Abstract
Purpose
Temporal lobe epilepsy (TLE) is thought to be a network disease and structural changes using diffusion tensor imaging (DTI) have been shown. However, lateralized differences in the structural integrity of TLE, as well as changes in structural integrity with longer disease duration, have not been well defined.
Methods
We examined the fractional anisotropy (FA) and mean diffusivity (MD) in the hippocampus, as well as its primary (cingulum and fornix) and remote (uncinate and external capsule) connections in both right and left TLE. Changes in diffusion measures over the disease course were examined by correlating FA and MD in the various structures with epilepsy duration. The potential for each measure of anisotropy and diffusivity as a biomarker of TLE laterality was investigated using random forest (RF) analysis.
Results
MD was increased in the bilateral hippocampus, cingulum, fornix and the right external capsule in both left and right TLE compared to controls. In addition, left TLE exhibited an increased MD in the ipsilateral uncinate fasciculus and bilateral external capsules. A decrease in FA was seen in the left cingulum in left TLE. RF analysis demonstrated that MD of the right hippocampus and FA of the left external capsule were the strongest predictors of TLE laterality. An association of increased MD with epilepsy duration was seen in the left hippocampus in left TLE.
Conclusion
Evidence of disrupted white matter architecture in the hippocampus and its primary and remote connections were demonstrated in TLE. While changes in the hippocampus and cingulum were more prominent in right TLE, remote changes were more prominent in left TLE. MD of the right hippocampus and FA of the left external capsule were found to be the strongest structural predictors of TLE laterality. Changes associated with duration of epilepsy indicated that changes in structural integrity may be progressive over the disease course. This study illustrates the potential of structural diffusion tensor imaging in elucidating pathophysiology, enhancing diagnosis and assisting prognostication.
Keywords: Temporal lobe epilepsy, diffusion tensor imaging, progression, mean diffusivity, fractional anisotropy
1. Introduction
Temporal lobe epilepsy (TLE) is the most common form of epilepsy in adults and is thought to be a network disease (Engel et al., 2013; Spencer, 2002). Diffusion tensor imaging (DTI) has been used to study the underlying white matter structural integrity in TLE, finding changes in temporal and extratemporal white matter structures (Arfanakis et al., 2002; Govindan et al., 2008; Gross, 2011; Gross et al., 2006; Thivard et al., 2005). The reason for white matter changes in TLE is unclear. However, various mechanisms have been suggested, including changes related to the underlying epileptogenic process, axonal degeneration due to seizures or epileptiform discharges, and compensatory white matter reorganization (Gross, 2011; Otte et al., 2012).
Measures often used to quantitate levels of white matter integrity include the fractional anisotropy (FA) and mean diffusivity (MD) (Le Bihan et al., 2001). FA is a measure of directional diffusivity in white matter, which is often decreased in white matter pathology including edema and inflammation. Normal white matter has axons arranged in tracts imparting a high directional diffusivity (high FA), while degenerated tracts have reduced directional diffusivity (low FA) (Basser and Pierpaoli, 1996). MD measures the overall motion of water molecules without respect to directionality (Le Bihan et al., 2001). A recent meta-analysis of 13 DTI studies in TLE found that the MD is increased and the FA reduced in TLE compared to healthy controls (Otte et al., 2012). DTI changes were more prominent in the tracts closely connected with the affected temporal lobe, including the cingulum and fornix (Otte et al., 2012). The major input to the hippocampus is the entorhinal cortex, which receives inputs from the cingulum, and the major output of the hippocampus is the fornix. We planned to study changes in (1) the hippocampus; (2) tracts directly connected to the hippocampus, including the cingulum and fornix; and (3) remote tracts previously identified as most significantly involved in DTI studies of TLE, including the external capsule and uncinate fasciculus (Otte et al., 2012). In addition to the recognized propensity to involve ipsilateral, rather than contralateral, white matter in TLE, there has been evidence to suggest that white matter changes may differ in left and right TLE, although studies investigating lateralized differences in structural integrity have been limited in both number and sample size (Ahmadi et al., 2009; Focke et al., 2008; Kemmotsu et al., 2011). Left TLE has been shown to have more structural compromise than right TLE, which has been suggested to be related to greater vulnerability of the left hemisphere to injury and progressive effects (Kemmotsu et al., 2011). Left TLE has also been noted to have more widespread involvement, compared to a restricted ipsilateral involvement in right TLE, using FA measurements (Ahmadi et al., 2009). These patterns, however, were restricted to the scope of the specific tracts studied, and the relationship of these patterns to hippocampal changes is not yet clear. Due to limited evidence on lateralized differences in structural integrity between left and right TLE (Besson et al., 2014), we examined lateralized changes separately. In addition, we investigated the relative importance of structural changes in primary versus remote hippocampal inputs and outputs for serving as a marker of TLE laterality. One study has found that FA measurements in the uncinate and parahippocampal gyrus may lateralize TLE into left and right TLE in 90% of cases (Ahmadi et al., 2009). However, despite emerging evidence that differences in anisotropy and diffusivity may be useful for lateralizing TLE, the relative importance of affected regions for lateralizing TLE remains relatively unexplored.
There has also been some evidence of white matter changes associated with the age of onset of epilepsy (Lin et al., 2008) and epilepsy duration (Govindan et al., 2008), although other studies failed to show evidence of such associations (Arfanakis et al., 2002; Gross et al., 2006; Thivard et al., 2005). In a recent study, however, Bernhardt et al. (2009) showed that structural changes exist in TLE beyond those attributable to normal aging. Improved understanding of progressive changes in the TLE network is of interest as (1) a measure of disease load, (2) a correlational tool with clinical measures including cognition and behavior (Yogarajah et al., 2010), and (3) identification of changes of interest that could help guide intervention using surgery or devices. However, few studies have investigated lateralized differences in the progression of structural integrity changes in left versus right TLE. Therefore, we further assessed for DTI changes correlated with the duration of epilepsy among separate left and right TLE groups.
The major aims of the current study were to (1) compare the MD and FA of the hippocampus, cingulum, fornix, uncinate fasciculus, and external capsule in left and right TLE to healthy controls; (2) investigate the potential of each of these measures for serving as a biomarker for TLE laterality; and (3) correlate these measures to epilepsy duration in left and right TLE.
2. Material and methods
2.1. Subjects
The study population included 28 controls (average age, 37.8±8.9 SD (y)) and 28 TLE patients (17 left TLE, average age, 37.3±11.6 SD (y); average epilepsy duration, 13.9±17.0 SD (y); average age of disease onset, 23.6±13.7 SD (y); 11 right TLE, average age, 44.6±12.8 SD (y); average epilepsy duration, 26.1±22.0 SD (y); average age of disease onset, 18.7±20.8 SD (y)). Epilepsy subjects were recruited from the Baylor College of Medicine comprehensive epilepsy center following clinical evaluation, video-EEG monitoring, and high-resolution MR imaging between July 2011 and June 2014. Exclusion criteria included patients with disabling cognitive impairment or other neurological co-morbidities. None of the patients had a seizure in the 24 hours preceding imaging. Control subjects were recruited through local advertisements and word-of-mouth, and were selected to match patient groups in age, gender, and educational background as closely as possible. The study was approved by the Institutional Review Board. Written informed consent was obtained from all subjects prior to scanning.
2.2. Image acquisition
Imaging was performed on a Philips Ingenia 3.0T MRI scanner (Philips Medical Systems, Best, Netherlands) equipped with a 16 channel digital radiofrequency coil for signal reception. T1-weighted imaging was performed as follows: TR = 2500 ms, TE = 4600 ms, FOV = 199 mm, matrix = 244 × 206, slice thickness = 1.4 mm, 284 slices. A spin-echo echo planar imaging based DTI sequence was acquired with the following acquisition parameters: FOV = 228mm × 228mm × 143mm; acquired voxel size = 2mm × 2mm × 2.2mm (i.e., 65 slices at 2.2 mm thick); TR/TE = 9400ms/75ms; parallel imaging acceleration factor = 2.5; b-values acquired: 0 (3 NSA) and 1000 (1 NSA along 32 directions) s/mm2; chemical shift selective fat suppression. Slices were acquired in axial-oblique orientation.
2.3. DTI processing
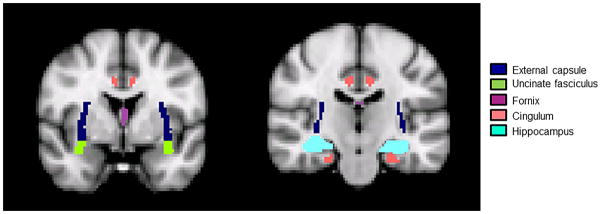
Imaging data were acquired in PAR/REC format (Philips Healthcare, Best, Netherlands) and underwent DICOM-to-NIfTI format conversion with dcm2nii (http://www.mccauslandcenter.sc.edu/mricro/mricron/dcm2nii.html). Diffusion gradient directions were extracted using CATNAP (Landman et al., 2013). The FMRIB Software Library (FSL)’s Diffusion Toolkit (FDT) was then used for diffusion-weighted image pre-processing and fitting of the diffusion tensor (http://www.fmrib.ox.ac.uk/fsl/fdt/index.htm) (Behrens et al., 2003). For each subject, brain extraction was performed on non-diffusion weighted b0 images (b=0 s/mm2) and T1-weighted images. Diffusion-weighted images were eddy-current corrected and co-registered to the b0 image in order to minimize head movement. Voxel-wise fitting of the diffusion tensor was then performed using FDT’s dtifit (http://www.fmrib.ox.ac.uk/fsl/fdt/fdt_dtifit.html) (Behrens et al., 2003). To delineate the fornix, cingulum, external capsule, and uncinate fasciculus, the white matter of each subject’s brain was parcellated in native diffusion space based on the anatomical labeling in the ICBM-DTI-81 atlas (Mori et al., 2008; Oishi et al., 2008). Right-left reversal and label-checking was manually performed after inspection (Rohlfing, 2013). To delineate the hippocampus, parcellation in native diffusion space based on the automated anatomical labeling (AAL) atlas was used (Tzourio-Mazoyer et al., 2002). Regions of interest in standard space were transformed to subject space using the inverse of the transformation matrix obtained by co-registering the subject’s T1-weighted image to the b0 image, followed by linear transformation to the MNI template. This parcellation procedure has been utilized by various studies (Gong et al., 2009; Li et al., 2009; Zhang et al., 2011). These structures defined the cingulum, fornix, external capsule, uncinate fasciculus, and hippocampal regions of interest (ROIs) from which mean value of FA and MD were calculated for each individual (Figure 1).
Figure 1. Regions of interest overlaid on a T1 weighted template in standard space.
Dark blue, external capsule; teal, hippocampus; green, uncinate fasciculus; purple, fornix; pink, cingulum.
2.4. Groupwise comparison of diffusion measures
Permutation testing with 10,000 resamples was used to compare DTI measures between controls and left/right TLE. Observations were excluded as outliers if located outside 1.5 times the interquartile range above/below the upper/lower quartiles. Significance was evaluated at the α=0.05 level after multiple testing correction by controlling the false discovery rate (FDR) at the 0.05 level (Benjamini and Hochberg, 1995).
2.5. Discriminatory importance of diffusion measures
To identify the hippocampal inputs and outputs that best distinguished left from right TLE, we used supervised machine learning through random forests (RF). RF is an ensemble learning method in which a large number of unpruned trees are grown, and their outputs aggregated to produce powerful learners. RF was chosen to assess variable importance due to several important characteristics, including (1) its ability to account for differences in scaling of predictor variables, (2) high tolerance for multicollinearity, (3) lack of parametric model assumptions due to binary partitioning, and (4) ability to provide measures of variable importance (Breiman, 2001). We grew 5000 trees in each forest with four predictors randomly selected at each node. Variable importance of each predictor was assessed using permutation importance. Permutation importance measures the difference in prediction accuracy before and after permuting the values of the predictor, with larger positive values indicating greater importance in discriminating left from right TLE. K-means cluster analysis using Hartigan’s method with K=3 and 100 random starts was used to identify predictors with high, moderate, and low levels of discriminatory importance. For regions with high discriminatory importance, ten-fold cross-validation was used to estimate the percentage of patients correctly lateralized using logistic regression.
2.6. Correlation of diffusion measures with epilepsy duration
Epilepsy duration was determined from the year when the first habitual seizure occurred to the time of DTI. Due to non-normality and small sample size, the Spearman correlation was used to assess the correlation of structural connectivity with epilepsy duration. Although the Spearman correlation is more robust to univariate outliers than the Pearson correlation due to its rank transformation, sensitivity to multivariate outliers remains an issue with the Spearman correlation. Therefore, influential observations with a Cook’s distance of >1 and outlying observations in either the X or Y direction outside 1.5 times the interquartile range above/below the upper/lower quartiles were removed. In order to adjust the estimates of correlation between epilepsy duration and structural integrity for the possible influence of age and gender as confounding variables, the change-in-estimate (CE) criterion at the conventional 10% level was used, with covariates considered to be confounders if the Spearman correlation changed by more than 10% when the covariate was added to the model. The CE criterion is less influenced by sample size than use of significance testing (ST) criteria for confounder identification (Tong and Lu, 2001). For correlation estimates that demonstrated significant association with age or gender, the partial Spearman correlation controlling for the identified confounder was used to evaluate the relationship between DTI measures and epilepsy duration. For all other measures, Spearman’s correlation coefficient was used to evaluate the relationship with epilepsy duration. Significance was evaluated at the α=0.05 level at both the exploratory level (prior to multiple testing correction) as well as after controlling the FDR at the 0.05 level (Benjamini and Hochberg, 1995). Due to the sensitivity of significance testing to sample size, effect sizes were also reported according to guidelines suggested by Cohen (1977), in order to facilitate comparison between left and right TLE progressive changes.
For significant correlates, the coefficient of determination was used to evaluate the percentage of variability in epilepsy duration explained by each diffusion measure. Because of the potential for age of disease onset to confound correlations with disease duration, the partial Spearman correlation coefficient was computed to determine whether correlations between diffusion measures and epilepsy duration were statistically significant after controlling for age of disease onset.
3. Results
No statistically significant differences in age, gender, handedness, education level, epilepsy duration, or age of disease onset were found between subject groups at the 0.05 level. The apparent difference in disease duration (left TLE, 13.9±17.0 SD (y); right TLE, 26.1±22.0 SD (y)) did not yield statistical significance (p=0.23) (Table 1). Among patients for whom language dominance was clinically assessed, atypical language dominance was present in one left-handed patient for whom language dominance was not lateralized (Table 2).
Table 1.
Summary demographic details for subject groups.
| Controls (n=28) | Left TLE (n=17) | Right TLE (n=11) | p-value | |
|---|---|---|---|---|
| Gender (M) | 15 | 5 | 5 | 0.29a |
| Handedness (right) | 25 | 15 | 9 | 0.88a |
| Age (years, mean±SD) | 37.8±8.9 | 37.3±11.6 | 44.6±12.8 | 0.16b |
| Education (years, mean±SD) | 14.3±2.6 | 13.2±4.2 | 12.3±2.4 | 0.22b |
| Epilepsy duration (years, mean±SD) | NA | 13.9±17.0 | 26.1±22.0 | 0.23c |
| Age of disease onset (years, mean±SD) | NA | 23.6±13.7 | 18.7±20.8 | 0.29c |
Chi-square test
Kruskal-Wallis test
Mann-Whitney U test
Abbreviations: M, male; SD, standard deviation
Table 2.
Baseline characteristics for temporal lobe epilepsy patients.
| # | Age (y) | Sex | Race | Handedness | Ictal EEG lateralization |
Interictal EEG lateralization |
Ictal semiology | MRI | Sz free post surgery |
Pathology | Language dominance |
Age of disease onset (y) |
Disease duration (y) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 | F | As | R | LTLE | LT sp. | Mesial (epigastric aura) | L MTS | Yes > 1 yr | MTS | L | 17 | 16 |
| 2 | 23 | F | C | R | LTLE | LT sp. | Mesial (epigastric, olfactory, gustatory aura) | L MT volume increase (cortical dysplasia / neoplasm) | No | HS | L | 19 | 4 |
| 3 | 67 | F | O | R | LTLE | BT sp. | Mesial (déjà vu) | L MTS | No surgery | No surgery | Unknown | 3 | 64 |
| 4 | 22 | M | A A |
R | LTLE | L F-T sp. | Temporal (AOC, automatisms) | Decreased volume and signal abnormality of splenium | No | No final report | L | 17 | 5 |
| 5 | 23 | F | H | R | LTLE | LT sp, LF>RF sp. | Temporal (AOC) | L MT volume increase (cortical dysplasia / neoplasm) | Lost to followup | HS | Predominant ly L | 12 | 11 |
| 6 | 39 | F | A A |
R | LTLE | LT>RT sp. | Temporal (AOC) | Subtle LT increased signal | No surgery | No surgery | Unknown | 12 | 27 |
| 7 | 32 | M | H | R | LTLE | LT sp. | Mesial (déjà vu) | Normal | Yes < 1 yr | No final report | L | 15 | 17 |
| 8 | 38 | F | A A |
R | LTLE | LT sp. | Temporal (olfactory) | L MT increased signal and volume increase | No surgery | No surgery | Unknown | 32 | 6 |
| 9 | 42 | F | A A |
R | LTLE | LT sp. | Temporal (AOC, automatisms) | L cortical dysplasia | No surgery | No surgery | Unknown | 34 | 9 |
| 10 | 33 | F | C | R | LTLE | LT sp. | Unclear (dizziness) | L cortical dysplasia | No surgery | No surgery | L | 23 | 11 |
| 11 | 28 | M | C | L | LTLE | LT sp. | Temporal (AOC, automatisms) | Normal | No surgery | No surgery | Unknown | 27 | 1 |
| 12 | 53 | F | C | R | LTLE | LT sp. | Temporal (AOC) | L increased volume | No | HS | L | 46 | 7 |
| 13 | 29 | F | A A |
R | LTLE | LT sp./sz | Temporal (AOC, automatisms) | R hippocampal atrophy | No surgery | No surgery | Unknown | 26 | 3 |
| 14 | 45 | F | C | R | LTLE | LT sp | Unclear (indescribable aura, lip pulling to right) | L increased hippocampal signal | No surgery | No surgery | Unknown | 45 | 1 |
| 15 | 43 | F | A A |
R | LTLE | LT sp | Temporal (AOC) | L parahippocampal increased signal | No | Chaslin’s gliosis | Unknown | 42 | 2 |
| 16 | 44 | M | As | R | LTLE | LT sp. | Unclear (dizziness) | L MTS | No surgery | No surgery | Unknown | 0 | 45 |
| 17 | 40 | M | C | L | LTLE | LT sp. | Temporal (AOC, automatisms, perseveration) | Normal | Yes < 1 yr | Result pending | Not lateralized | 32 | 8 |
| 18 | 47 | F | H | R | RTLE | RT>LT sp. | Mesial (epigastric aura) | R MTS | No surgery | No surgery | Unknown | 44 | 3 |
| 19 | 36 | M | C | L | RTLE | RT sp. | Mesial (deja vu) | Mild diffuse volume loss | Yes < 1 yr | HS, CD | Unknown | 6 | 30 |
| 20 | 21 | M | O | R | RTLE | RT sp. | Temporal (AOC, tinnitus) | Normal | No surgery | No surgery | Unknown | 12 | 9 |
| 21 | 28 | M | A A |
R | RTLE | BT sp. | No data | R hippocampal increased signal and volume increase | No surgery | No surgery | Unknown | 24 | 4 |
| 22 | 57 | M | C | R | RTLE | RT sp. | Unclear (sz starts with yelling and followed by extension of all 4 extremities) | R MTS | No | No final report | Unknown | 52 | 5 |
| 23 | 49 | F | H | R | RTLE | RT sp./sz | Unclear (felt like ‘6th sense’) | R hippocampal increased signal and volume loss, L MT increased signal | No surgery | No surgery | Unknown | 1 | 49 |
| 24 | 38 | F | H | R | RTLE | RT sp. | Temporal (AOC) | R MTS | Lost to followup | HS, mild right lateral temporal CD | Unknown | 6 | 32 |
| 25 | 61 | F | H | R | RTLE | RT sp. | Temporal (AOC) | R MTS | No surgery | No surgery | Unknown | 5 | 56 |
| 26 | 56 | M | A A |
R | RTLE | RT sp. | Temporal (AOC, automatisms) | R hippocampal atrophy | No surgery | No surgery | Unknown | 0 | 56 |
| 27 | 43 | F | C | R | RTLE | RT >bifrontal sp. | Temporal (AOC, dryness in throat) | Possible R hippocampal atrophy/signal change | No surgery | No surgery | Unknown | 4 | 40 |
| 28 | 55 | F | C | R | RTLE | RT TIRDA/sz | Mesial (olfactory aura) | FCD vs. glioma, R MTL | No | No final report | Unknown | 52 | 3 |
Abbreviations: #, subject number; L, left; R, right; F, female; M, male; As, Asian; AA, African-American; H, Hispanic; C, Caucasian; O, Other; sp, spikes; LT, left temporal; BT, bitemporal; RT, right temporal; AOC, alteration of consciousness; HS, hippocampal sclerosis; MTS, mesial temporal sclerosis; MT, mesial temporal; CD, cortical dysplasia; sz, seizure; f/u, followup; TBD, to be determined (these are patients who did not return for followup); y, years. Language dominance was assessed through Wada testing.
3.1. Groupwise comparison of diffusion measures
Table 3 shows the groupwise differences in DTI measures of right and left TLE patients as compared to healthy controls. Among left TLE patients, MD was increased in the bilateral hippocampi, bilateral cingulate gyrus, fornix, ipsilateral uncinate, and bilateral external capsules. FA of the ipsilateral cingulate gyrus was also decreased. Trends toward decreased FA of the ipsilateral external capsule and increased MD of the contralateral uncinate were also observed. Among right TLE patients, an increase in the MD of the bilateral hippocampi, bilateral cingulate gyrus, and ipsilateral external capsule were observed. A trend toward increased MD of the fornix in right TLE was also observed.
Table 3. DTI measures of structural integrity of external capsule, hippocampus, uncinate fasciculus, cingulum, and fornix.
p-values are after multiple testing correction. Up and down arrows signify direction of change in TLE groups compared to HC.
| HC Mean ± SD |
LTLE Mean ± SD |
p-value (LTLE vs HC) | RTLE Mean ± SD |
p-value (RTLE vs HC) | |
|---|---|---|---|---|---|
| MD | |||||
| L Hip | 0.00098 ± 0.00006 | 0.00111 ± 0.00013 | 0.002** ↑ | 0.00109 ± 0.00014 | 0.02* ↑ |
| R Hip | 0.00097 ± 0.00007 | 0.00104 ± 0.00008 | 0.01* ↑ | 0.00123 ± 0.00025 | 0.002** ↑ |
| L Cng | 0.00078 ± 0.00004 | 0.00081 ± 0.00003 | 0.02* ↑ | 0.00083 ± 0.00006 | 0.04* ↑ |
| R Cng | 0.00079 ± 0.00004 | 0.00084 ± 0.00006 | 0.01* ↑ | 0.00094 ± 0.00019 | 0.01* ↑ |
| FOR | 0.00151 ± 0.00019 | 0.00171 ± 0.0003 | 0.02* ↑ | 0.00179 ± 0.00047 | 0.06 |
| L Unc | 0.0009 ± 0.00011 | 0.001 ± 0.00014 | 0.02* ↑ | 0.00092 ± 0.00007 | 0.37 |
| R Unc | 0.00077 ± 0.00006 | 0.00081 ± 0.00006 | 0.07 | 0.0008 ± 0.00005 | 0.21 |
| L EC | 0.00079 ± 0.00004 | 0.00082 ± 0.00004 | 0.02* ↑ | 0.00081 ± 0.00003 | 0.21 |
| R EC | 0.00074 ± 0.00002 | 0.00078 ± 0.00003 | 0.003** ↑ | 0.00079 ± 0.00005 | 0.006* ↑ |
| FA | |||||
| L Hip | 0.22487 ± 0.03312 | 0.22323 ± 0.02797 | 0.45 | 0.21926 ± 0.05262 | 0.46 |
| R Hip | 0.2583 ± 0.04456 | 0.24938 ± 0.0329 | 0.28 | 0.23243 ± 0.03961 | 0.16 |
| L Cng | 0.40797 ± 0.07567 | 0.34327 ± 0.07501 | 0.02* ↓ | 0.36532 ± 0.134 | 0.21 |
| R Cng | 0.3567 ± 0.06111 | 0.32717 ± 0.07005 | 0.12 | 0.30906 ± 0.08959 | 0.13 |
| FOR | 0.40394 ± 0.0752 | 0.37717 ± 0.08272 | 0.18 | 0.39552 ± 0.12872 | 0.46 |
| L Unc | 0.27422 ± 0.08889 | 0.24858 ± 0.07102 | 0.2 | 0.28281 ± 0.09271 | 0.46 |
| R Unc | 0.39105 ± 0.08584 | 0.38083 ± 0.06095 | 0.35 | 0.39663 ± 0.03556 | 0.47 |
| L EC | 0.34768 ± 0.05949 | 0.31521 ± 0.046 | 0.07 | 0.34808 ± 0.03452 | 0.50 |
| R EC | 0.38814 ± 0.04356 | 0.36973 ± 0.03195 | 0.11 | 0.37242 ± 0.05033 | 0.30 |
Significant at 0.05 level after multiple testing correction
Significant at 0.01 level after multiple testing correction
Abbreviations: HC, healthy controls; LTLE, left TLE; RTLE, right TLE; L, left hemisphere; R, right hemisphere; EC, External capsule; Hip, hippocampus; Unc, uncinate; Cng, Cingulum; For, Fornix; MD, mean diffusivity; FA, fractional anisotropy; SD, standard deviation.
3.2. Discriminatory importance of diffusion measures
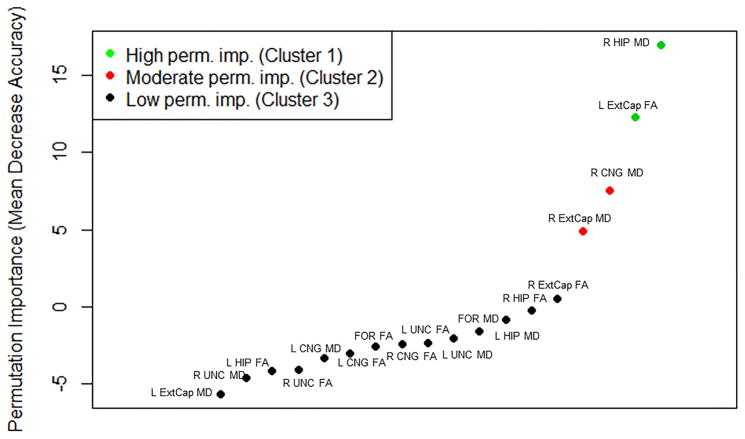
The estimated variable importance of the FA and MD of each hippocampal input and output for distinguishing left from right TLE is shown in Figure 2. Among the diffusion measures of hippocampal inputs and outputs, MD of the right hippocampus and FA of the left external capsule had the highest discriminatory ability between left and right TLE (Figure 2), with higher levels of right hippocampal MD present in right TLE and lower levels of left external capsule FA present in left TLE (Table 3). 71.4% (95% CI, 51.3–86.8) and 64.3% (95% CI, 44.1–81.4) of patients were correctly lateralized using right hippocampal MD and left external capsule FA based on 10-fold cross validation of logistic regression, respectively.
Figure 2.
Permutation importance learned by random forests, based on variable importance of FA and MD of hippocampal inputs and outputs for discriminating left from right TLE. Mean decrease in accuracy upon permutation of each predictor is shown, with higher values indicating greater variable importance. Negative values indicate an increase in prediction accuracy upon permutation of the predictor and are indicative of irrelevant predictors. From left to right: left external capsule MD, right uncinate MD, left hippocampal FA, right uncinate FA, left cingulum MD, left cingulum FA, fornix FA, right cingulum FA, left uncinate FA, left uncinate MD, fornix MD, left hippocampal MD, right hippocampal FA, right external capsule FA, right external capsule MD, right cingulum MD, left external capsule FA, right hippocampal MD.
Abbrevations: FOR, fornix; Cng, cingulum; UNC, uncinate; HIP, hippocampus, ExtCap, external capsule; MD, mean diffusivity; FA, fractional anisotropy.
3.3. Correlation of diffusion measures with epilepsy duration
Table 4 shows the effect sizes of the correlations between epilepsy duration and DTI measures of structural integrity. Large effect sizes were found for the correlation between epilepsy duration and increased MD of the bilateral hippocampi and right cingulate gyrus for left TLE patients. Among right TLE patients, large effect sizes were found for the correlation between epilepsy duration and FA and MD of the right hippocampus, FA of the bilateral cingulate gyri as well as MD of the right cingulate gyrus, FA and MD of the fornix, and FA and MD of the right external capsule. Null hypothesis testing yielded a significant correlation prior to multiple testing correction for MD of the bilateral hippocampi and right cingulum among left TLE patients, and for FA and MD of the right hippocampus, MD of the right cingulum, FA of the right external capsule, and MD of the fornix among right TLE patients. Of these changes, a significant positive correlation among left TLE patients between longer epilepsy duration and increased left hippocampal MD remained after FDR control. This correlation remained significant after controlling for age of disease onset (p=0.01). The coefficient of determination for the association between epilepsy duration and left hippocampal MD among left TLE patients was 0.792, indicating that an estimated 79.2% of the variability in epilepsy duration among left TLE patients was explainable by left hippocampal MD.
Table 4.
Effect sizes of correlations between epilepsy duration and DTI measures of structural integrity.
| LTLE | RTLE | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect size1 | Effect size1 | |||||||
| Negligible (<0.1) | Small (0.1–0.3) | Medium (0.3–0.5) | Large (>0.5) | Negligible (<0.1) | Small (0.1–0.3) | Medium (0.3–0.5) | Large (>0.5) | |
| MD | L CNG MD (r = 0.227) FOR MD (r = 0.164) R UNC MD (r = 0.281) |
L UNC MD (r = −0.335) L ExtCap MD (r = 0.428) R ExtCap MD (r = 0.318) |
L HIP MD (r = 0.782)* R HIP MD (r = 0.614) R CNG MD (r = 0.621) |
L UNC MD (r = −0.090) | L HIP MD (r = 0.136) L CNG MD (r = 0.123) R UNC MD (r = 0.152) |
L ExtCap MD (r = −0.336) |
R CNG MD (r = 0.849) FOR MD (r = 0.714) R ExtCap MD (r = 0.626) R HIP MD (r = 0.756) |
|
| FA | R HIP FA (r = −0.0698) R CNG FA (r = −0.0841) L UNC FA (r = 0.0449) L CNG FA (r = 0.094) |
L HIP FA (r = −0.159) FOR FA (r = 0.140) L ExtCap FA (r = −0.208) |
R ExtCap FA (r = −0.411) R UNC FA (r = −0.312) |
L ExtCap FA (r = −0.0295) | L UNC FA (r = 0.235) | L HIP FA (r = −0.504) R UNC FA (r = 0.411) |
R HIP FA (r = −0.857) L CNG FA (r = −0.594) R CNG FA (r = −0.563) FOR FA (r = −0.573) R ExtCap FA (r = −0.790) |
|
Here, we used the suggested effect size guidelines for correlations suggested by Cohen (1988).
Bold = Significant at 0.05 level before multiple testing correction
Significant at 0.05 level after multiple testing correction
Abbreviations: LTLE, left TLE; RTLE, right TLE; L, left hemisphere; R, right hemisphere; EC, External capsule; Hip, hippocampus; Unc, uncinate; Cng, Cingulum; For, Fornix; MD, mean diffusivity; FA, fractional anisotropy; r, correlation coefficient
4. Discussion
We investigated the white matter structural integrity of the hippocampus and associated white matter tracts in TLE through cross-sectional analysis of mean diffusivity and fractional anisotropy using DTI. Compared to controls, we found that the mean diffusivity was increased in multiple structures bilaterally in TLE, and is increased more diffusely in left TLE than in right TLE. Fractional anisotropy reduction was most evident in ipsilateral hippocampal inputs for left TLE. Among the areas tested, the right hippocampus and left external capsule had the highest discriminatory ability between left and right TLE. A significant positive correlation was identified between the mean diffusivity of the left hippocampus and longer epilepsy duration among left TLE patients.
4.1. Diffusion changes in TLE
Several studies have shown altered functional connectivity in TLE (Engel et al., 2013), with implications in seizure generation/ propagation and associated neurobehavioral comorbidity (McDonald et al., 2008). DTI provides a method to study the structural correlate of these connectivity changes. A recent meta-analysis found that DTI studies of TLE patients have generally shown an increase in MD and decrease in FA among multiple white matter regions in TLE, with involvement of both ipsilateral and contralateral hemispheres (Otte et al., 2012).
In the present study, the hippocampus, cingulum, and fornix were found to have an increased MD bilaterally for both left and right TLE. Given that the hippocampus is typically involved with seizures in TLE, involvement of its primary output (fornix) and input (cingulum via entorhinal cortex) tracts is unsurprising (Mayanagi et al., 1996). Consistent with our finding, previous studies have observed that the MD is more likely to be involved closer to the presumed epileptogenic location (Otte et al., 2012). The involvement of the cingulum and fornix observed in our study may have resulted from downstream Wallerian degeneration or upstream disuse atrophy, and may be clarified by additional translational investigation.
The finding of bilaterally increased MD of the hippocampi, cingulum, and fornix is consistent with prior reports of increased MD in the bilateral hippocampus (Thivard et al., 2005), cingulum (Concha et al., 2009; Nilsson et al., 2008), and fornix (Concha et al., 2009; McDonald et al., 2008), and supports the concept of unilateral TLE as a network disease. Bilateral temporal epileptiform discharges are also often seen in depth electrode recordings in unilateral TLE, suggesting bilateral hippocampal involvement (So et al., 1989). The reason for bilateral hippocampal involvement in unilateral TLE is unknown, but may involve spread to the contralateral hippocampus through the hippocampal commissure, or may involve bilateral damage which is present from onset (Araujo et al., 2006; Bernasconi et al., 1999). Our data also show that MD is increased bilaterally in both left and right TLE, but occurs asymmetrically and is greater ipsilaterally. This may reflect the pattern of hippocampal cell loss in TLE which has been found to be bilateral but asymmetric in TLE (Babb and Brown, 1987).
Overall, we found more changes in MD than in FA. MD is a measure of the total diffusion in all directions, whereas FA characterizes the directional similarity of diffusion. Therefore, MD is generally more sensitive to changes in fiber density, while FA is more sensitive to changes in fiber orientation (Kubicki et al., 2003). The greater number of changes in MD than in FA may suggest that changes in fiber density play a large role in the white matter changes present in hippocampal inputs and outputs in TLE.
4.2 Lateralization of TLE
Both hippocampi demonstrated increased MD for both left and right TLE, with greater increases ipsilaterally. However, lateralizing ability of right hippocampus structural integrity was greater than the left hippocampus. The difference in variable importance between the right and left hippocampus may reflect a later and slower course of maturation of the left hemisphere (Corballis and Morgan, 1978). This has been postulated to lead to a greater baseline vulnerability of the left hemisphere to early damage in both left and right TLE (Kemmotsu et al., 2011), which may underlie its lower discriminatory power for TLE laterality. Evidence that structural integrity of the right hippocampus may have greater lateralizing ability than the left hippocampus has been indirectly suggested by connection-wise group analysis of DTI, in which the left hippocampus was found to be strongly affected in both left and right TLE, whereas the right hippocampus was strongly affected in only right TLE (Besson et al., 2014).
The general pattern of results that we observed suggests that white matter changes in the temporal lobe and its direct connections may be more prominently affected in right TLE, whereas both direct and remote connections are affected in left TLE. In particular, we found that right TLE patients experienced changes primarily in the hippocampi, cingula, and fornix. One remote region, the ipsilateral external capsule, was affected in right TLE patients. In comparison, left TLE patients experienced white matter changes in the hippocampi, cingula, and fornix, in addition to changes in the bilateral external capsules and uncinate fasciculi. Using random forest analysis, we also found that the presence of remote white matter changes (left external capsule) had high discriminatory ability between left and right TLE. These findings suggest that the presence or absence of remote white matter changes may have potential clinical utility for aiding in focus lateralization. Other DTI studies have also identified patterns of greater and more distant changes in left TLE, compared to less pronounced and more restricted changes in right TLE (Ahmadi et al., 2009; Besson et al., 2014; Kemmotsu et al., 2011). Patterns of more widespread atrophic changes in left compared to right TLE have also been observed using cortical thickness analyses from structural MRI (Bernhardt et al., 2010; Kemmotsu et al., 2011).
4.3. Correlation of diffusion measures with disease duration in TLE
We observed a significant correlation between longer epilepsy duration and higher MD of the ipsilateral hippocampus among left TLE patients after adjusting for age. Although structural abnormalities in TLE are widespread, the progressive nature of these changes remains under investigation. A previous longitudinal study spanning 3.5 years of pharmacologically-controlled epilepsy patients concluded that brain volume reduction in epilepsy is the result of an initial precipitating injury and age-related atrophy, and not the progressive effects of epilepsy (Liu et al., 2005). In another longitudinal study spanning 2.5 years of intractable TLE patients, however, a cortical thickness study directly disassociated the effects of age and disease duration, concluding that there are effects of progressive atrophy in TLE distinct from aging (Bernhardt et al., 2009). Other cross-sectional (Govindan et al., 2008; Lin et al., 2008) as well as longitudinal (Bernhardt et al., 2010) studies have also demonstrated an association between structural changes and epilepsy duration. Our results support the hypothesis of progressive white matter changes in TLE. As a cross-sectional investigation, our study has the advantage of spanning a longer range of disease duration, although the need to correct statistically for age may result in decreased effect size.
We found that a large proportion of the variability in epilepsy duration for left TLE patients was explained by left hippocampal MD, suggesting that quantitative measurements of left hippocampal MD may be a potentially useful clinical marker for disease load in left TLE patients. The correlation between left hippocampal mean diffusivity changes and longer epilepsy duration makes it tempting to speculate that this may be related to other long-term changes in TLE, such as memory. It is generally thought that memory decline worsens with longer epilepsy duration in mesial TLE patients (Jokeit et al., 1999), although another study of mesial and non-mesial TLE patients showed no evidence of memory decline with longer disease duration other than that attributable to normal aging (Helmstaedter and Elger, 2009). One possible reason for previous discrepancies may involve the attenuation of significant correlations through inclusion of heterogeneous TLE subpopulations, as hippocampal sclerosis is associated with both poorer memory performance (Helmstaedter and Elger, 2009) and more extensive DTI white matter changes (Concha et al., 2009) than other pathologies. Further investigation on more homogeneous subgroups is needed to identify TLE subtypes for whom markers, such as left hippocampal MD, may serve as a clinically useful correlational tool.
Our observation that white matter changes were significantly correlated with disease duration in left, but not right, TLE patients is consistent with research from structural MRI, which also found a significant correlations between disease duration and cortical thinning in left, but not right, TLE patients (Kemmotsu et al., 2011). This may be related to a greater susceptibility of the left hemisphere to longer disease duration. In particular, the left hemisphere exhibits more widespread white matter connectivity in left hemisphere dominant patients, leading seizure activity to propagate more diffusely through the left hemisphere and causing increased excitotoxicity from repeated seizures (Powell et al., 2007). Future subgroup analysis to compare longitudinal patterns between subgroups defined by hemispheric language dominance will be useful to evaluate this hypothesis.
Lastly, we note that effect sizes provide an estimate of the magnitude of an effect which is independent of sample size, and have the additional advantage of being independent of the original scale of the variable. We found that, although correlations of white matter changes with epilepsy duration were particularly strong for the ipsilateral hippocampus, a large effect size was also identified among left TLE patients for an increase in MD of the contralateral hippocampus with longer epilepsy duration. Among right TLE patients, only small to moderate effect sizes were identified for the contralateral hippocampus. Bilateral decreases in hippocampal volume with increased epilepsy duration have been observed in left and right TLE using MRI volumetry and PET (Jokeit et al., 1999). However, results based on significance testing have been mixed depending on the imaging modality: a previous DTI study found correlated changes in left but not right TLE (Kemmotsu et al., 2011), while a voxel-based morphometry study found no evidence of gray or white matter changes in a combined group of left and right TLE patients relation to longer epilepsy duration (Bernasconi et al., 2004). Our consideration of effect sizes in addition to significance testing supports a hypothesis that progressive changes in hippocampal structural integrity may be greatest ipsilaterally, with possible progressive changes also occurring contralaterally in left TLE. Other diffusion measures with a large effect size for correlation with epilepsy duration may also be worthwhile to further investigate for utility as markers of disease load. We identified a large effect size for increased MD of the right cingulum with longer epilepsy duration among both left and right TLE patients. As mentioned previously, the cingulum is the primary input into the hippocampus and a major tract of the limbic system. The correlation of increased MD of the cingulum with longer epilepsy duration may suggest that the level of disruption in tracts connecting limbic structures becomes more severe with longer epilepsy duration. Left-right asymmetry in correlational changes of the cingulum with longer epilepsy duration may stem from a weaker baseline level of structural integrity of the right compared to left cingulum, as identified through previous investigations of healthy controls (Gong et al., 2005). A large effect size for decreased FA and increased MD of the fornix with longer epilepsy duration was also found for right TLE patients. A previous study found that FA of the fornix is negatively correlated with epilepsy duration in patients with non-lesional TLE, but not for TLE with mesial temporal sclerosis (Concha et al., 2009). Inclusion of both pathologies in our study may lead to mitigation of these effects.
4.3. Limitations
One strength of the current study is the large number of TLE patients (n=28) investigated compared to prior studies (Ahmadi et al., 2009; Arfanakis et al., 2002; Concha et al., 2009; Govindan et al., 2008; Knake et al., 2009; Lin et al., 2008; McDonald et al., 2008; Meng et al., 2010; Nilsson et al., 2008; Rodrigo et al., 2007; Wang et al., 2010). This enabled separate investigation of left and right TLE patients, and was more powerful in examining progressive changes in TLE compared to prior studies. Several methodological factors should also be considered. One limitation of this study is the cross-sectional study design. An advantage of cross-sectional over longitudinal study designs is the ability to investigate changes over a wider range of time than is typically feasible with longitudinal studies. Generally, however, cross-sectional designs are not ideal for assessing the effects of TLE progression on the structural integrity of hippocampal inputs and outputs due to potential confounding effects of age. In order to mitigate these effects, we corrected for age as well as age of disease onset in correlational assessment with epilepsy duration. However, decreased effect size may have contributed to decreased power for detecting subtle progressive changes.
Second, automatic registration of individual brains to a normalized template was used to delineate regions investigated in this study. Manual delineation is historically considered the gold standard technique for region delineation, and several studies have shown that regions obtained through automatic parcellation techniques and manual delineation may differ systematically (Morey et al., 2009; Pardoe et al., 2009). However, manual delineation of multiple brain structures is less practical in larger datasets and may lead to greater intra-observer variability. Furthermore, the use of automated techniques allows for inter-study comparability, in particular to the large number of neuroimaging studies which have used the AAL atlas to understand changes in brain topology and connectivity. Our use of automated parcellation techniques to segment structures that traditionally difficult to delineate, such as the hippocampus, is expected to tend to label more tissue as hippocampus than if manual delineation were used (Morey et al., 2009; Pardoe et al., 2009). However, a recent study showed that the intraclass correlation coefficient (ICC) between manual delineation and automatic segmentation of the hippocampus is reasonably acceptable, with ICC values of 0.7–0.8 for healthy controls, and ICC values of 0.65–0.75 for patients. Moreover, the systematic differences present in automated parcellation methods were found not to impact the detection of group-wise differences (Nugent et al., 2013).
Third, right TLE had slightly longer disease duration than left TLE. Although this difference was not significant (p=0.23), it is possible that the difference may have influenced some left-right differences observed in white matter integrity.
Fourth, use of antiepileptic drugs (Gunbey et al., 2011) and heterogeneity in TLE etiology (Scanlon et al., 2013) have also been shown to affect DTI. Earlier age of onset has also been associated with decreased hippocampal volumes (Trenerry et al., 1993). As a population-based study of TLE, our patient sample inevitably includes patients with several different pathologies. This allowed us to evaluate patterns in diffusion that occur consistently across a wide range of TLE pathologies and may be more relevant to the general TLE population. However, heterogeneity of pathology, disease duration, seizure frequency and severity, and age of disease onset may also lead to attenuated detection of changes in TLE. Further studies on more homogeneous groups are needed to control for these potential confounds.
5. Conclusions
The white matter structural integrity of the hippocampus and associated white matter tracts is disrupted in TLE, evident as increased mean diffusivity in several structures bilaterally. We also found an increase in the mean diffusivity of the left hippocampus correlating with epilepsy duration in left TLE, suggesting an ongoing pathological process as the disease progresses. This study illustrates the potential of structural DTI imaging in TLE diagnosis and prognostication.
Highlights.
DTI analysis in TLE showed increased MD and decreased FA of hippocampal connections.
Increased left hippocampal MD correlated strongly with epilepsy duration in left TLE.
Right hippocampal MD had the strongest discriminatory ability between left and right TLE.
We find a reduction in white matter integrity with increasing duration of TLE that is distinct based on TLE laterality.
Acknowledgments
Sharon Chiang’s contributions to this article include study concept or design, statistical analysis, drafting/revising the manuscript for content, and analysis/interpretation of data. Harvey S. Levin’s contributions to this article include revising the manuscript for content. Elisabeth Wilde’s contributions to this article include revising the manuscript for content. Zulfi Haneef’s contributions to this article include study concept or design, drafting/revising the manuscript for content, analysis/interpretation of data, study supervision, and obtaining funding. The authors acknowledge Raja Muthupillai, Claudio Arenas, Cynthia Calija, Sikawat Thanaviratananich, and Christina Thomas for help with methodology, recruitment, and data collection. Funding for the design and conduct of this study; for the collection, management, analysis, and interpretation of the data; and for the preparation, review, and approval of the manuscript was provided by the Epilepsy Foundation of America. Support for this publication was provided by the National Library of Medicine Training Fellowship in Biomedical Informatics, Gulf Coast Consortia for Quantitative Biomedical Sciences (Grant #2T15LM007093-21) (SC); the National Institute of Health (Grant #5T32CA096520-07) (SC); the Moody Foundation (HSL); Epilepsy Foundation of America (Research Grants Program) (ZH); and the Baylor College of Medicine Computational and Integrative Biomedical Research Center Seed Grant Awards (ZH).
Abbreviations
- TLE
temporal lobe epilepsy
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- MD
mean diffusivity
- FDR
false discovery rate
- CE
change-in-estimate
- ST
significance testing
- RF
random forests
- EC
external capsule
- TR
repetition time
- TE
echo time
- FOV
field of view
- NSA
number of signals averaged
Footnotes
Conflicts of interest
None of the authors have any disclosures/conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sharon Chiang, Email: sc4712@rice.edu.
Harvey S. Levin, Email: hlevin@bcm.edu.
Elisabeth Wilde, Email: ewilde@bcm.edu.
Zulfi Haneef, Email: zulfi.haneef@bcm.edu.
References
- Ahmadi ME, Hagler DJ, Jr, McDonald CR, Tecoma ES, Iragui VJ, Dale AM, Halgren E. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR American journal of neuroradiology. 2009;30:1740–1747. doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo D, Santos AC, Velasco TR, Wichert-Ana L, Terra-Bustamante VC, Alexandre V, Jr, Carlotti CG, Jr, Assirati JA, Jr, Machado HR, Walz R, Leite JP, Sakamoto AC. Volumetric evidence of bilateral damage in unilateral mesial temporal lobe epilepsy. Epilepsia. 2006;47:1354–1359. doi: 10.1111/j.1528-1167.2006.00605.x. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Hermann BP, Rogers BP, Carew JD, Seidenberg M, Meyerand ME. Diffusion tensor MRI in temporal lobe epilepsy. Magnetic resonance imaging. 2002;20:511–519. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- Babb T, Brown W. Pathological findings in epilepsy. In: Engel J Jr, editor. Surgical treatment of the epilepsies. Raven Press; New York: 1987. pp. 511–540. [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of magnetic resonance Series B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Bernasconi N, Bernasconi A, Andermann F, Dubeau F, Feindel W, Reutens DC. Entorhinal cortex in temporal lobe epilepsy: a quantitative MRI study. Neurology. 1999;52:1870–1876. doi: 10.1212/wnl.52.9.1870. [DOI] [PubMed] [Google Scholar]
- Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004;23:717–723. doi: 10.1016/j.neuroimage.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology. 2010;74:1776–1784. doi: 10.1212/WNL.0b013e3181e0f80a. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology. 2009;72:1747–1754. doi: 10.1212/01.wnl.0000345969.57574.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson P, Dinkelacker V, Valabregue R, Thivard L, Leclerc X, Baulac M, Sammler D, Colliot O, Lehericy S, Samson S, Dupont S. Structural connectivity differences in left and right temporal lobe epilepsy. Neuroimage. 2014;100:135–144. doi: 10.1016/j.neuroimage.2014.04.071. [DOI] [PubMed] [Google Scholar]
- Breiman L. Random forests. Machine learning. 2001;45:5–32. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Academic Press; New York, New York: 1977. [Google Scholar]
- Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. Journal of neurology, neurosurgery, and psychiatry. 2009;80:312–319. doi: 10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- Corballis MC, Morgan MJ. On the biological basis of human laterality: I. Evidence for a maturational left–right gradient. Behavioral and Brain Sciences. 1978;1:261–269. [Google Scholar]
- Engel J, Jr, Thompson PM, Stern JM, Staba RJ, Bragin A, Mody I. Connectomics and epilepsy. Current Opinion in Neurology. 2013;26:186–194. doi: 10.1097/WCO.0b013e32835ee5b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage. 2008;40:728–737. doi: 10.1016/j.neuroimage.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cerebral Cortex. 2009;19:524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Jiang T, Zhu C, Zang Y, He Y, Xie S, Xiao J. Side and handedness effects on the cingulum from diffusion tensor imaging. Neuroreport. 2005;16:1701–1705. doi: 10.1097/01.wnr.0000183327.98370.6a. [DOI] [PubMed] [Google Scholar]
- Govindan RM, Makki MI, Sundaram SK, Juhasz C, Chugani HT. Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy research. 2008;80:30–41. doi: 10.1016/j.eplepsyres.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DW. Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia. 2011;52(Suppl 4):32–34. doi: 10.1111/j.1528-1167.2011.03149.x. [DOI] [PubMed] [Google Scholar]
- Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–1363. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Gunbey HP, Ercan K, Findikoglu AS, Bilir E, Karaoglanoglu M, Komurcu F, Alhan A. Secondary corpus callosum abnormalities associated with antiepileptic drugs in temporal lobe epilepsy. A diffusion tensor imaging study. The neuroradiology journal. 2011;24:316–323. doi: 10.1177/197140091102400223. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain. 2009;132:2822–2830. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Ebner A, Arnold S, Schuller M, Antke C, Huang Y, Steinmetz H, Seitz RJ, Witte OW. Bilateral reductions of hippocampal volume, glucose metabolism, and wada hemispheric memory performance are related to the duration of mesial temporal lobe epilepsy. J Neurol. 1999;246:926–933. doi: 10.1007/s004150050484. [DOI] [PubMed] [Google Scholar]
- Kemmotsu N, Girard HM, Bernhardt BC, Bonilha L, Lin JJ, Tecoma ES, Iragui VJ, Hagler DJ, Halgren E, McDonald CR. MRI analysis in temporal lobe epilepsy: Cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia. 2011;52:2257–2266. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knake S, Salat DH, Halgren E, Halko MA, Greve DN, Grant PE. Changes in white matter microstructure in patients with TLE and hippocampal sclerosis. Epileptic disorders : international epilepsy journal with videotape. 2009;11:244–250. doi: 10.1684/epd.2009.0272. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biological psychiatry. 2003;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman BA, Bogovic JA, Carass A, Chen M, Roy S, Shiee N, Yang Z, Kishore B, Pham D, Bazin PL, Resnick SM, Prince JL. System for integrated neuroimaging analysis and processing of structure. Neuroinformatics. 2013;11:91–103. doi: 10.1007/s12021-012-9159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. Journal of magnetic resonance imaging : JMRI. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput Biol. 2009;5:e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Riley JD, Juranek J, Cramer SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy research. 2008;82:162–170. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Sisodiya SM, Bartlett PA, Shorvon SD, Sander JW, Duncan JS. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia. 2005;46:1482–1494. doi: 10.1111/j.1528-1167.2005.51603.x. [DOI] [PubMed] [Google Scholar]
- Mayanagi Y, Watanabe E, Kaneko Y. Mesial temporal lobe epilepsy: clinical features and seizure mechanism. Epilepsia. 1996;37(Suppl 3):57–60. doi: 10.1111/j.1528-1157.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Xiang J, Kotecha R, Rose D, Zhao H, Zhao D, Yang J, Degrauw T. White matter abnormalities in children and adolescents with temporal lobe epilepsy. Magnetic resonance imaging. 2010;28:1290–1298. doi: 10.1016/j.mri.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, LaBar KS, Styner M, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson D, Go C, Rutka JT, Rydenhag B, Mabbott DJ, Snead OC, 3rd, Raybaud CR, Widjaja E. Bilateral diffusion tensor abnormalities of temporal lobe and cingulate gyrus white matter in children with temporal lobe epilepsy. Epilepsy research. 2008;81:128–135. doi: 10.1016/j.eplepsyres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA, Jr, Drevets WC. Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Hum Brain Mapp. 2013;34:2313–2329. doi: 10.1002/hbm.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, Akhter K, Hua K, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Zhang J, Huang H, Miller MI, van Zijl PC, Mazziotta J, Mori S. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43:447–457. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte WM, van Eijsden P, Sander JW, Duncan JS, Dijkhuizen RM, Braun KP. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia. 2012;53:659–667. doi: 10.1111/j.1528-1167.2012.03426.x. [DOI] [PubMed] [Google Scholar]
- Pardoe HR, Pell GS, Abbott DF, Jackson GD. Hippocampal volume assessment in temporal lobe epilepsy: How good is automated segmentation? Epilepsia. 2009;50:2586–2592. doi: 10.1111/j.1528-1167.2009.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, Duncan JS. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007;36:209–221. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Rodrigo S, Oppenheim C, Chassoux F, Golestani N, Cointepas Y, Poupon C, Semah F, Mangin JF, Le Bihan D, Meder JF. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. European radiology. 2007;17:1663–1668. doi: 10.1007/s00330-006-0558-x. [DOI] [PubMed] [Google Scholar]
- Rohlfing T. Incorrect ICBM-DTI-81 atlas orientation and white matter labels. Frontiers in neuroscience. 2013;7:4. doi: 10.3389/fnins.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon C, Mueller SG, Cheong I, Hartig M, Weiner MW, Laxer KD. Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. J Neurol. 2013;260:2320–2329. doi: 10.1007/s00415-013-6974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So N, Gloor P, Quesney LF, Jones-Gotman M, Olivier A, Andermann F. Depth electrode investigations in patients with bitemporal epileptiform abnormalities. Annals of neurology. 1989;25:423–431. doi: 10.1002/ana.410250502. [DOI] [PubMed] [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Thivard L, Lehericy S, Krainik A, Adam C, Dormont D, Chiras J, Baulac M, Dupont S. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2005;28:682–690. doi: 10.1016/j.neuroimage.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Tong IS, Lu Y. Identification of confounders in the assessment of the relationship between lead exposure and child development. Ann Epidemiol. 2001;11:38–45. doi: 10.1016/s1047-2797(00)00176-9. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Jack CR, Jr, Sharbrough FW, Cascino GD, Hirschorn KA, Marsh WR, Kelly PJ, Meyer FB. Quantitative MRI hippocampal volumes: association with onset and duration of epilepsy, and febrile convulsions in temporal lobectomy patients. Epilepsy Res. 1993;15:247–252. doi: 10.1016/0920-1211(93)90062-c. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Lang SY, Hong LU, Lin MA, Yan-ling MA, Yang F. Changes in extratemporal integrity and cognition in temporal lobe epilepsy: a diffusion tensor imaging study. Neurology India. 2010;58:891–899. doi: 10.4103/0028-3886.73739. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, Alexander DC, Symms MR, Koepp MJ, Duncan JS. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133:2348–2364. doi: 10.1093/brain/awq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liao W, Chen H, Mantini D, Ding JR, Xu Q, Wang Z, Yuan C, Chen G, Jiao Q, Lu G. Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain. 2011;134:2912–2928. doi: 10.1093/brain/awr223. [DOI] [PubMed] [Google Scholar]