Abstract
Risk factors for non-Aspergillus mold infection (NAMI) and the impact on transplant outcome are poorly assessed in the current era of antifungal agents. Outcomes of 124 patients receiving allogeneic HCT diagnosed with either mucormycosis [n=72] or fusariosis [n=52] between days 0-365 after HCT are described and compared to a control cohort (n=11856). Patients with NAMI had more advanced disease [mucormycois 25%, fusariosis 23%, controls 18%; p = 0.004] and were more likely to have a KPS<90% at HCT [mucormycosis 42%, fusariosis 38%, controls 28%; p=0.048]. The 1-year survival after HCT was 22% (15–29%) for cases and was significantly inferior compared to controls [65%(64–65%); p < 0.001]. Survival from infection was similarly dismal regardless of mucormycosis [15% (8-25%)] and fusariosis [21% (11-33%)].
In multivariable analysis, NAMI was associated with a 6-fold higher risk of death (p<0.0001) regardless of the site or timing of infection. Risk factors for mucormycosis include preceding acute GVHD, prior aspergillus infection, and older age. For fusariosis, increased risks including receipt of cord blood, prior CMV infection, and transplant prior to May 2002. In conclusion, NAMI occurs infrequently, is associated with high mortality, and appears with similar frequency in the current antifungal era.
Introduction
Infections remain a significant cause of morbidity and mortality following both autologous and allogeneic hematopoietic cell transplantation (HCT). Registry data indicate that 13 - 17% of patients receiving allogeneic HCT have infection as a primary cause of death(1). However, these data neither define the extensive morbidity nor the frequency of opportunistic infections and, they likely underestimate the full contribution of infection as a cause of death(2).
The etiology of infectious complications after transplantation is multifactorial and prevention of infection remains crucial to improve outcomes(3). Routine prophylaxis with fluconazole results in decreased Candida spp. infections compared to placebo(4); however since its institution, increasing Aspergillus spp. infections were noted(5). Since the approval of agents with anti-aspergillosis activity, case reports from individual institutions have suggested breakthrough infections of mucormycosis in patients receiving anti-aspergillus azoles or echinocandins(6-10). This was not seen in a randomized control trial comparing fluconazole to voriconazole for prophylaxis (11).
The frequency of pathologically or microbiologically confirmed post-transplant mold infections not due to Aspergillus spp. (non-Aspergillus mold infections, NAMI) fortunately remains rare. An analysis from the Transplant Associated Infection Surveillance Network (TRANSNET) identified 639 patients with invasive fungal infection out of 16,200 receiving hematopoietic cell transplant procedures between March 2001 and September 2005(7). The majority of these infections were due to Aspergillus spp. and Candida spp. with only 66 reported cases of mucormycosis and 22 cases of fusariosis.
In order to better understand the impact of mucormycosis and fusariosis on transplant outcomes and to identify risk factors, potentially including antifungal prophylaxis, we designed and executed a retrospective observational study using the Center for International Blood and Marrow Transplant Research (CIBMTR) database.
Methods
Patients
This analysis includes all patients receiving an allogeneic hematopoietic cell transplant for any indication and reported to the CIBMTR between 1995 and 2008. Patients received either conventional myeloablative or a reduced intensity/non-myeloablative conditioning from any donor source. Patients were considered cases if they were reported with either fusariosis (n=52) or mucormycosis (n = 72) (including Mucorales spp., Rhizopus spp., and Zygomycetes not otherwised specified) at any site occurring from day 0 up to one year following transplantation; additional NAMI were not included due to lack of sufficient numbers of cases reported of other organisms such as Scedosporium spp. All identified cases came from 66 transplant centers predominantly in North America (n=50) with additional centers in Europe (n=5), Asia (n=2), Australia/New Zealand (n=4), Middle East/Africa (n=2) and Central/South America (n=3). To minimize ascertainment bias since the CIBMTR does not collect data using EORTC-MSG criteria, a comparison control cohort (n=11,856) included all patients from the same centers as cases who met all other inclusion criteria but did not have a NAMI reported in the first year after transplant(12). This restriction potentially minimizes prophylaxis, diagnostic, and treatment biases as well.
Data Sources
Data were obtained from the CIBMTR, a research affiliate of the International Bone Marrow Transplant Registry, Autologous Blood and Marrow Transplant Registry, and the National Marrow Donor Program (NMDP) established in 2004. It comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute data on consecutive allogeneic and autologous HCT procedures to a statistical center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers report longitudinal data on all transplants and compliance is monitored by on-site audits. Transplant essential data, collected for consented patients participating in CIBMTR data collection, include demographic, disease type and stage, survival, relapse, graft type, the presence of GVHD, and cause of death data. A subset of CIBMTR participants are selected for comprehensive research level data collection by weighted randomization. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information used in the performance of such research is collected and maintained in CIBMTR's capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule. Studies conducted by the CIBMTR are performed under guidance and review of the Institutional Review Board of the National Marrow Donor Program.
Definitions and Endpoints
Conditioning intensity was defined as myeloablative or non-myeloablative using criteria defined by CIBMTR(13). Performance status at the time of HCT was defined according to the Karnofsky scale for patients 16 and older, or the Lansky scale for those younger than 16 years. Leukemia disease stage for patients with AML, ALL, and CML was categorized as early [CR1, CP1], intermediate [≥ CR2/CP2, Rel 1, or AP], or advanced [all other stages]. HLA-match grade categories for unrelated donors were categorized as previously published (14). Infection data are reported by centers with an organism code, a date of onset, and a site of infection. Sites of infection are reported as disseminated if the organism is found in 3 or more non-contiguous sites. Data regarding method of diagnosis, severity of infection, and treatment are not captured.
Outcomes of interest included overall survival (OS) from the time of transplant, OS from the time of NAMI, and risk factors for each infection. Infections with cytomegalovirus (CMV) and aspergillosis were assessed for both cases and controls. Acute graft versus host disease (GVHD) was defined as the occurrence of grade II-IV skin, gastrointestinal, and/or liver abnormalities fulfilling the Glucksberg criteria of acute GVHD (15). Chronic GVHD was defined using the NIH consensus criteria (16).
Statistical Analysis
Descriptive statistics for patient, disease, and transplant-related factors were compared using Chi-squared analysis or Mann-Whitney testing as appropriate. A Kaplan Meyer analysis for OS from the time of transplant was determined. Cox modeling was employed to determine the effect of NAMI on OS adjusted for factors at the time of transplant as well as the time dependent co-variates of acute and chronic GVHD. Cox models also determined the effect of infection site and the time of infection onset following transplant on OS.
To account for multiple infectious episodes occurring in a single patient and adjusted for a period of time at risk, infection density was determined (17). This was calculated specifically for CMV infection and aspergillosis for the first year after transplant. A recurrent infection required a period of 60 days without evidence of CMV or 90 days without evidence of aspergillosis following an initial infectious event (18).
Estimation of OS from the time of NAMI was determined within the infection cohort. Additionally, a Cox model was used to identify risk factors for the diagnosis of mucormycosis and fusariosis. Variables included age; stem cell source; disease and disease status at transplant; co-morbid conditions of endocrine, pulmonary, and/or autoimmune disease; Karnofsky performance status; acute GVHD before infection; chronic GVHD before infection; CMV reactivation before NAMI; aspergillosis before NAMI; and year of transplant prior to or after May 2002 as a surrogate for antifungal prophylaxis. These variables had a p-value ≤0.1 in univariate analysis between cases and controls or were incorporated as time dependent variables that may impact the likelihood of NAMI.
Median follow-up of the survivors was 61 months (range, 13 – 132 months) for cases and 62 months (range, 1 – 186 months) for controls.
Results
Patients
Key characteristics of patients with mucormycosis, fusariosis, and controls are shown in Table 1. Patients with mucormycosis were older [median, 47 years (range, 3-68 years)] compared to either the cases with fusariosis or the controls [fusariosis: 32 years (range, 1–63 years); controls: 34 years (<1–79 years); p <0.001]. Patients in the control cohort were more likely to have a KPS ≥ 90% at the time of transplant [mucormycosis: 56%, fusariosis: 60%; controls: 68%; p = 0.048]. Although there was no difference in the disease indication for transplant, cases were less likely to have early stage leukemia compared to controls [mucormycosis: 38%; fusariosis: 42%; controls: 53%; p = 0.004]. The frequency of certain co-morbid conditions including pulmonary disease, endocrine diseases, and autoimmune disease was assessed as these conditions may be associated with increased risk of infection. There was no difference in the frequency of these comorbidities although pulmonary disease was slightly more common for patients with mucormycosis and autoimmune diseases for patients with fusariosis.
Table 1.
Pre-transplant characteristics of patients with mucormycosis, fusariosis, and controls
| Variable N (%) | Mucormycosis (n =72) | Fusariosis (n =52) | Controls (n = 11856) | p-value |
|---|---|---|---|---|
| Age, yrs [median (range)] | 47 (3 – 68) | 32 (1 – 63) | 34 (<1 – 79) | <0.001 |
| Gender, male | 47 (65%) | 31 (60%) | 6924 (58%) | 0.491 |
| KPS at HCT | 0.048 | |||
| ≥ 90% | 40(56%) | 31 (60%) | 8049 (68%) | |
| < 90% | 30 (42%) | 20 (38%) | 3330 (28%) | |
| unknown | 2 ( 3%) | 1 ( 2%) | 477 ( 4%) | |
| Co-morbid illness at HCT | ||||
| Pulmonary | 11 (15%) | 5 (10%) | 1059 (9%) | 0.183 |
| Endocrine | 9 (13%) | 5 (10%) | 888 (7%) | 0.353 |
| Autoimmune | 1 ( 1%) | 4 ( 8%) | 266 (2%) | 0.060 |
| Disease | 0.233 | |||
| AML/ALL/MDS | 42 (58%) | 24 (46%) | 6216 (52%) | |
| NHL/HL | 8 (11%) | 7 (13%) | 1088 ( 9%) | |
| CML | 7 (10%) | 4 ( 8%) | 1511 (13%) | |
| CLL | 5 ( 7%) | 1 ( 2%) | 375 ( 3%) | |
| SAA | 4 ( 6%) | 5 (10%) | 926 ( 8%) | |
| Plasma cell disorders | 0 | 3 ( 6%) | 207 ( 2%) | |
| Other diseases | 6 ( 8%) | 8 (15%) | 1455 (12%) | |
| Leukemia Disease Stage | 0.004 | |||
| Early | 15 (38%) | 11 (42%) | 3500 (53%) | |
| Intermediate | 11 (28%) | 9 (35%) | 1800 (27%) | |
| Advanced | 10 (25%) | 6 (23%) | 1162 (18%) | |
| Missing | 4 (10%) | 0 | 121 ( 2%) | |
| Donor/Recipient Gender Match | 0.293 | |||
| Female/Male | 21 (29%) | 17 (33%) | 2805 (24%) | |
| Female/Female | 8 (11%) | 9 (17%) | 2147 (18%) | |
| Male/Female | 13 (18%) | 9 (17%) | 2424 (20%) | |
| Male/Male | 20 (28%) | 13 (25%) | 3610 (30%) | |
| UNK/Male | 6 ( 8%) | 1 ( 2%) | 511 ( 4%) | |
| UNK/Female | 4 ( 6%) | 3 ( 6%) | 359 ( 3%) | |
| Donor/Recipient CMV status | 0.571 | |||
| Positive/Positive | 29 (40%) | 16 (31%) | 4289 (36%) | |
| Positive/Negative | 4 ( 6%) | 5 (10%) | 1189 (10%) | |
| Negative/Positive | 12 (17%) | 14 (27%) | 2395 (20%) | |
| Negative/Negative | 18 (25%) | 9 (17%) | 2799 (24%) | |
| Missing | 9 (13%) | 8 (15%) | 1184 (10%) | |
| Donor/Recipient HLA match | 0.229 | |||
| HLA-identical siblings | 42 (58%) | 26 (50%) | 7608 (64%) | |
| Other related | 4 ( 6%) | 5 (10%) | 591 ( 5%) | |
| Well-matched unrelated | 12 (17%) | 4 ( 8%) | 1439 (12%) | |
| Partially matched unrelated | 3 ( 4%) | 6 (12%) | 708 ( 6%) | |
| Mismatched unrelated | 2 ( 3%) | 1 ( 2%) | 190 ( 2%) | |
| Umbilical cord blood | 9 (13%) | 10 (19%) | 1228 (10%) | |
| Stem cell source | 0.037 | |||
| Bone marrow | 23 (32%) | 19 (37%) | 5408 (46%) | |
| Peripheral blood | 40 (56%) | 23 (44%) | 5220 (44%) | |
| Umbilical cord blood | 9 (13%) | 10 (19%) | 1228 (10%) | |
| GvHD Prophylaxis | 0.300 | |||
| T-cell depletion | 6 ( 8%) | 2 ( 4%) | 475 ( 4%) | |
| Tacrolimus ± Other | 24 (33%) | 14 (27%) | 3032 (26%) | |
| Cyclosporine ± Other | 39 (54%) | 35 (67%) | 7887 (66%) | |
| Other | 3 ( 4%) | 1 ( 2%) | 462 ( 4%) | |
| Year of Transplant | 0.044 | |||
| 1995 – April 2002 | 28 (39%) | 32 (62%) | 5632 (48%) | |
| May 2002 – 2008 | 44 (61%) | 20 (38%) | 6224 (52%) | |
Transplant related variables, such as donor/recipient gender match, CMV serostatus match, and HLA match were similar between cases and controls (Table 1). Additionally, the use of myeloablative conditioning was similar between the groups [mucormycosis: n = 46 (64%); fusariosis: n = 39 (75%), controls: n = 8075 (68%), p = 0.43].
Infection
The rate of NAMI was 10.35 cases per 1000 patients transplanted (mucormycosis: 6.01/1000; fusariosis: 4.34/1000) in these 66 centers. In comparison, the rate of Aspergillus occurring in the first year after transplant was 45.9 cases per 1000 patients transplanted. When analyzed by stem cell source, there were 7.71 cases per 1000 bone marrow grafts, 11.93 cases per 1000 peripheral blood stem cell grafts, and 15.24 cases per 1000 umbilical cord blood grafts (p = 0.02). There was also a slightly higher rate of NAMI in recipients of adult unrelated donor grafts (11.53/1000 transplants) compared to patients receiving related donor allografts (9.31/1000 transplants, p = 0.19).
Information on systemic antifungal prophylaxis is shown in Table 2. The antifungal prophylaxis did not differ between the groups (p = 0.549). No systemic antifungal prophylaxis was administered to 13 (18%) mucormycosis cases, 6 (12%) fusariosis cases, and 2000 (17%) controls. The predominant antifungal agent used was fluconazole [mucormycosis: 40%; fusariosis: 54%; controls: 53%] and there was no difference in the use of any azole (p=0.148). Information on dosing and duration of antifungal prophylaxis was not available.
Table 2.
Frequency of systemic antifungal prophylaxis administered.
| Antifungal Prophylaxis N (%) | Mucormycosis (n = 72) | Fusariosis (n = 52) | Controls (n =11856) | p-value |
|---|---|---|---|---|
| 0.549 | ||||
| Azoles | 0.148 | |||
| Fluconazole | 29 (40%) | 28 (54%) | 6264 (53%) | |
| Voriconazole | 8 (11%) | 1 ( 2%) | 683 ( 6%) | |
| Itraconazole | 5 ( 7%) | 2 ( 4%) | 512 ( 4%) | |
| Posaconazole | 1 ( 1%) | 0 | 74 (<1%) | |
| Amphotericin | 14 (19%) | 13 (25%) | 1782 (15%) | |
| Echinocandin | 1 ( 1%) | 0 | 158 ( 1%) | |
| Other agent (including clinical trial) | 1 ( 1%) | 2 ( 4%) | 319 ( 3%) | |
| None | 13 (18%) | 6 (12%) | 2000 (17%) | |
The median time from transplant to infection onset was 48 days (range, 1-363) for all NAMI (mucormycosis: 75 days (1-360); fusarium: 28 days (0-363). This was earlier than the onset for Aspergillus infections, which occurred at a median of 96 days (range, 14-230). The majority of NAMI occurred in the first 100 days post-transplant with only 35 (28%) cases occurring between 3 months and one year post-transplant. In the first 3 months post-transplant, infections occurred primarily between day 0 - 30 [n = 49 (40%)] with a similar distribution occurring from day 31 – 60 [n=18 (15%)] and day 61 – 100 [n = 22 (18%)]. Only 14 (11%) cases experienced relapse of the underlying hematologic malignancy prior to the diagnosis of NAMI occurring at a median of 2.3 months.
As expected, sinus/respiratory was the most common site of infection reported occurring in 55 (44%) cases. The remaining cases occurred in the blood/marrow (14%), GI tract (14%), skin only (6%), CNS (2%), and musculoskeletal system (<1%). Five (4%) patients were reported with disseminated infection, and 18 patients had no site of infection reported.
Since patients are at risk for multiple infections during the first year after HCT, we determined the infection density for CMV and aspergillosis and compared between the cases and controls. There was no difference in the rate of CMV infection between cases and controls [cases: 0.57 per patient vs controls: 0.29 per patient; p = 0.55]; however, cases had a 4-fold higher rate of aspergillosis compared to controls [cases: 0.29 per patient vs controls: 0.07 per patient; p <0.001] The number of NAMI patients with aspergillosis was small (n = 14) and only 7 patients were diagnosed prior to or concomitant with the NAMI diagnosis.
In multivariable analyses (Tables 3, 4), prior aspergillosis was the greatest risk factor for development of mucormycosis in the first year after transplant. Other risk factors included age > 50 years and prior acute GVHD. The risks for fusariosis included receipt of umbilical cord blood, prior CMV infection, and transplant between 1995 and April 2002. Notably, year of transplant did not increase the risk of mucormycosis. Other factors not increasing the risk for either mucormycosis or fusariosis include KPS, prior chronic GVHD, disease and disease status at transplant, nor co-morbid conditions of prior pulmonary, endocrine, or autoimmune disease.
Table 3.
Multivariable analysis for risk factors for development of mucormycosis in the first year after transplant
| Variable | RR for mucormycosis (95% confidence interval) | p-value |
|---|---|---|
| Acute GvHD grade II – IV | 0.027 | |
| No | 1.00 | |
| Yes | 1.78 (1.07 – 2.98) | |
| Prior Aspergillus infection | 0.0007 | |
| No | 1.00 | |
| Yes | 4.91 (1.96 – 12.28) | |
| Age | 0.0006 | |
| ≤ 50 years | 1.00 | |
| > 50 years | 2.28 (1.42-3.65) | |
Table 4.
Multivariable analysis for risk factors for development of fusariosis in the first year after transplant
| Variable | RR for fusariosis (95% confidence interval) | p-value |
|---|---|---|
| Prior CMV infection | 0.013 | |
| No | 1.00 | |
| Yes | 2.72 (1.24 – 5.97) | |
| Stem cell source | 0.017 | |
| Bone marrow | 1.00 | |
| Peripheral blood | 1.61 (0.86 – 3.02) | 0.136 |
| Umbilical cord blood | 3.11 (1.14 – 6.81)* | 0.004 |
| Year of Transplant | 0.009 | |
| 1995 – April 2002 | 1.00 | |
| May 2002 – 2008 | 0.46 (0.26 – 0.82) | |
Peripheral blood vs Umbilical Cord blood: 0.52 (0.25 – 1.09), p = 0.082
Survival
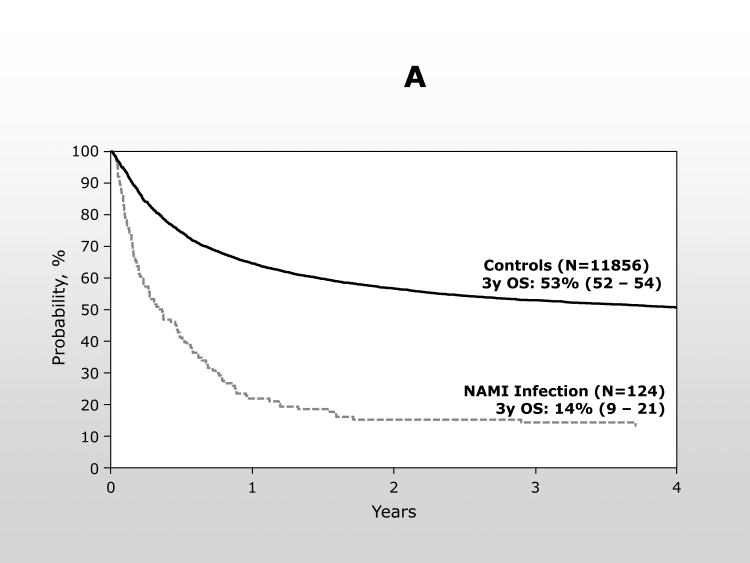
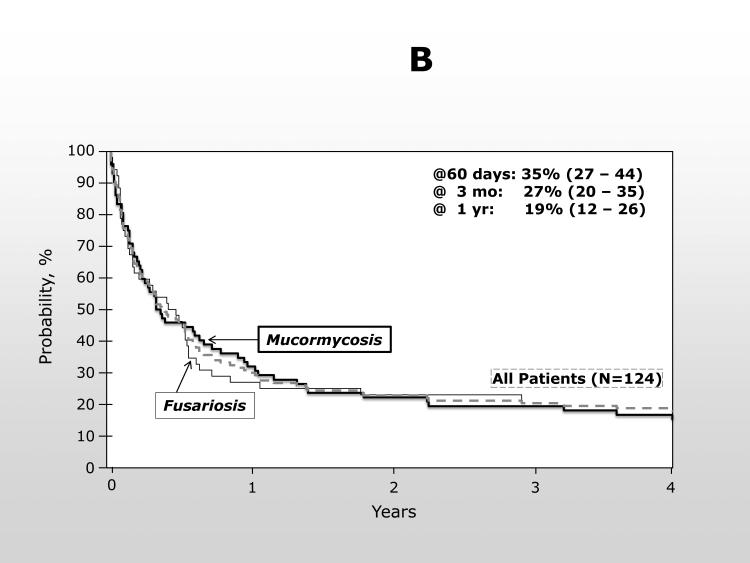
The overall survival from transplant was significantly inferior for patients developing a NAMI with only 22% (95% confidence interval (CI): 15 – 29%) of cases alive at 1 year compared to 65% (95% CI: 64 – 65%) of controls [p <0.0001] (Figure 1A). For patients with NAMI, most died within 30 days of the diagnosis and the probability of survival at 100 days following NAMI was only 27% (95% CI: 20 – 35%) (Figure 1B). The type of NAMI did not change the poor outcome [100 day OS, mucormycosis: 29% (95% CI: 19-40%); fusariosis: 25% (14-37%)], and, therefore, fusariosis and mucormycosis were combined for the multivariable analysis of OS.
1A.
Overall survival from the time of transplantation for patients with NAMI compared to the control population from the same centers.
1B.
Overall survival from the time of AMI. Represented are all patients (---), patients with mucormycosis ( ), and with fusariosis (
), and with fusariosis ( ).
).
In multivariable analysis, patients with a NAMI had a 6-fold greater risk of death compared to the controls and this was not impacted by the site of the infection [bloodstream: 6.41 (95% CI: 3.69 – 11.13) vs. GI: 6.34 (95% CI: 4.67 – 8.59) vs. respiratory: 6.43 (95% CI: 3.74 – 11.23) vs other site: 5.30 (95% CI: 3.59 – 7.81)]. Infections diagnosed beyond day 100 post-transplant [11.78 (95% CI: 7.76 – 17.87)] were associated with a higher risk of death compared to infections in the first 100 days [0 – 30 days: 5.67 (95% CI: 4.14 – 7.77), p = 0.006; 31 – 60 days: 7.67 (95% CI: 4.67 – 12.60), p = 0.19; 61 – 100 days: 4.36: (95% CI: 2.65 – 7.18), p = 0.002]. Other factors increasing the risk of death were as expected and included acute GvHD grade 2 – 4, advanced leukemia, older age, KPS <90%, greater degree of HLA-mismatch, and transplant between 1995 – April 2002. GvHD prophylaxis with T-cell depletion methods or methotrexate without calcineurin inhibitors also resulted in inferior survival. Infection was reported as the primary cause of death in 51% of patients with NAMI and only 19% of controls (Table 5).
Table 5.
Primary causes of death (COD) reported for deceased patients in this analysis
| Primary COD | Mucormycosis (n =62) | Fusariosis (n =46) | Controls (n =5947) |
|---|---|---|---|
| Infection | 32 (52%) | 23 (50%) | 1141 (19%) |
| Relapse | 10 (16%) | 5 (11%) | 2227 (37%) |
| GVHD | 6 (10%) | 5 (11%) | 690 (12%) |
| Organ failure | 11 (18%) | 6 (13%) | 877 (15%) |
| Other | 3 ( 5%) | 7 (15%) | 898 (15%) |
Discussion
Our data represent a large cohort of patients receiving allogeneic HCT from 66 centers reporting to the CIBMTR. Within a cohort of 11,980 patients, only 124 (1%) developed an NAMI with either mucormycosis or fusariosis between the time of stem cell infusion and one year post transplant. However for that 1% of patients, outcomes were dismal with only 22% of patients alive one year after transplant and only 27% of patients alive 3 months after the diagnosis of NAMI. Risk factors increasing the likelihood of mucormycosis included prior GVHD, older age, and prior Aspergillus spp. infection. Notably, there was no difference based on year of transplant preceding or following May 2002 as a surrogate for potential increased use of voriconazole in the risk of developing mucormycosis. The risks were different for fusariosis and included use of umbilical cord blood, prior CMV infection, and earlier transplant period.
Neutropenia and impaired cell-mediated immunity are known risk factors for the development of invasive fungal infections. Other risk factors include iron overload, poorly controlled diabetes mellitus, and prolonged use of corticosteroids (19-21). Many of these factors are clearly present in an allogeneic HCT population. However, in general, these risk factor analyses have focused on all invasive fungal infections which are generally predominated by aspergillosis and candida infections (5, 19, 20). In recent years, single center publications document breakthrough mucormycosis in patients receiving anti-aspergillosis prophylaxis (6, 8, 22). Large multicenter randomized controlled trials did not support this finding (11, 23). Our data also dispute evidence of increasing NAMI in the recent antifungal era. In fact, the overall incidence and rate per 1000 allogeneic transplants from our analysis is similar to that reported by Garcia-Vidal and colleagues for patients transplanted prior to 2003 in two centers, a single center publication for patients transplanted from 1985 through 1989 by Marr et al., and the more recent TRANSNET prospective surveillance for patients receiving either autologous or allogeneic transplant at 22 centers between March 2001 and March 2006 (5, 7, 20). Consequently, it does not appear that the use of voriconazole increases the risk of mucormycosis; however, further analyses in which larger numbers of patients are exposed to voriconazole are warranted to confirm this finding. Conversely, it appears that the FDA approval of voriconazole corresponds with a decreased risk of fusariosis in our population although voriconazole prophylaxis was rare in our study. Data suggests improved survival in patients with disseminated fusariosis treated with voriconazole.(24) It is possible that the decreased risk of fusariosis after May 2002 may reflect an earlier transition to voriconazole for empiric therapy in a neutropenic patient; unfortunately, we do not have treatment data to confirm this hypothesis.
Despite the small number of patients diagnosed with aspergillosis either preceding or concomitant with the NAMI diagnosis, aspergillosis was associated with more than a 4-fold increased risk of mucormycosis in multivariable analysis. As previously noted, risk factors for invasive fungal infections are likely similar regardless of the causative organism; therefore, this risk factor may simply serve as a surrogate for extensive defects in both innate and adoptive immunity resulting in increased risk of any opportunistic infection. Alternatively, it is possible that patients were mis-diagnosed or mis-reported with aspergillosis in the setting of a mold infection. However, this finding may also explain the suggested breakthrough of mucormycosis with the use of voriconazole since patients may be surviving the aspergillosis due to use of voriconazole and ultimately live long enough to manifest an alternative NAMI (6, 8, 22).
As expected, survival following NAMI is quite poor. Unfortunately, and more concerning, this does not appear to have improved in the era of newer antifungal agents. Based on our analysis, the one-year survival following infection with mucormycosis is approximately 15%. For fusariosis, 1 year survival following infection is approximately 21%. These data do not differ from outcomes published in 2002 for patients receiving HCT from 1985 through 1999 (5). Until better therapies are identified, our ability to improve outcomes from NAMI will require prevention of exposure, continued improvement in diagnostic techniques, and modification of risk factors in the allogeneic HCT population.
Our study has clear limitations due to its observational cohort design. A major limitation is the reported data for antifungal prophylaxis. Notably, 15% of cases and 17% of controls were reported to receive no antifungal prophylaxis. Additionally, several patients were reported to receive more than one antifungal agent as “prophylaxis”. Data are lacking regarding timing and duration of antifungal prophylaxis as well as length of empiric antifungal therapy and changes in therapy that occurred during the transplant course. Furthermore, we have no data regarding duration of corticosteroid use in either the cases or controls. Finally, the infections are reported by organism, date of onset, and site of infection is requested, but the CIBMTR does not collect diagnostic criteria to determine “proven” or “probable” by EORTC-MSG criteria; therefore, there is the possibility of both under- and over-reporting which could affect a risk factor analysis. (12) We have no information on the clinical symptoms or severity of the infection; therefore, it is possible that some cases represent colonization or contaminants rather than an invasive infection. We attempted to account for this by limiting our control cohort to those transplant centers that had cases making the assumption that transplant centers generally employ similar prophylaxis, treatment paradigms, and diagnostic and data reporting criteria across the transplant patient population.
Our data demonstrate that NAMI occur infrequently but are most common early after HCT. This observation is not surprising since most patients have multiple risk factors including impaired cellular immunity as well as neutropenia during this time (21). However, those NAMI which occurred beyond 100 days post-transplant were twice as likely to be fatal as the patients diagnosed during the first 30 days post-transplant. This may be due to relapse of the underlying hematologic malignancy preceding the infection. Alternatively, this outcome may be due to lack of awareness of ongoing risk and exposures to these ubiquitous microbes as patients are commonly seen less frequently at the transplant centers later in the transplant course. Education of patients and referring physicians about the on-going risk of NAMI and adjustments in prophylaxis may be necessary to decrease these infections (25).
In summary, NAMI remain uncommon but highly fatal infections following allogeneic HCT. Prolonged vigilance for the development of NAMI is required, particularly after development of acute GVHD or other opportunistic infections and may require novel antifungal prophylaxis schedules. Detailed accurate information on prophylaxis and antifungal therapy is required to better understand the implications of these agents on the development and outcome of NAMI. The CIBMTR is now collecting detailed information on all research patients reported with invasive mold infections.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pasquini M, Zhu X. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2014. 2014 Available from: http://www.cibmtr.org.
- 2.Copelan E, Casper JT, Carter SL, van Burik JA, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(12):1469–76. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 3.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(10):1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. The New England journal of medicine. 1992;326(13):845–51. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 5.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 6.Imhof A, Balajee SA, Fredricks DN, Englund JA, Marr KA. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis. 2004;39(5):743–6. doi: 10.1086/423274. [DOI] [PubMed] [Google Scholar]
- 7.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 8.Trifilio SM, Bennett CL, Yarnold PR, McKoy JM, Parada J, Mehta J, et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant. 2007 doi: 10.1038/sj.bmt.1705614. [DOI] [PubMed] [Google Scholar]
- 9.Pang KA, Godet C, Fekkar A, Scholler J, Nivoix Y, Letscher-Bru V, et al. Breakthrough invasive mould infections in patients treated with caspofungin. J Infect. 2012;64(4):424–9. doi: 10.1016/j.jinf.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Sugawara Y, Sekine T, Nakase K, Katayama N. Breakthrough disseminated zygomycosis induced massive gastrointestinal bleeding in a patient with acute myeloid leukemia receiving micafungin. J Infect Chemother. 2009;15(1):42–5. doi: 10.1007/s10156-008-0657-5. [DOI] [PubMed] [Google Scholar]
- 11.Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–8. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(7):748–58. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Tomblyn M, Young JA, Haagenson MD, Klein JP, Trachtenberg EA, Storek J, et al. Decreased infections in recipients of unrelated donor hematopoietic cell transplantation from donors with an activating KIR genotype. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(8):1155–61. doi: 10.1016/j.bbmt.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Burik JA, Carter SL, Freifeld AG, High KP, Godder KT, Papanicolaou GA, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(12):1487–98. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102(3):827–33. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47(8):1041–50. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagano L, Akova M, Dimopoulos G, Herbrecht R, Drgona L, Blijlevens N. Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. Journal of Antimicrobial Chemotherapy. 2011;66(suppl 1):i5–i14. doi: 10.1093/jac/dkq437. [DOI] [PubMed] [Google Scholar]
- 22.Siwek GT, Dodgson KJ, de Magalhaes-Silverman M, Bartelt LA, Kilborn SB, Hoth PL, et al. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis. 2004;39(4):584–7. doi: 10.1086/422723. [DOI] [PubMed] [Google Scholar]
- 23.Marks DI, Pagliuca A, Kibbler CC, Glasmacher A, Heussel CP, Kantecki M, et al. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. British journal of haematology. 2011;155(3):318–27. doi: 10.1111/j.1365-2141.2011.08838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lortholary O, Obenga G, Biswas P, Caillot D, Chachaty E, Bienvenu AL, et al. International retrospective analysis of 73 cases of invasive fusariosis treated with voriconazole. Antimicrob Agents Chemother. 2010;54(10):4446–50. doi: 10.1128/AAC.00286-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. The New England journal of medicine. 2007;356(4):335–47. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]