4.Cellini M, Strobbe E, Gizzi C, et al. Endothelin-1 plasma levels and vascular endothelial dysfunction in primary open-angle glaucoma. Life Sci. 2012;15:699–702. doi: 10.1016/j.lfs.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Evans DE, Harris A, Garrett M, et al. Glaucoma patients demonstrate faulty autoregulation of ocular blood flow during posture change. Br J Ophthalmol. 1999;83:809–813. doi: 10.1136/bjo.83.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feke G, Pasquale LR. Retinal blood flow response to posture change in glaucoma patients compared to healthy subjects. Ophthalmology. 2008;115:246–52. doi: 10.1016/j.ophtha.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 7.Feke GT, Rhee DJ, Turalba AT, Pasquale LR. Effects of dorzolamide-timolol and brimonidine-timolol on retinal vascular autoregulation and ocular perfusion pressure in primary open-angle glaucoma. J Ocul Pharmcol Ther. 2013;29:639–45. doi: 10.1089/jop.2012.0271. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich A, Ulrich C, Barth T, Ulrich WD. Detection of disturbed autoregulation of the peripapillary choroid in primary open angle glaucoma. Ophthalmic Surg Lasers. 1996;27(9):746–57. [PubMed] [Google Scholar]
- 9.Gugleta K, Orgul S, Hasler PW, et al. Choroidal vascular reaction to hand-grip stress in subjects with vasospasm and its relevance in glaucoma. Invest Ophthalmol Vis Sci. 2003;44(4):1573–80. doi: 10.1167/iovs.02-0521. [DOI] [PubMed] [Google Scholar]
- 10.Fuchsjager-Mayrl G, Wally B, Georgopoulos M, et al. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2004;45(3):834–9. doi: 10.1167/iovs.03-0461. [DOI] [PubMed] [Google Scholar]
- 11.Okuno T, Sugiyama T, Kojima S, et al. Diurnal variation in microcirculation of ocular fundus and visual field change in normal-tension glaucoma. Eye. 2004;18(7):697–702. doi: 10.1038/sj.eye.6700749. [DOI] [PubMed] [Google Scholar]
- 12.Galambos P, Vafiadis J, Vilchez SE, et al. Compromised autoregulatory control of ocular hemodynamics in glaucoma patients after postural change. Ophthalmology. 2006;113(10):1832–6. doi: 10.1016/j.ophtha.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Grunwald JE, Riva CE, Stone RA, et al. Retinal autoregulation in open-angle glaucoma. Ophthalmology. 1984;91(12):1690–4. doi: 10.1016/s0161-6420(84)34091-x. [DOI] [PubMed] [Google Scholar]
- 14.Tutaj M, Brown CM, Brys M, et al. Dynamic cerebral autoregulation is impaired in glaucoma. J Neurol Sci. 2004;220:49–5. doi: 10.1016/j.jns.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Weiss J, Frankl SA, Flammer J, et al. No difference in genotype frequencies of polymorphisms of the nitric oxide pathway between Caucasian normal and high tension glaucoma patients. Mol Vis. 2012;18:2174–2181. [PMC free article] [PubMed] [Google Scholar]
- 16.Magalhaes da Silva T, Rocha AV, Lacchini R, et al. Association of polymorphisms of endothelial nitric oxide synthase (eNOS) gene with the risk of primary open angle glaucoma in a Brazilian population. Gene. 2012;502:142–146. doi: 10.1016/j.gene.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Logan JF, Chakravarthy U, Hughes AE, et al. Evidence for association of endothelial nitric oxide synthase gene in subjects with glaucoma and a history of migraine. Invest Ophthalmol Vis Sci. 2005;46:3221–3226. doi: 10.1167/iovs.05-0368. [DOI] [PubMed] [Google Scholar]
- 18.Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995;36:1774–1784. [PubMed] [Google Scholar]
- 19.Polak K, Luksch A, Berisha F, et al. Altered nitric oxide system in patients with open-angle glaucoma. Arch Ophthalmol. 2007;125:494–498. doi: 10.1001/archopht.125.4.494. [DOI] [PubMed] [Google Scholar]
- 20.Tunny TJ, Richardson KA, Clark CV. Association study of the 5′ flanking regions of endothelial-nitric oxide synthase and endothelin-1 genes in familial primary open-angle glaucoma. Clin Exp Pharmacol Physiol. 1998;25:26–29. doi: 10.1111/j.1440-1681.1998.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 21.Javadiyan S, Burdon KP, Whiting MJ, et al. Elevation of serum asymmetrical and symmetrical dimethylarginine in patients with advanced glaucoma. Invest Ophthalmol Vis Sci. 2012;53:1923–1927. doi: 10.1167/iovs.11-8420. [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Wiggs JL, Rosner BA, et al. The relation between endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: Interactions with gender and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010;51:971–9. doi: 10.1167/iovs.09-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emam WA, Zidan HE, Abdulhalim BE, et al. Endothelial nitric oxide synthase polymorphisms and susceptibility to high-tension primary open-angle glaucoma in an Egyptian cohort. Mol Vis. 2014;20:804–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto Y, Saito Y, Nakayama M, et al. Replication protein A1 reduces transcription of the endothelial nitric oxide synthase gene containing -786→C mutation associated with coronary spastic angina. Hum Mol Gen. 2000;9:2629–37. doi: 10.1093/hmg/9.18.2629. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J Biol Chem. 1998;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- 26*.Loomis SJ, Kang JH, Weinreb RN, et al. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and by pattern of visual field loss. Ophthalmology. 2014;121:508–516. doi: 10.1016/j.ophtha.2013.09.012. This paper demonstrates that the association between selected CAV1/CAV2 polymorphisms was particularly strong among women who present with early paracentral loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SC, DeMoraes CG, Teng CC, et al. Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology. 2011;118:1782–9. doi: 10.1016/j.ophtha.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Iester M, DeFeo F, Douglas GR. Visual field morphology in high and normal tension glaucoma. J Ophthalmology. 2012;2012:327326. doi: 10.1155/2012/327326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Buys ES, Ko Y-C, Alt C, et al. Soluble guanylate cyclase α1-deficient mice: a novel murine model for primary open angle glaucoma. PLoS One. 2013;8:e60156. doi: 10.1371/journal.pone.0060156. This is the first paper to show that impairment of nitric oxide signaling produced by knocking out soluble guanylate cyclase (sGC) in a murine model produced elevated IOP and optic nerve degeneration resembling POAG. More remarkably, a gene variant in an intergenic region between regions coding for sGC subunits was associated with POAG among women who presented with early paracentral visual loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehret GB, Munro PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–9. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Durango R, Rollin R, Mediero A, et al. Localization of endothelin-1 mRNA and immunoreactivity in the anterior segment of human eye: expression of ETA and ETB receptors. Mol Vis. 2003;9:103–9. [PubMed] [Google Scholar]
- 32.Cioffi GA, Sullivan P. The effect of chronic ischemia on the primate optic nerve. Eur J Ophthalmol. 1999;9(Suppl):S34–6. doi: 10.1177/112067219900901S12. [DOI] [PubMed] [Google Scholar]
- 33.Chen HY, Chang YC, Chen WC, Lane HY. Association between plasma endothelin-1 and severity of different types of glaucoma. J of Glaucoma. 2013;22:117–22. doi: 10.1097/IJG.0b013e31822e8c65. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser HJ, Flammer J, Wenk M, Lüscher T. Endothelin-1 plasma levels in normal-tension glaucoma: abnormal response to posture change. Graefes Arch Clin Exp Ophthalmol. 1995;233:484–8. doi: 10.1007/BF00183429. [DOI] [PubMed] [Google Scholar]
- 35.Kunimatsu S, Mayama C, Tomidokoro A, Araie M. Plasma endothelin-1 in Japanese normal tension glaucoma patients. Curr Eye Res. 2006;31:727–31. doi: 10.1080/02713680600837382. [DOI] [PubMed] [Google Scholar]
- 36.Nicolela MT, Ferrier SN, Morrison CA, et al. Effects of cold-induced vasospasm in glaucoma: the role of endothelin-1. Invest Ophthalmol Vis Sci. 2003;44:2565–72. doi: 10.1167/iovs.02-0913. [DOI] [PubMed] [Google Scholar]
- 37.Emre M, Orgül S, Haufschild T, et al. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br J Ophthalmol. 2005;89:60–3. doi: 10.1136/bjo.2004.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry E, Newby DE, Webb DJ, et al. Altered endothelin-1 vasoreactivity in patients with untreated normal-pressure glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2528–2532. doi: 10.1167/iovs.05-0240. [DOI] [PubMed] [Google Scholar]
- 39.Verhaar MC, Strachan FE, Newby DE, et al. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation. 1998;97:752–56. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa K, Funayama T, Ohtake Y, et al. Association between glaucoma and gene polymorphism of endothelin type A receptor. Mol Vis 2005. 2005;111:431–7. [PubMed] [Google Scholar]
- 41.Kim SH, Kim JY, Kim DM, et al. Investigations on the association between normal-tension glaucoma and singl nucleotide polymorphisms and the endothelin-1 and endothelin receptor genes. Mol Vis. 2006;12:1016–21. [PubMed] [Google Scholar]
- 42*.Kang JH, Loomis SJ, Yaspan BL, et al. Vascular tone pathway polymorphisms in relation to primary open-angle glaucoma. Eye. 2014;28:662–71. doi: 10.1038/eye.2014.42. This was a large study that evaluated several of the endothelin processing enzymes in relation to POAG stratified by early paracentral loss (n=224) and isolated peripheral loss cases (n=993) compared to 3430 controls. Only one gene variant in the endothelin B receptor was associated with POAG with paracentral loss but this result could be due to the multiple comparisons made. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorleifsson G, Waiters GB, Hewitt AW, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42:906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiggs JL, Kang JH, Yaspan BL, et al. Common Variants Near CAV1 and CAV2 are Associated with Primary Open-Angle Glaucoma. Human Molecular Genetics. 2011;20(23):4707–4713. doi: 10.1093/hmg/ddr382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feke GT, Hazin R, Grosskreutz CL, Pasquale LR. Effect of brimonidine on retinal blood flow autoregulation in primary open-angle glaucoma. J Ocul Pharmcol Ther. 2011;27(4):347–52. doi: 10.1089/jop.2011.0014. [DOI] [PubMed] [Google Scholar]
- 46.Budenz DL, Anderson DR, Feuer WJ, et al. Ocular Hypertension Treatment Study Group. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113:2137–2143. doi: 10.1016/j.ophtha.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson DR, Normal Tension Glaucoma Study Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14:86–90. doi: 10.1097/00055735-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Leske MC, Heijl A, Hussein M, Early Manifest Glaucoma Trial Group Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 49.Hseih JW, Lan YW, Wang IJ, Sun FJ. Clinical characteristics and prognostic significance of disc hemorrhage in open-angle and angle closure glaucoma. J Glaucoma. 2010;19:483–7. doi: 10.1097/IJG.0b013e3181b6e5ea. [DOI] [PubMed] [Google Scholar]
- 50.Krupin T, Liebmann JM, Greenfield DS, the Low-Pressure Glaucoma Study Group A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151:671–81. doi: 10.1016/j.ajo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 51.Rosa RH, Jr, Hein TW, Yuan Z, et al. Brimonidine evokes heterogeneous vasomotor response of retinal arterioles: diminished nitric oxide-mediated vasodilation when size goes small. Am J Physiol Heart Circ Physiol. 2006;291:H231–H238. doi: 10.1152/ajpheart.01281.2005. [DOI] [PubMed] [Google Scholar]
- 52*.Furlanetto RL, DeMoraes CG, Teng CC, Low-Pressure Glaucoma Treatment Study Group Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am J Ophthalmol. 2014;157:945–52. doi: 10.1016/j.ajo.2014.02.009. This paper demonstrated a trend toward the occurrence of a disc hemorrhage in patients treated with brimonidine, ana agent which has retinal vasomodulatory acitivity. [DOI] [PubMed] [Google Scholar]
- 53.The Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressure. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 54.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 55.Samuels BC, Hammes NM, Johnson PL, et al. Dorsomedial/Perifornial hypothalamic stimulation increases intraocular pressure, intracranial pressure and the translaminar pressure gradient. Invest Ophthalmol Vis Sci. 2012;53:7328–35. doi: 10.1167/iovs.12-10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agnifili L, Mastropasqua R, Frezzotti P, et al. Circadian intraocular pressure in healthy subjects, primary open-angle and normal tension glaucoma patients with a contact lens sensor. Acta Ophthalmol. 2015;93:e14–21. doi: 10.1111/aos.12408. [DOI] [PubMed] [Google Scholar]
- 57.Ye X, Laties AM, Stone RA. Peptidergic innervation of the retinal vasculature and optic nerve head. Invest Ophthalmol Vis Sci. 1990;31:1731–37. [PubMed] [Google Scholar]
- 58.Mendoza-Sentiesteban CE, Hedge TR, 3rd, Norcliffe-Kaufman L, et al. Clinical neuro-ophthalmic findings in familial dysautonomia. J Neuroophthalmol. 2012;32:23–6. doi: 10.1097/WNO.0b013e318230feab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59**.Mendoza-Sentiesteban CE, Hedge TR, 3rd, Norcliffe-Kaufman L, et al. Selective retinal ganglion cell loss in familial dysautonomia. J Neurology. 2014;261:702–9. doi: 10.1007/s00415-014-7258-2. This paper is noteworthy for its description of the optic nerve phenotype associated with familial dysautonomia, also known as Reilly Day syndrome. [DOI] [PubMed] [Google Scholar]
- 60.Hennessey TA, Backman SB, Meterissian SH, et al. Asystole during combined epidural and general anesthesia in Nail Patella syndrome: a case report and anesthetic implications. Can J Anesth. 2007;54:835–9. doi: 10.1007/BF03021712. [DOI] [PubMed] [Google Scholar]
- 61.Miniwati Z, Mackey DA, Craig JE, et al. Nail-patella syndrome and its association with glaucoma: a review of eight families. Br J Ophthalmol. 2006;90:1505–09. doi: 10.1136/bjo.2006.092619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail patella syndrome. Hum Mol Genet. 1998;7:1091–8. doi: 10.1093/hmg/7.7.1091. [DOI] [PubMed] [Google Scholar]
- 63.Park S, Jamshidi Y, Valeanu D, et al. Genetic risk for primary open-angle glaucoma determined by LMX1B haplotype. Invest Ophthalmol Vis Sci. 2009;50:1522–30. doi: 10.1167/iovs.08-2483. [DOI] [PubMed] [Google Scholar]
- 64.Kashiwagi K, Tsumura T, Ishii H, et al. Circadian rhythm of autonomic nervous function in patients with normal-tension glaucoma compared with normal subjects using ambulatory electrocardiography. J Glaucoma. 2000;9:239–46. doi: 10.1097/00061198-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Riccadonna M, Covi G, Pancera P, et al. Autonomic systemic activity and 24-hour blood pressure variations in subjects with normal- and high-tension glaucoma. J Glaucoma. 2003;12:156–63. doi: 10.1097/00061198-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Na KS, Lee NY, Park SH, Park CK. Autonomic dysfunction in normal tension glaucoma: the short-term heart rate variability analysis. J Glaucoma. 2010;19:377–81. doi: 10.1097/IJG.0b013e3181c4ae58. [DOI] [PubMed] [Google Scholar]
- 67.Wierzbowska J, Wierzbowska R, Stankiewicz A, et al. Cardiac autonomic dysfunction in patients with normal tension glaucoma: 24-h heart rate and blood pressure variability analysis. Br J Ophthalmol. 2012;96:624–8. doi: 10.1136/bjophthalmol-2011-300945. [DOI] [PubMed] [Google Scholar]
- 68.Brown CM, Dütsch M, Michelson G, et al. Impaired cardiovascular responses to baroreflex stimulation in open-angle and normal pressure glaucoma. Clin Sci (Lond) 2002;102:523–30. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 69.Gherghel D, Hosking SL, Armstrong R, Cunliffe IA. Autonomic dysfunction in unselected and untreated primary open-angle glaucoma patients: a pilot study. Ophthalmic Physiol Opt. 2007;27:336–41. doi: 10.1111/j.1475-1313.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 70.Visontai Z, Horváth T, Kollai M, Holló G. Decreased cardiovagal regulation in exfoliation syndrome. J Glaucoma. 2008;17:133–8. doi: 10.1097/IJG.0b013e3181379d67. [DOI] [PubMed] [Google Scholar]
- 71.Park HY, Jung KI, Na KS, et al. Visual field characteristics in normal-tension glaucoma patients with autonomic dysfunction and abnormal peripheral microcirculation. Am J Ophthalmol. 2012;154:466–75. doi: 10.1016/j.ajo.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 72.Park HY, Jung KI, Na KS, et al. Association between heart rate variability and systemic endothelin-1 concentration in normal tension glaucoma. Curr Eye Res. 2013;38:516–9. doi: 10.3109/02713683.2012.745881. [DOI] [PubMed] [Google Scholar]
- 73.deVoogd S, Wolfs RC, Jansonius NM, et al. Atherosclerosis. C-reactive protein, and risk for open-angle glaucoma: the Rotterdam study. Invest Ophthalmol Vis Sci. 2008;47:3772–6. doi: 10.1167/iovs.05-1278. [DOI] [PubMed] [Google Scholar]
- 74.Akbari M, Akbari S, Pasquale LR. The association of primary open-angle glaucoma with mortality: A meta-analysis of observational studies. Arch Ophthalmol. 2009;127:204–210. doi: 10.1001/archophthalmol.2008.571. [DOI] [PubMed] [Google Scholar]


