Abstract
Highly aggressive cancers “entrain” innate and adaptive immune cells to suppress anti-tumor lymphocyte responses. Circulating myeloid-derived suppressor cells (MDSCs) constitute the bulk of monocytic immunosuppressive activity in late stage melanoma patients. Previous studies revealed that monocyte-derived macrophage migration inhibitory factor (MIF) is necessary for the immune suppressive function of tumor-associated macrophages (TAMs) and MDSCs in mouse models of melanoma. In the current study we sought to determine whether MIF contributes to human melanoma MDSC induction and T-cell immunosuppression using melanoma patient-derived MDSCs and an ex vivo co-culture model of human melanoma-induced MDSC. We now report that circulating MDSCs isolated from late stage melanoma patients are reliant upon MIF for suppression of antigen-independent T-cell activation and that MIF is necessary for maximal reactive oxygen species (ROS) generation in these cells. Moreover, inhibition of MIF results in a functional reversion from immune suppressive MDSC to an immunostimulatory dendritic cell (DC)-like phenotype that is at least partly due to reductions in MDSC prostaglandin E2 (PGE2). These findings indicate that monocyte-derived MIF is centrally involved in human monocytic MDSC induction/immune suppressive function and that therapeutic targeting of MIF may provide a novel means of inducing anti-tumor DC responses in late stage melanoma patients.
Keywords: Melanoma, MDSC, MIF, immune suppression, prostaglandin E2
Introduction
Stage IV melanoma is a highly aggressive and resistance prone malignancy that carries a 5 year survival rate of ~ 15%. Melanoma cells are unusually immunogenic and, consequently, are adept at inducing host innate and adaptive immune suppressive mechanisms that, collectively, serve to attenuate anti-tumor lymphocyte responses (1). Adaptive cell types and effectors involved in melanoma-associated immune suppression include regulatory T lymphocytes (Treg), cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death 1 (PD-1) – of which the latter two are currently being evaluated as therapeutic targets in late stage melanoma patients (2).
It is becoming increasingly evident that tumor-entrained innate immune effector cells – e.g., tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), tolerogenic dendritic cells, and MDSCs – also provide highly significant degrees of immune escape to aggressive malignancies (3-7). In patients with advanced melanoma, circulating monocytic MDSCs provide the bulk of monocyte-associated immune suppression (8), negatively impact patient survival and inversely correlate with the presence of functional antigen-specific T cells (9).
Previous studies from our laboratory established a novel functional role for monocyte-derived MIF in dictating alternative activation phenotypes in mouse melanoma TAMs; loss or inhibition of MIF reduces melanoma TAM and MDSC-mediated immune suppression (10). In a related study using the 4T1 mouse model of breast cancer, Simpson and colleagues showed that tumor-derived MIF promotes MDSC accumulation and immunosuppressive activity (11). Reconstitution of wildtype MIF cDNA into 4T1 MIF shRNA knockdown cells, but not an enzymatically inactive MIF mutant (proline-2 to serine-2, P2S) cDNA, was capable of reconstituting tumor-derived, MIF-dependent MDSC induction. This was in line with our finding that small molecule inhibitors of MIF’s enzymatic activity fully phenocopy MIF-deficiency in their ability to dictate the immune suppressive activities of monocytes/macrophages in tumor-bearing hosts (10).
Studies by the Dranoff laboratory have identified MIF as a target of naturally developing auto-antibodies in late stage melanoma patients who had successfully responded to a trial immunotherapy consisting of autologous GM-CSF secreting tumor cell vaccines followed by CTLA-4 blockade (Ipilimumab) (12). MIF auto-antibodies disrupted MIF-dependent effects on human monocytes/macrophages, suggesting that the beneficial effects of these MIF-targeting auto-antibodies in advanced melanoma patients are due to inhibition of MIF-dependent innate immune stromal cell phenotypes. Although this finding suggests a clinically relevant role for MIF in human melanoma disease progression/survival, no studies have been done to directly investigate the functional and/or mechanistic contributions of MIF to innate immune cell-mediated immune suppression in melanoma patients.
Using our well-characterized, small-molecule MIF enzymatic antagonist (4-iodo-6-phenylpyrimidine, 4-IPP) (10,13-15), we investigated MIF contributions to human melanoma MDSC induction, phenotype, differentiation status, and mechanistic effectors. We show that human MDSCs derived from late stage melanoma patients and those induced in vitro by tumor cells, rely on MIF to suppress T cell activation. MIF reliance corresponds with reactive oxygen species (ROS) and cyclooxygenase-2 (COX-2)/PGE2 production elicited by MDSCs. Unexpectedly, when MDSC-derived MIF is inhibited during short-term ex vivo culture of MDSCs, their differentiation is redirected toward a more DC-like phenotype. These MIF-inhibited monocytic MDSCs induce antigen-specific T cell stimulatory function in these cells.
Combined, our results support a crucial pro-tumorigenic contribution by MIF to the immune suppression and differentiation of circulating melanoma MDSC and provide justification for therapeutic targeting of MIF in patients with advanced melanoma disease.
Materials and Methods
Patient samples and cell lines
Peripheral blood was collected from 27 patients with metastatic melanoma stage III to IV, and from 12 healthy donors. Melanoma patients included in this study were not undergoing therapy when their samples were collected and they all had progressive disease. Patient samples were collected after receiving informed consent by staff of the JG Brown Cancer Center Biorepository and covered under University of Louisville IRB protocol number 08.0388. Melanoma cell line [A375] (ATCC® CRL-1619™) was purchased from ATCC (Manassas, VA) and maintained in DMEM containing 10% (v/v) FBS. We do not culture this cell line longer than 6-8 weeks and all of our stocks come from thawed vials that were frozen at passage two after receiving from ATCC. A375 cell line was authenticated by ATCC cell bank using the Short Tandem Repeat (STR) profiling.
Mice
Wildtype male C57BL/6 mice (MIF+/+) were obtained from Harlan Laboratories. OT-1 and OT-II transgenic mice were obtained from Jackson Laboratory. All mice were handled with the approval of the Institutional Animal Care and Use Committee at University of Louisville.
MDSC isolation and 4-IPP treatment
Monocytic CD14+ MDSCs from melanoma patients were isolated from PBMCs using anti-CD14 magnetic microbeads and the autoMACS Pro Separator (Miltenyi Biotec, Auburn, CA), per manufacturer’s instructions. One million MDSCs were plated in complete IMDM medium (supplemented with 10% human AB serum [Sigma-Aldrich, St. Louis, MO], 2 mM L-glutamine, and penicillin/streptomycin) per well in a 6-well plate (BD Falcon) and treated with 4-IPP (50 μM) or DMSO (vehicle control) for 24 hours. For functional experiments, autologous T cells were isolated from PBMCs using the Pan T-cell Isolation kit (Miltenyi Biotec).
In vitro generation of human MDSC
CD14+ cells (1 × 106) isolated from PBMCs obtained from healthy donors were co-cultured with 5 × 105 A375 tumor cells in complete IMDM medium per well in a 6-well plate (16). Tumor/monocyte co-cultures were treated twice with 4-IPP (100 μM on day 0 and 50 μM on day 2) or 0.1% DMSO (vehicle control). A375 co-cultured monocytes (both untreated and 4-IPP treated) and control monocytes cultured without tumor cells were harvested by gently scraping after 64-68 hours of culture and CD11b+ cells were purified. Details for cell isolation techniques used are provided in the Supplemental Methods section.
Mouse bone marrow-derived MDSC
Tibias and femurs from MIF+/+ and MIF−/− C57BL/6 mice were removed using sterile techniques and bone marrow (BM) was flushed. To obtain BM-derived MDSCs, BM cells were cultured for 4 days with GM-CSF (40 ng/mL), and IL-6 (40 ng/mL) cytokines as previously described (17). MIF+/+ and MIF−/− BM cultures were treated with 0.1% DMSO (vehicle control) or with 4-IPP (50 μM) during the last 48 hours of the culture period. For functional assays, CD11b+GR1+ BM-MDSCs were isolated from BM cultures using CD11b and GR1 microbeads followed by magnetic separation (Miltenyi).
Antibodies and flow cytometry
Untreated or 4-IPP-treated melanoma patient MDSCs and tumor cell line-induced MDSCs (A375-MDSCs) were stained with anti-human antibodies according to the manufacturer’s recommendations. Details on flow cytometry staining and antibody panels are provided in Supplemental Methods section and in Supplemental Table 1.
Functional Studies
To evaluate the suppressive functions of melanoma patient-derived MDSCs and A375-MDSCs, autologous T cells were labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE, Invitrogen, Grand Island, NY) and seeded at 100,000 cells per well in a 96-well U bottom plate. For patient samples, freshly purified CD14+ cells or CD14+ cells that were pre-treated with or without 4-IPP for 24 hours were added to T cells at ratios of 2:1, 1:1, or 1:2. T cells were activated by addition of anti-CD3/CD28 mAb-coated beads (Invitrogen) per well for 4 days. T-cell activation was measured by flow cytometry and IFN-γ concentrations in the supernatants were determined by ELISA. Controls included non-activated T cells or T cells activated with beads alone. For A375-MDSCs, CD11b+ monocytes or CD11b+HLA-DR− cells purified from tumor co-cultures with or without 4-IPP treatment for 64 hours were added to T cells at ratios of 1:2 or 1:4 and T-cell activation was measured as above.
DC phenotype and function
Melanoma patient MDSCs and A375-MDSCs were either untreated or treated with 4-IPP (50 μM), PGE2 (10 μM) or with 4-IPP plus PGE2 for 72 hours and were analyzed for the expression of human DC markers by flow cytometry. For mouse DC phenotype studies, BM-MDSCs from MIF+/+ and MIF−/− mice were cultured for 48 hours and analyzed for mouse DC marker expression by flow cytometry. DC function in A375-MDSCs was analyzed using Tetanus Toxoid (TT) antigen presentation assays. A375-MDSCs were either untreated or treated with 4-IPP (50 μM) or 4-IPP (50 μM) plus PGE2 (10 μM) for 72 hours. MDSCs were added to autologous, CFSE-labeled T cells at ratios of 1:5 (20,000 MDSCs) or 1:10 (10,000 MDSCs). T cells were activated with 1.0 μg/mL of TT per well for 5 days. T-cell proliferation was measured by flow cytometry. DC function in mouse BM-MDSCs was determined using the OT-II TCR transgenic mice and ovalbumin (OVA) antigen presentation. MIF+/+ and MIF−/− BM-MDSCs harvested post 48 hour culture period were added to CFSE labeled CD4+ T cells purified from OT-II splenocytes at 1:5 and 1:10 ratio in the presence of 200 μg/mL of OVA (Sigma-Aldrich) per well for 5 days. CD4+ T-cell activation was estimated by flow cytometry.
Quantitative PCR analysis
Total RNA and real-time analysis was performed as previously described (10). Taqman probes (Applied Biosystems) for genes 18S (Hs99999901.s1; VIC), MIF (Hs00236988_g1; FAM), COX-2 (Hs00153133_m1; FAM), and NOX4 (Hs00418356_m1; FAM), were used according to manufacturer’s instructions.
Microarray Analysis
Total RNA from cultured monocytes, vehicle-treated and 4-IPP-treated A375-MDSCs were isolated and microarray analysis was performed according to the manufacturer's instructions. Details of the instrumental set up and analysis are described in Supplemental Methods section. The microarray datasets discussed in current study have been deposited in NCBI's Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo) and are accessible through GEO Series accession number GSE73333.
ROS detection
The oxidation-sensitive dye DCF-DA was used to measure ROS production in untreated or 4-IPP-treated melanoma patient MDSCs or A375-MDSCs. Details of ROS estimation by flow cytometry are provided in Supplemental Methods section.
Western blotting
Lysates of cultured monocytes and A375-MDSC were probed with antibodies that recognize human MIF and human GAPDH (Santa Cruz Biotechnology, Inc.).
ELISAs
Cytokines were measured by ELISA in supernatants from T cell:MDSC co-cultures and from MDSC cultures. ELISA kits used were the human IFN-γ and PGE2 kits obtained from R&D Systems.
Statistical analysis
GraphPad Prism 5.0 software (GraphPad Prism Software, Inc., La Jolla, CA) was used for all statistical analyses. Two-group comparisons between control and test samples (groups compared are indicated in the respective figures) were done by two-tailed Student’s t tests. Multiple data comparisons were derived by one-way ANOVA followed by Tukey’s post-hoc test. For all tests, statistical significance was assumed where p<0.05.
Results
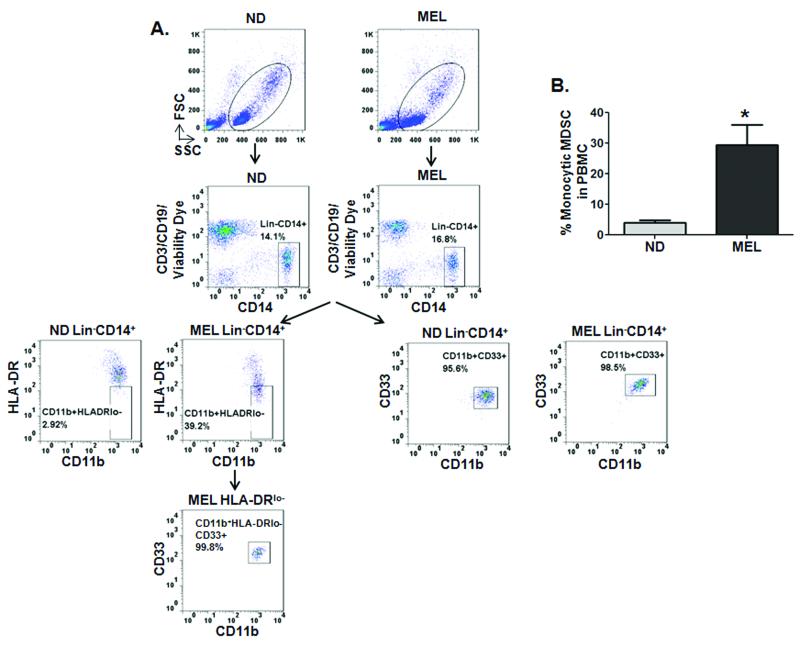
Circulating CD14+HLADR−/low MDSCs with potent immunoregulatory activities have been identified in the peripheral blood of ovarian (18), hepatocellular (19) and late stage melanoma patients (20-22). In an effort to extend our previous findings that monocytic cell-derived MIF provides functional contributions to MDSC immune suppressive activity (10), we first analyzed the frequency and phenotype of circulating monocytic MDSCs in stage III/IV metastatic melanoma patients using multicolor FACS analysis. Representative dot plots for one of the melanoma patients and one of the normal donors included in the study to illustrate the gating strategy used (Fig. 1A). The percentage of circulating lineage−(Lin−)CD14+CD11b+CD33+HLADR−/low monocytic MDSCs is significantly elevated in melanoma patients’ freshly isolated peripheral blood compared to that of normal donors (Fig. 1A and B) (21,22).
Figure 1. CD14+HLA-DR−/low MDSCs are increased in the peripheral blood of patients with advanced melanoma.
(A) Flow cytometry evaluation of expression of Lineage (Lin; CD3/CD19), CD11b, CD14, HLA-DR and CD33 in PBMCs obtained from normal donor (ND) and melanoma patient (MEL). An example of representative dot plots after excluding aggregates and dead cells is shown (top panel). Numbers represent the percentages from the populations gated. Names above FACS plots indicate the population gated that was analyzed. Markers analyzed are indicated in the axis of each FACS plot. The gating strategy used to analyze the samples is illustrated. Gates were set based on isotype controls (B) Bar graph showing the percentage of CD14+CD11b+HLA-DR–/lo MDSCs in late stage melanoma patients (MEL; n = 5) versus healthy donor (ND; n = 5) PBMCs. Data represents the average ± SEM of 5 independent experiments. P values = *, p≤0.05.
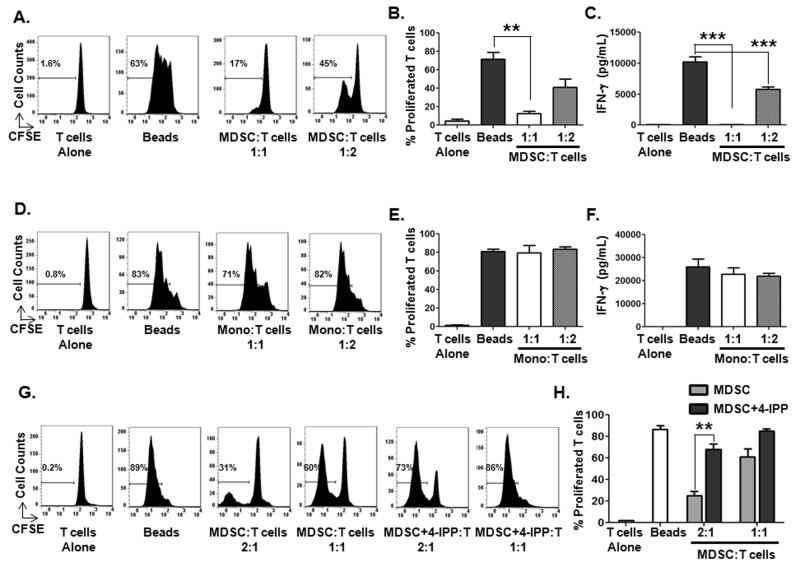
Consistent with prior studies (21,22), purified CD14+ melanoma monocytes exhibit potent inhibitory activity against autologous T-cell activation (Fig. 2A and B) and IFN-γ production (Fig. 2C) induced by anti-CD3/anti-CD28, compared to cultured CD14+ monocytes from normal donors (Fig. 2D-F). This finding also is consistent with prior studies (8) demonstrating that both HLADR+ and HLADR–/lo CD14+ circulating myeloid cell populations represent highly immunsuppressive MDSCs. We next determined whether inhibition of melanoma MDSC MIF with our small molecule MIF enzymatic antagonist, 4-IPP, (10,13-15) affected MDSC immune suppressive activity. Treatment of melanoma patient-derived CD14+ monocytic MDSCs with 4-IPP for 24 hours significantly reduces their T cell inhibitory activity (Figs. 2G and H). No toxicity or loss of viability was observed in MDSCs treated with either 4-IPP or vehicle (Supplemental Fig. S1A) although there was a slight decrease in MDSC suppressive activity compared to freshly isolated MDSCs (compare Fig. 2A to Fig. 2G). This loss of T cell suppressive activity in short-term, cytokine free cultures of MDSCs is likely a result of ex vivo culture in the absence of tumor-derived MDSC polarizing factors.
Figure 2. Melanoma MDSCs suppress autologous T-cell activation in an MIF-dependent manner.
(A-C) Melanoma patient-derived CD14+ MDSCs were cultured with CFSE-labeled autologous T cells and anti-CD3/anti-CD28 beads for 4 days and T-cell activation was determined. Representative histograms (A) and bar graphs showing the percentage of proliferated T cells (B) and IFN-γ production (C). (D-F) Healthy donor CD14+ monocytes were cultured with CFSE-labeled autologous T cells and anti-CD3/anti-CD28 beads for 4 days and T-cell activation was determined. Representative histograms (D) and bar graphs showing the percentage of proliferated T cells (E) and IFN-γ production (F). (G, H) Melanoma MDSCs were pre-treated with or without 50 μM 4-IPP for 24 hours and then added to CFSE-labeled autologous T cells and anti-CD3/anti-CD28 beads for 4 days. Representative histograms (G) and bar graphs (H) showing the percentage of proliferated T cells. Data represents the average ± SEM of three independent experiments. P values = **, p≤0.005; ***, p≤0.0005.
Because we were interested in pursuing validation and mechanism-based studies—both of which necessitate greater numbers of cells than would be practical using patient-derived peripheral blood samples—we established an in vitro model of melanoma cell-line–induced MDSCs that faithfully recapitulates patient-derived CD14+HLADR−/low monocytic MDSC phenotype and function (16). This model utilizes a co-culture system consisting of A375 human melanoma cells and normal donor CD14+ monocytes co-cultured for ~ 68 hours (16). We characterized the phenotype of the monocytic MDSC-like cells induced during the A375-monocyte co-culture, with multicolor flow cytometry. The percentage of CD14+CD11b+CD33+HLADR−/low cells was substantially increased in A375-monocyte co-cultures in comparison to that in monocytes cultured in the absence of melanoma cells, (Supplemental Fig. S2). Furthermore, CD11b+ cells purified from the A375 monocyte co-cultures exhibit a significant reduction in HLA-DR and increases in CD14, CD33, PD-L1, and DC-SIGN markers (Supplemental Fig. S3A and B) – an expression signature that closely corresponds to monocytic MDSCs isolated from late stage melanoma patients (16,21,23). Although we refer to the CD11b+ cells isolated from the co-cultures of A375 cells and monocyte as “A375-MDSCs”, these cells represent a heterogenous population of cells, similar to the MDSCs isolated from melanoma patients (8). MIF mRNA and protein expression was increased in A375-MDSCs compared to monocytes cultured without tumor cells for the same period of time (Supplemental Fig. S3C and D).
To determine whether MIF inhibition during MDSC induction influences the acquisition of MDSC phenotype/function, 4-IPP was added at the beginning of the A375:monocyte co-culture. Changes in cell surface marker expression of A375-MDSCs and relative T cell suppressive activity were assessed 68 hours later. MIF inhibition during the MDSC induction phase by melanoma cells resulted in reductions of CD14, CD33, and PD-L1 and an increase in DC-SIGN expression, whereas HLA-DR or CD11c expression was not significantly altered (Supplemental Figs. S3A and B).
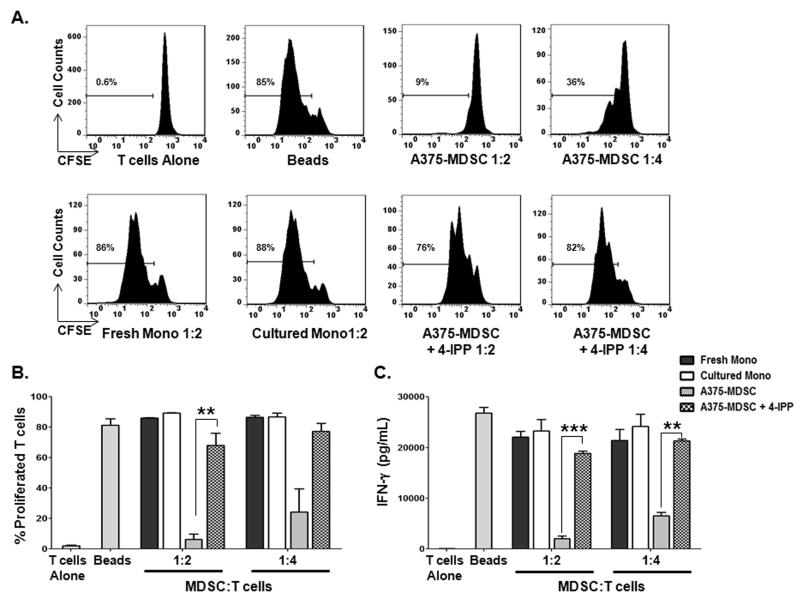
With respect to functional immune suppressive activities, A375-MDSCs were potent suppressors of autologous T-cell activation and IFN-γ production compared to fresh monocytes and tumor cell-free cultured monocytes, whereas A375-MDSCs from 4-IPP-treated co-cultures (A375-MDSCs + 4-IPP) possessed little to no suppressive activity on T cell proliferation/IFN-γ production (Fig. 3). The induction of suppressive function in monocytes relied upon direct cell contact with the tumor cells: monocytes cultured in the presence of A375 tumor cell conditioned media did not suppress autologous T-cell activation (Supplemental Fig. S4A). This finding is consistent with previous observations using the same A375-monocyte co-culture model system to induce MDSCs (16). To evaluate whether the diminished suppressive activity observed with MDSCs from 4-IPP-treated A375-monocyte co-cultures was simply due to a reduced number of MDSCs present in the 4-IPP-treated cultures, we isolated CD11b+HLA-DR− MDSCs from both untreated and 4-IPP-treated A375-monocyte co-cultures and compared their respective immunosuppressive functions. CD11b+HLA-DR− MDSCs from 4-IPP-treated co-cultures were significantly less suppressive compared to CD11b+HLA-DR− MDSCs from untreated co-cultures (Supplemental Fig. S4B). Neither melanoma nor monocyte cell viability was significantly affected by the presence of 4-IPP during co-culture (Supplemental Fig. S1B) but the possibility that 4-IPP may be influencing the expression/secretion of tumor-derived MDSC-polarizing factors including active, tumor cell-derived, MIF, was not ruled out (11).
Figure 3. MIF inhibition during melanoma cell line-induced MDSC reduces MDSC suppressive activity.
(A-C) Autologous CFSE-labeled T cells were cultured in the presence of fresh healthy donor monocytes (fresh mono), with monocytes cultured for 64 hours in the absence of melanoma cells (cultured mono), or with monocytes co-cultured with A375 cells in the absence (A375-MDSC), or presence of 4-IPP (A375-MDSC+4-IPP; 100 μM, day 0 and 50 μM, day 2). T cells were activated with anti-CD3/anti-CD28 beads in the absence or presence of the indicated monocytes/MDSCs for 4 days. Representative histograms (A) and bar graphs showing the percentage of proliferated CFSE-labeled T cells (B) and IFN-γ production (C). Data represents the average ± SEM of three independent experiments. P values = **, p≤0.005; ***, p≤0.0005.
To determine whether MIF was necessary for the suppressive function of established A375-MDSCs, isolated A375-MDSCs from A375:monocyte co-cultures were treated with 4-IPP for 24 hours. This treatment partially attenuated established A375-MDSC inhibition of T cell proliferation (Supplemental Fig. S5A and B), suggesting that MDSC-derived MIF was necessary for maximal MDSC immune suppressive functions. The effects of 4-IPP recapitulated those previously observed in established murine MDSCs (10).
To validate these observations, we turned to a murine in vitro model of bone marrow (BM)-derived MDSC induction using GM-CSF and IL-6 (BM-MDSCs) (17). Similar to 4-IPP treatment during the induction of human MDSCs by a melanoma cell line (Fig. 3), murine BM-MDSCs from MIF-deficient mice were significantly less immunosuppressive than their wildtype counterparts (Supplemental Fig. S6C). MIF-deficient BM-MDSCs expressed less of the prototypical murine MDSC marker GR1 and more CD11c when compared to BM-MDSCs derived from MIF wildtype mice – a finding suggestive of a broader defect in MDSC induction associated with loss of MIF (Supplemental Figs. S6A and B). To rule out the possibility that the lower suppressive activity observed with MIF-deficient BM-MDSCs was not simply due to fewer MDSCs present in the differentiated MIF-deficient BM-MDSCs, we isolated CD11b+GR1+ MDSCs from both MIF wildtype and MIF-deficient BM cultures and compared their immunosuppressive functions. CD11b+GR1+ MDSCs from wildtype BM-MDSCs exhibited potent inhibitory activity on antigen-specific T cell proliferation, compared to MIF-deficient CD11b+GR1+ MDSCs (Supplemental Fig. S7A).When 4-IPP was added during differentiation of wildtype CD11b+GR1+ BM-MDSCs, they were significantly less immunosuppressive compared to vehicle-treated CD11b+GR1+ BM-MDSCs (Supplemental Fig. S7B)—effectively phenocopying 4-IPP–treated human MDSCs and MIF-deficient BM-MDSCs (Supplemental Fig. S7A). To confirm that 4-IPP treatment had no off-target effects, MIF-deficient BM-MDSC were treated with 4-IPP or vehicle control. No difference in immunosuppressive activity was observed between vehicle control and 4-IPP-treated MIF-deficient BM-MDSCs (Supplemental Fig. 7A) confirming the lack of any residual in vitro 4-IPP activity in the absence of its target – MIF.
To identify potential mechanistic effectors and/or pathways associated with melanoma monocytic MDSCs, we performed mRNA microarray analyses on cultured monocytes and on A375-MDSCs obtained from either untreated or 4-IPP-treated A375:monocyte co-cultures. Expression profiles from A375-MDSCs were markedly different from cultured monocytes (Supplemental Fig. S8A).
When MIF was inhibited during A375-MDSC induction, a large subset of gene products reverted back to levels observed in monocytes cultured in the absence of tumor cells (Supplemental Fig. S8B). Inflammatory cytokines, chemokines/chemokine receptors, matrix metalloproteases, angiogenic growth factors and arachidonic acid/prostaglandin-generating enzymes were all differentially expressed in A375-MDSCs and restored to “normal” expression by MIF inhibition (Supplemental Fig. S8C).
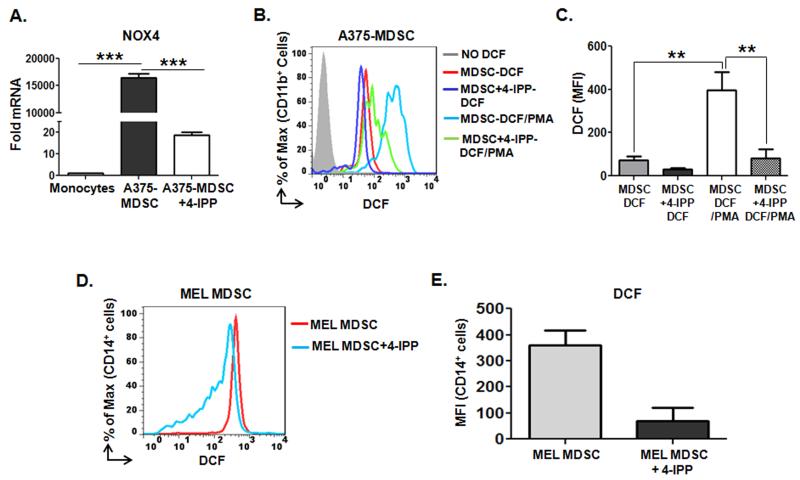
One gene product of particular interest is that of the NADPH oxidase 4 enzyme (NOX4 – Supplemental Fig. 8C). Because NADPH oxidases are centrally involved in mediating MDSC immune suppressive activities (21,24) we next validated by qPCR that NOX4 is induced in A375-MDSCs, but not in A375-MDSCs from 4-IPP-treated co-cultures (Fig. 4A). NADPH oxidases convert molecular oxygen into superoxide anion upon activation by PKC (25) so we next evaluated the relative ability of phorbol myristic acid (PMA) to induce dichlorofluorescein (DCF)-detectable ROS in A375-MDSCs. DCF fluorescence was more strongly induced by PMA in A375-MDSCs than in A375-MDSCs obtained from 4-IPP-treated co-cultures (Figs. 4B and C). In accordance with published results (21), CD14+ monocytes from freshly isolated peripheral blood mononuclear cells from melanoma patients had more ROS than healthy donor CD14+ monocytes (Supplemental Fig. S1C), and treatment of isolated melanoma MDSCs with 4-IPP for 24 hours significantly reduced DCF-detectable ROS in these cells (Fig. 4D and E).
Figure 4. MIF maintains NADPH oxidase 4 (NOX4) expression and reactive oxygen species (ROS) levels in melanoma MDSCs.
(A) Quantitative PCR analysis of NOX4 mRNA in healthy donor monocytes (n = 3) cultured for 64 hours in the absence (cultured monocytes) or presence of A375 cells. A375:monocyte co-cultures were either untreated (A375-MDSCs) or treated with 4-IPP (A375-MDSCs+4-IPP; 100 μM, day 0 and 50 μM, day 2). Data represents the average ± SEM of triplicate samples. (B, C) Representative histogram (B) and bar graph (C) of mean fluorescent intensities (MFI) of DCF-detectable ROS in untreated and PMA-treated A375-MDSCs and A375-MDSC+4-IPP. (D, E) DCF-detectable ROS levels in CD14+ late stage melanoma patient-derived MDSCs (n = 3) that were pre-treated with either DMSO or 4-IPP (50 μM) for 24 hours. Representative histogram (D) and bar graph (E) representing expression of DCF-detectable ROS in melanoma MDSCs and 4-IPP-treated MDSCs. Data represents the average ± SEM of three independent experiments. P values = **, p≤0.005; ***, p≤0.0005.
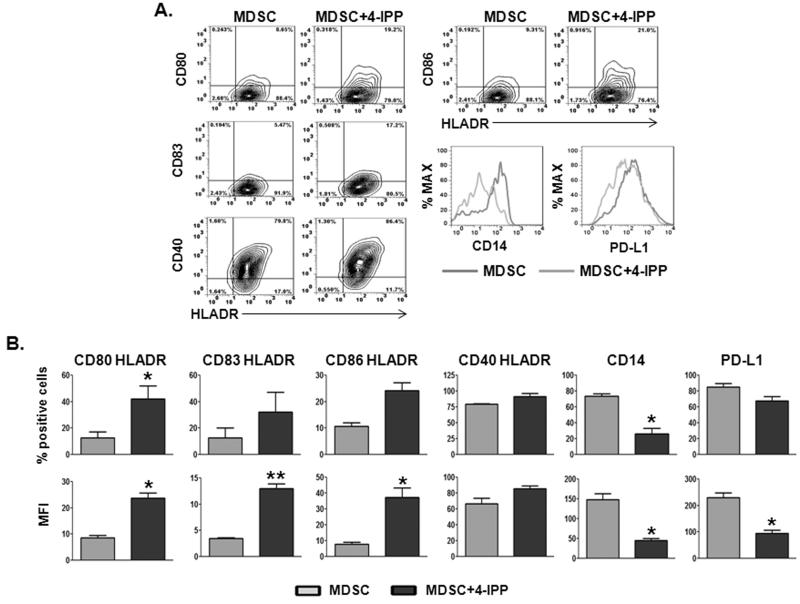
Murine MDSCs, when cultured in the presence of appropriate growth factors, can differentiate into dendritic cells (DCs) (26,27). Human melanoma MDSCs are characterized by higher levels of the DC markers, CD80, CD83 and DC-SIGN compared to normal human monocytes (21). Short-term, cytokine- free, culture of human melanoma MDSCs moderately increases the expression of DC markers—including and especially HLA-DR—but without loss of CD14 expression. The retention of CD14 expression on these cells is indicative of a lack of lineage-specific differentiation (21). In an effort to determine whether MIF inhibition influences MDSC → DC differentiation phenotypes, purified MDSCs from melanoma patients were cultured in the presence and absence of 4-IPP for 72 hours (Fig. 5). Treatment with 4-IPP resulted in significant increases in percent of cells (Fig. 5B, upper panel) and relative expression (MFI; Fig. 5B, lower panel) of DC markers CD80, CD83, CD86, CD40, and perhaps more importantly, significant reductions in CD14 and PD-L1 on HLA-DR+ MDSCs.
Figure 5. MIF inhibition induces a dendritic cell (DC) phenotype in melanoma patient-derived MDSCs.
(A, B) CD14+ melanoma patient-derived MDSCs (n = 3) were cultured ex vivo for 72 hours in the absence or presence of 4-IPP (50 μM) and analyzed for the expression of DC markers by flow cytometry. Representative dot plots (A) and bar graphs representing percentages and mean fluorescent intensities (MFI) (B) of DC marker expression on HLADR+ MDSC and 4-IPP-treated MDSC. Data represents the average ± SEM of three independent experiments. P values = *, p≤0.05; **, p≤0.005.
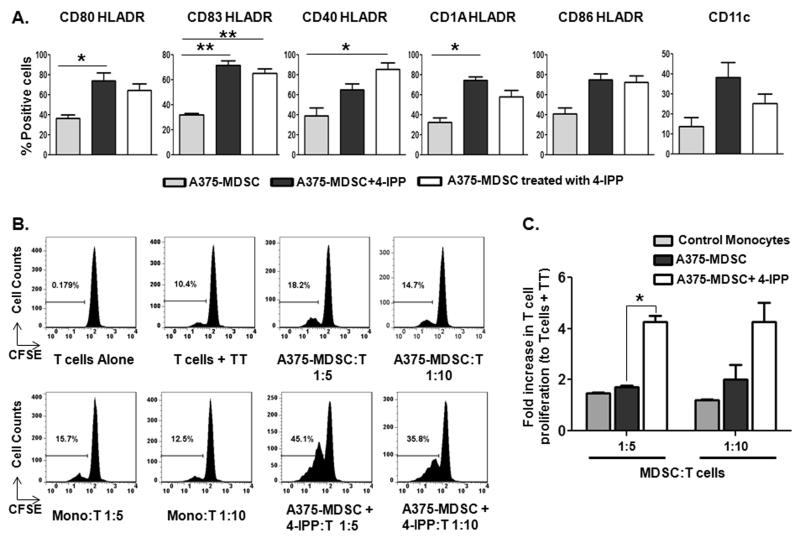
We next sought to recapitulate these findings using MDSCs derived from the A375:monocyte co-culture model. We tested two independent models (please see diagram; Supplemental Fig. S9) for different timing of MIF inhibition by 4-IPP: 1) Culturing A375 with monocyte MDSC in the prescence of 4-IPP during the induction phase, followed by culturing purified A375-MDSCs for an additional 72 hours with no other treatments (4-IPP during MDSC induction = A375-MDSC+4-IPP; Supplemental Fig. S9B), and 2) Addition of 4-IPP after MDSC induction during the 72 hour differentiation phase (4-IPP after MDSC induction = A375-MDSC treated with 4-IPP; Supplemental Fig. S9C). Changes in DC marker upregulation were similar whether 4-IPP was added during MDSC induction (A375-MDSC+4-IPP) or after MDSC induction (A375-MDSC treated with 4-IPP) compared to control, untreated A375-MDSCs (Fig. 6A). Specifically, markers associated with dendritic cells—CD80, CD83, CD40, CD1A, CD86, and CD11c—trended toward increased expression in both 4-IPP treatment conditions of HLA-DR+ A375-MDSCs although not all conditions resulted in statistically significant increases (Fig. 6A).
Figure 6. MIF inhibition in melanoma cell line-educated MDSCs induces an immunostimulatory dendritic cell (DC) phenotype and function.
CD11b+ MDSCs were isolated from untreated or 4-IPP-treated A375:monocyte co-cultures (A375-MDSC or A375-MDSC+4-IPP). Isolated cells were cultured ex vivo for an additional 72 hours. Alternatively, 4-IPP (50 μM) was added to established A375-MDSCs for a 72-hour culture period (A375-MDSC treated with 4-IPP). Cells were then analyzed for the expression of DC markers by flow cytometry. (A) Bar graphs showing the percentages of DC marker expressing HLADR+ cells in indicated cells post 72-hour culture period. (B, C) Control monocytes and established A375-MDSCs were cultured ex vivo for 72 hours in the absence or presence of 4-IPP (50 μM – as shown in Supplemental Fig. 9C). Indicated cells were added to autologous CFSE-labeled T cells in the presence of Tetanus Toxoid (TT; 1.0 μg/mL) for 5 days. Representative histograms (B) and bar graphs (C) showing the percentage of proliferated T cells. Data from (B) represents the average ± SEM of two independent experiments. P values = *, p≤0.05; **, p≤0.005.
To determine whether these phenotypic marker changes correspond to an increase in antigen-specific T cell functional responses, established A375-MDSCs were cultured with or without 4-IPP for 72 hours (per Supplemental Fig. S9C) followed by assessment of tetanus toxoid-induced T-cell activation. Neither normal donor cultured monocytes (Mono:T) nor untreated A375-MDSCs could induce tetanus toxoid-specific T-cell activation to any appreciable extent (Fig. 6B and C). In contrast, 4-IPP-treated A375-MDSCs induced a ~4-fold increase in tetanus toxoid–specific T-cell proliferation, indicating that MIF inhibition promotes established MDSC differentiation that results in antigen-specific T cell responses.
We next validated these findings in the murine MIF-deficient model, using BM-MDSCs. Like human melanoma MDSCs treated with 4-IPP, murine MIF-deficient BM-MDSCs cultured for 48 hours in cytokine-free media express elevated CD80, CD83, CD86, and MHC II-IA-IE compared to MIF wildtype BM-MDSCs (Supplemental Fig. S10A and B). These MIF-deficient BM-MDSCs could to induce ovalbumin-specific CD4+ T-cell proliferation to a significantly greater extent than BM-MDSCs from MIF wildtype mice (Supplemental Fig. S10C and D). Combined, these findings indicate that loss or inhibition of MIF promotes MDSC differentiation towards a more DC-like cell phenotype that results in noticeably improved T cell–mediated antigen-specific immune responses.
Prostaglandin E2 (PGE2) is a critical determinant of MDSC immune suppressive activity and, perhaps more importantly, can redirect the differentiation of human DC toward functionally stable MDSCs (16,28,29). PGE2 is generated from a prostaglandin synthase 2 (PTGS2 - aka COX-2)-dependent conversion of arachidonic acid (released as a product of phospholipase A2 catalysis) to PGH2 which is then converted to PGE2 by prostandin E synthase (PTGES). Because the expression of cytosolic PLA2 (PLA2G4), COX-2 (PTGS2) and PTGES are all increased in A375-MDSCs in an MIF-dependent manner (Supplemental Fig. S8C), we next sought to determine whether reductions in PGE2 in 4-IPP-treated melanoma MDSCs was mechanistically linked to MDSC differentiation towards DC-like cells.
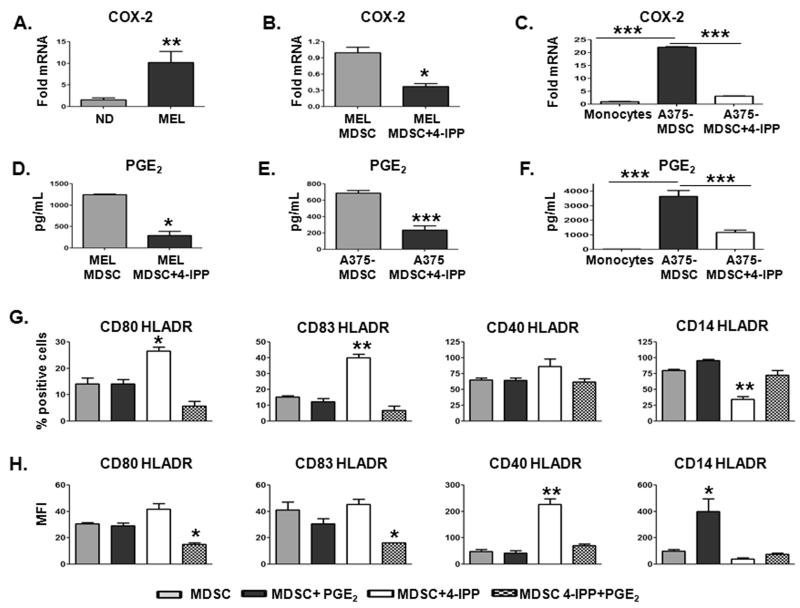
As COX-2 is generally considered to be the rate limiting step associated with PGE2 production and release, we first determined whether COX-2 expression is elevated in MDSCs from patients with late stage melanoma. The average mRNA expression of COX-2 in peripheral blood CD14+ cells isolated from advanced melanoma patients (n = 5) was ~ 10 fold greater than that of CD14+ cells isolated from normal donors (n = 5) (Fig. 7A). Inhibition of MIF with 4-IPP in both patient-derived MDSCs (Fig. 7B) and A375-MDSCs (Fig. 7C) significantly reduced COX-2 mRNA expression, consistent with several studies that demonstrated a central regulatory role for MIF in dictating COX-2 expression (30-32). The reduced COX-2 expression in melanoma patient-derived MDSCs treated with 4-IPP established that both A375-MDSCs and A375:monocyte co-cultures correlated with significant reductions in PGE2 concentrations (Fig. 7D–F).
Figure 7. MIF maintains melanoma MDSC suppressive phenotype through COX-2/PGE2 production.
(A-C) COX-2 mRNA expression was analyzed using qPCR. Bar graphs showing the relative mRNA expression of COX-2 in freshly isolated CD14+ cells from melanoma patients (MEL; n = 7) vs. healthy donors (ND; n = 5) (A), in melanoma patient-derived MDSCs pre-treated for 24 hours with DMSO or 4-IPP (50 μM) (B), and in A375-MDSCs obtained from untreated or 4-IPP-treated A375:monocyte co-cultures (C). (D-F) PGE2 levels in supernatants from melanoma patient-derived MDSCs treated for 24 hours with DMSO or 4-IPP (D), from A375-MDSCs treated for 24 hours with DMSO or 4-IPP (E), and from A375-MDSCs obtained from untreated or 4-IPP-treated A375:monocyte co-cultures (F). (G, H) Melanoma patient-derived CD14+ MDSCs (MEL; n = 2) were either untreated or treated with 4-IPP (50 μM), PGE2 (10 μM) or with 4-IPP plus PGE2 for 72 hours and analyzed for DC marker expression. Percentages (G) and MFI (H) of DC marker expressing HLADR+ cells in MDSC, MDSC+4-IPP, MDSC+PGE2, and MDSC+4-IPP+PGE2 cells. Data represent the average ± SEM of two independent experiments. P values = *, p≤0.05; **, p≤0.005; ***, p≤0.0005.
Next, we asked whether reconstituting PGE2 to 4-IPP-treated MDSC cultures was sufficient to reverse the effects of MIF inhibition on MDSC → DC-like differentiation (Figs. 7G and 7H). PGE2 added to 4-IPP-treated melanoma MDSCs efficiently reduced the 4-IPP-mediated increases in both the proportion of HLADR+ MDSCs (% positive cells – Fig. 7G) and the expression (MFI – Fig. 7H) of CD80, CD83 and CD40 markers. It also increased the 4-IPP-dependent reductions in percentages and expression of CD14 on HLADR+ MDSCs (Figs. 7G and H). Consistent with the observed reversion in immunophenotype, PGE2 reconstitution of 4-IPP-treated MDSCs effectively inhibited their ability to induce tetanus toxoid-specific T-cell proliferation (Supplemental Fig. S11). Taken together, these data suggest that MIF is an important and previously unrecognized determinant of human melanoma monocytic MDSC induction and immunosuppressive function. Perhaps more importantly, inhibition of MIF in established melanoma MDSCs induces the differentiation of immunosuppressive MDSCs into cells with DC-like phenotype and function. These findings provide compelling justification and rationale for therapeutic targeting of MIF in immunosuppressive human malignancies.
Discussion
Our data describe the important functional contribution made by MIF to human monocytic MDSCs. We show that MIF is necessary for CD14+HLADRlow MDSC induction, immune suppression, and in vitro differentiation. Using CD14+ MDSCs derived from advanced-stage melanoma patients, we show that the small molecule MIF antagonist, 4-IPP, strongly reduced MDSC-mediated suppression of T-cell activation and IFN-γ production. MIF inhibition in short-term, cytokine free, MDSC cultures led to the reduction of MDSC-associated cell surface markers and the induction of DC markers. MIF inhibitor-treated MDSCs in vitro acquire antigen-specific T-cell stimulatory potential, suggesting a functionally immunosuppressive MDSC → immunostimulatory, DC-like differentiation by MIF antagonism. MIF-deficient mouse MDSCs phenocopy this DC differentiation and acquisition of DC antigen-presentation functionality. It will be of interest to determine if the maturation status and immunostimulatory capacity of these DC-like cells can be further influenced by culturing them with DC maturation-inducing cytokine cocktails such as TNFα/IL1β/IL6 (33,34).
In cancer patients, defective DCs have been implicated in promoting tumor growth and adversely impacting anti-tumor efficacy of vaccines. Inhibition of VEGF signaling with VEGF-Trap treatment improves DC differentiation/maturation in cancer patients; these effects, however, are insufficient to improve antigen-specific immune responses (35). This lack of immune response is linked to the increased presence of MDSCs in the peripheral blood of the treated patients. Our findings that a safe, bioavailable, and highly efficacious in vivo pharmacologic MIF inhibitor is sufficient to induce MDSC differentiation into functionally immunostimulatory DCs, suggest that a multifaceted approach combining anti-MIF therapeutics with established DC maturation strategies could be highly effective in the treatment of late stage cancer patients (35).
Although several studies describe important functional contributions by MIF to murine innate immune tumor stromal cell phenotypes, none of these previous studies—including our own (10)—have identified mechanistic effectors and signaling pathways of MIF-dependent functions (11,36). In an attempt to identify downstream MIF effectors that could be responsible for MIF-dependent phenotypic and/or functional contributions to human MDSCs, we did a microarray analysis of normal monocytes, A375-MDSCs and A375-MDSCs + 4-IPP cells. Although several candidate effector mRNAs were identified that are potentially regulated by MIF, we initially chose to focus on ROS and PGE2 regulatory gene products for the following reasons: 1) Reactive oxygen species are a necessary component of MDSC-dependent immune suppression (21,24,26), 2) COX-2 inhibitors attenuate human monocytic MDSC immunosuppression (16), 3) COX-2-dependent PGE2 generation maintains MDSC phenotype and function while inhibiting DC development (28,29), and 4) MIF is well-documented to regulate ROS-regulatory and PGE2 regulatory mechanisms in a variety of cell types (31,37-39). Our current findings indicate that MIF is an important determinant of several PGE2 regulatory enzymes’ expression—most notably, COX-2. MIF inhibitor treatment reduces MDSC PGE2 levels and exogenously reconstituted PGE2 maintains MDSC marker expression while reducing DC-like phenotype in these cells. These findings suggest that MIF maintains MDSC suppressive phenotype, at least in part, via PGE2 production.
It is less clear how MIF mechanistically dictates MDSC immune suppressive activity. Our results clearly indicate an important functional role for MIF in maintaining NOX-4 expression and DCF-detectable ROS in MDSCs. At the same time, MIF is centrally important to COX-2 expression and maintaining PGE2 levels that are necessary for MDSC immunosuppressive function (16). It is likely that both of these effector mechanisms (ROS and PGE2 maintenance)—and potentially others—are involved in MIF-dependent MDSC suppression of T-cell activation, but what is less clear is how MIF regulates such a broad array of immunosuppressive and differentiation-regulating gene products. Although we are currently evaluating the signaling requirements for the MIF receptor, CD74 (39), in MIF-dependent MDSC phenotypes, we cannot rule out the possibility that MIF’s influences on these cells may be receptor-independent. This is based on the fact that enzymatically inactive, CD74-binding competent, N-terminal proline MIF mutants are entirely unable to reconstitute MIF-dependent MDSC induction/function (11,40). This, coupled with the fact that the MIF enzymatic inhibitor, 4-IPP, reportedly has little to no MIF:CD74 antagonist activity (41) but very effectively phenocopies MIF-deficiency in monocytes/macrophages [current study and (10)], is highly suggestive of a CD74-independent signaling function. If this is, in fact, the case, alternative mechanisms for MIF-dependent modulation of MDSC functionality include alternative outside-in signaling via non-cognate MIF receptors (42,43) or an intracellular mechanism of action via known (44), or presently unknown, pathways. It will be important to identify the precise mechanism of action going forward as it will not only provide important information regarding a seemingly central node of control for MDSC immunoregulatory functions in both mice and humans, but also because it could point to a previously unknown function for the highly druggable enzymatic active site of MIF.
Although MIF has been shown to regulate MDSC induction and suppressive activity in murine models of cancer (10,11), these results illuminate the functional contribution by MIF to human MDSCs. Our findings introduce a role for MIF in regulating human melanoma MDSC differentiation. Our data demonstrating that 4-IPP effectively reduces in vitro MDSC immune suppression while increasing DC-like antigen-specific T-cell responses suggests that in vivo MIF therapeutic targeting may simultaneously reduce cancer-induced MDSC-mediated T cell inactivation while enhancing anti-tumor antigen-specific T cell responses in metastatic melanoma patients.
It is not yet known whether current therapies that target adaptive immune tumor suppressive checkpoints such as anti-CTLA-4 (ipilimumab) and anti-PD-1 (pembrolizumab or nivolumab) could act in synergy with 4-IPP targeting of MIF. Some evidence supports the former possibility: a trial immunotherapy consisting of ipilimumab (in combination with irradiated, GM-CSF-expressing, autologous tumor cells) provokes a humoral response resulting in the elicitation of clinically relevant anti-MIF autoantibodies (12). Given that the targeting of CTLA-4 with ipilimumab is efficacious in patients with metastatic melanoma (45,46), it is possible that combinatorial targeting of adaptive immune suppressive mechanisms (anti-CTLA-4) and innate immune suppressive mechanisms (anti-MIF) may provide synergistic clinical responses in patients with advanced stage melanoma.
Supplementary Material
Acknowledgements
The authors would like to thank Melissa B. Hall for collecting and providing us with de-identified melanoma patient peripheral blood samples. We would also like to thank Drs. Jun Yan and Jill Suttles for helpful discussions and their insightful comments on the manuscript.
Support: This work was supported in part by NIH CA186661 to R. A. Mitchell and K. Yaddanapudi and NIH CA102285 to R. A. Mitchell
Footnotes
Conflict of Interest Statement: R.A.M is an inventor on patents pertaining to 4-IPP as an anti-cancer therapeutic agent targeting MIF.
References
- 1.Dranoff G. Targets of protective tumor immunity. AnnNYAcadSci. 2009;1174:74–80. doi: 10.1111/j.1749-6632.2009.04938.x. [DOI] [PubMed] [Google Scholar]
- 2.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–09. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–37. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167(2):195–205. doi: 10.1111/j.1365-2249.2011.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. The dendritic cell-regulatory T lymphocyte crosstalk contributes to tumor-induced tolerance. Clin Dev Immunol. 2011;2011:430394. doi: 10.1155/2011/430394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71(7):2411–6. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 8.Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res. 2012;18(19):5212–23. doi: 10.1158/1078-0432.CCR-12-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. 2014;20(6):1601–09. doi: 10.1158/1078-0432.CCR-13-2508. [DOI] [PubMed] [Google Scholar]
- 10.Yaddanapudi K, Putty K, Rendon BE, Lamont GJ, Faughn JD, Satoskar A, et al. Control of Tumor-Associated Macrophage Alternative Activation by Macrophage Migration Inhibitory Factor. J Immunol. 2013 Mar 15;190(6):2984–93. doi: 10.4049/jimmunol.1201650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson KD, Templeton DJ, Cross JV. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol. 2012;189(12):5533–40. doi: 10.4049/jimmunol.1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenfeld J, Jinushi M, Nakazaki Y, Wiener D, Park J, Soiffer R, et al. Active Immunotherapy Induces Antibody Responses That Target Tumor Angiogenesis. Cancer Res. 2010;70(24):10150–60. doi: 10.1158/0008-5472.CAN-10-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winner M, Meier J, Zierow S, Rendon BE, Crichlow GV, Riggs R, et al. A novel, macrophage migration inhibitory factor suicide substrate inhibits motility and growth of lung cancer cells. Cancer Res. 2008;68(18):7253–57. doi: 10.1158/0008-5472.CAN-07-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadjeva M, Nagashima J, Zaidi T, Mitchell RA, Pier GB. Inhibition of macrophage migration inhibitory factor ameliorates ocular Pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 2010;6(3):e1000826. doi: 10.1371/journal.ppat.1000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kindt N, Laurent G, Nonclercq D, Journe F, Ghanem G, Duvillier H, et al. Pharmacological inhibition of macrophage migration inhibitory factor interferes with the proliferation and invasiveness of squamous carcinoma cells. Int J Oncol. 2013;43(1):185–93. doi: 10.3892/ijo.2013.1944. [DOI] [PubMed] [Google Scholar]
- 16.Mao Y, Poschke I, Wennerberg E, Pico de CY, Egyhazi BS, Schultz I, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73(13):3877–87. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 17.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32(6):790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Gordon IO, Freedman RS. Defective antitumor function of monocyte-derived macrophages from epithelial ovarian cancer patients. Immunity. 2006;12(5):1515–24. doi: 10.1158/1078-0432.CCR-05-2254. [DOI] [PubMed] [Google Scholar]
- 19.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 21.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–45. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 22.Ugurel S, Uhlig D, Pfohler C, Tilgen W, Schadendorf D, Reinhold U. Down-regulation of HLA class II and costimulatory CD86/B7-2 on circulating monocytes from melanoma patients. Cancer Immunol Immunother. 2004;53(6):551–59. doi: 10.1007/s00262-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185(4):2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182(9):5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nauseef WM, Volpp BD, McCormick S, Leidal KG, Clark RA. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991;266(9):5911–17. [PubMed] [Google Scholar]
- 26.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–53. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obermajer N, Kalinski P. Generation of myeloid-derived suppressor cells using prostaglandin E2. Transplant Res. 2012;1(1):15. doi: 10.1186/2047-1440-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118(20):5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Wu H, Xu S, Guo X, Yang J, Shen X. Macrophage migration inhibitory factor activates cyclooxygenase 2-prostaglandin E2 in cultured spinal microglia. Neurosci Res. 2011;71(3):210–18. doi: 10.1016/j.neures.2011.07.1821. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA. 2002;99(1):345–50. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampey AV, Hall PH, Mitchell RA, Metz CN, Morand EF. Regulation of synoviocyte phospholipase A2 and cyclooxygenase 2 by macrophage migration inhibitory factor. Arthritis Rheum. 2001;44(6):1273–80. doi: 10.1002/1529-0131(200106)44:6<1273::AID-ART219>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27(12):3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 34.Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, et al. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170(8):4069–76. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 35.Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13(16):4840–8. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Chen T, Leng L, Fan J, Cao K, Duan Z, et al. MIF Produced by Bone Marrow-Derived Macrophages Contributes to Teratoma Progression after Embryonic Stem Cell Transplantation. Cancer Res. 2012;72(11):2867–78. doi: 10.1158/0008-5472.CAN-11-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuang YC, Su WH, Lei HY, Lin YS, Liu HS, Chang CP, et al. Macrophage migration inhibitory factor induces autophagy via reactive oxygen species generation. PLoS ONE. 2012;7(5):e37613. doi: 10.1371/journal.pone.0037613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koga K, Kenessey A, Powell SR, Sison CP, Miller EJ, Ojamaa K. Macrophage migration inhibitory factor provides cardioprotection during ischemia/reperfusion by reducing oxidative stress. Antioxid Redox Signal. 2011;14(7):1191–202. doi: 10.1089/ars.2010.3163. [DOI] [PubMed] [Google Scholar]
- 39.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, et al. MIF Signal Transduction Initiated by Binding to CD74. J Exp Med. 2003;197(11):1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fingerle-Rowson G, Kaleswarapu DR, Schlander C, Kabgani N, Brocks T, Reinart N, et al. A tautomerase-null macrophage migration-inhibitory factor (MIF) gene knock-in mouse model reveals that protein interactions and not enzymatic activity mediate MIF-dependent growth regulation. Mol Cell Biol. 2009;29(7):1922–32. doi: 10.1128/MCB.01907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cournia Z, Leng L, Gandavadi S, Du X, Bucala R, Jorgensen WL. Discovery of human macrophage migration inhibitory factor (MIF)-CD74 antagonists via virtual screening. J Med Chem. 2009;52(2):416–24. doi: 10.1021/jm801100v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13(5):587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 43.Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J, et al. Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol Cancer Res. 2010;8(10):1328–43. doi: 10.1158/1541-7786.MCR-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000;408(6809):211–16. doi: 10.1038/35041591. [DOI] [PubMed] [Google Scholar]
- 45.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lebbe C, Weber JS, Maio M, Neyns B, Harmankaya K, Hamid O, et al. Survival follow-up and ipilimumab retreatment for patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann Oncol. 2014 doi: 10.1093/annonc/mdu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.