Abstract
Objective
Physiologic dead space is associated with mortality in acute respiratory distress syndrome (ARDS), but its measurement is cumbersome. Alveolar dead space fraction (AVDSf) relies on the difference between arterial and end-tidal carbon dioxide (AVDSf = (PaCO2 − PETCO2)/PaCO2). We aimed to assess the relationship between AVDSf and mortality in a cohort of children meeting criteria for ARDS (both Berlin 2012 and AECC 1994 acute lung injury) and pediatric ARDS (PARDS, as defined by PALICC in 2015).
Design
Secondary analysis of a prospective, observational cohort.
Setting
Tertiary care, university affiliated pediatric intensive care unit.
Patients
Invasively ventilated children with PARDS.
Interventions
None.
Measurements and Main Results
Of the 283 children with PARDS, 266 had available PETCO2. AVDSf was lower in survivors (median 0.13 [IQR 0.06, 0.23]) than non-survivors (0.31 [0.19, 0.42], p < 0.001) at PARDS onset, but not 24 hours after (survivors 0.12 [0.06, 0.18], non-survivors 0.14 [0.06, 0.25], p=0.430). AVDSf at PARDS onset discriminated mortality with an area under receiver operating characteristic curve of 0.76 (95% CI 0.66–0.85, p < 0.001), better than either initial oxygenation index or PaO2/FIO2. In multivariate analysis, AVDSf at PARDS onset was independently associated with mortality, after adjustment for including severity of illness, immunocompromised status, and organ failures.
Conclusions
AVDSf at PARDS onset discriminates mortality, and is independently associated with non-survival. AVDSf represents a single, useful, readily obtained clinical biomarker reflective of pulmonary and non-pulmonary variables associated with mortality.
Keywords: pediatric, acute respiratory distress syndrome, ARDS, PARDS, alveolar dead space fraction, AVDSf
INTRODUCTION
Measures of oxygenation, including PaO2/FIO2 (1, 2) and oxygenation index (OI)(3), are surrogates for intrapulmonary shunt, and used to stratify severity of acute respiratory distress syndrome (ARDS). However, degree of hypoxemia has an inconsistent association with mortality (4–6), and aberrations of pulmonary blood flow and microcirculation characteristic of ARDS (7, 8) are not entirely reflected by PaO2/FIO2 or OI. Physiologic dead space is the alveolar and anatomic components of ventilation not participating in gas exchange. Dead space is elevated in adult ARDS and correlated with risk of death (9). Dead space increases with decreasing pulmonary blood flow, including peri-cardiac arrest and other low cardiac output states, from pulmonary embolus, in pulmonary hypertension, and with alveolar over-distension. However, measurement of physiologic dead space is cumbersome, requiring specialized equipment including a large-volume Douglas bag, metabolic carts or volumetric capnography, and is infrequently performed in routine care (10). The alveolar dead space fraction (AVDSf), which relies on the difference between the PaCO2 and the end-tidal CO2 (PETCO2), serves as a reasonable estimate of alveolar dead space (11–14). As most patients are ventilated with time-based capnography, AVDSf is readily obtainable. A single institution has reported in children with acute hypoxemic respiratory failure (15), and in a separate population of intubated children (16), that elevated AVDSf early in the course of respiratory failure was associated with increased mortality
Recently, we described a prospective observational study of pediatric ARDS (PARDS) with the aim of assessing the discriminative ability of traditional oxygenation based metrics of severity (PaO2/FIO2 and OI) for survival (17). We did not assess dead space in that report. In the current study, we tested the hypothesis that AVDSf measured early in the course of PARDS was associated with mortality in this prospectively collected cohort.
METHODS
Study Design and Patient Selection
This study was approved by the Children’s Hospital of Philadelphia’s (CHOP) Institutional Review Board, and requirement for informed consent waived. The cohort has been previously described in detail (17). Briefly, consecutive patients in the pediatric intensive care unit (PICU) were screened daily for eligibility between July 1, 2011 and June 30, 2014. Children (> 1month and < 18 years) undergoing invasive mechanical ventilation meeting American-European Consensus Conference (AECC) criteria for acute lung injury (ALI, PaO2/FIO2 ≤ 300 on 2 consecutive arterial blood gases separated by ≥ 1 hour and bilateral parenchymal infiltrates) were included. Exclusion criteria were 1) respiratory failure exclusively from cardiac failure (determined by echocardiography) or fluid overload, 2) exacerbation of underlying chronic respiratory disease, 3) chronic ventilator dependence, 4) mixing cyanotic heart disease, 5) mechanical ventilation for > 7 days before PaO2/FIO2 ≤ 300, and 6) PARDS established outside of the CHOP PICU. Determination of bilateral infiltrates was made independently by a PICU attending and a pediatric radiologist blinded to clinical data; only cases agreed to by both as consistent with AECC-defined ALI met inclusion (2). Determination of hydrostatic pulmonary edema (from either heart failure or anuric/oliguric renal failure) as the sole etiology of respiratory failure was made in consultation with the PICU attending using available data. As the study was initiated prior to 2012 Berlin updated definitions of ARDS (1), we did not specify a minimum positive end-expiratory pressure (PEEP); however, our institutional practice does not utilize PEEP < 5 cmH2O, and all patients therefore met Berlin criteria. Similarly, as these data were collected prior to the 2015 Pediatric Acute Lung Injury Consensus Conference (PALICC) definitions of PARDS (3), children who met PARDS criteria by non-invasive (Spo2) criteria were not enrolled; however, all children met PARDS criteria by invasive (OI) criteria.
Data Collection and PARDS Management
Demographics at admission, ventilator settings at PARDS onset and 24 hours after, and laboratory data and medications for the first 3 days of PARDS were recorded. We recorded first qualifying values (after initiation of ventilation) of PaO2/FIO2 and OI at PARDS diagnosis and 24 hours after diagnosis.
Absent a standardized ventilator protocol, our institutional practice is to initiate conventional ventilation with a minimum 5 cmH2O of PEEP, and attempt to wean FIO2 to ≤ 0.60. There is no specific target PaO2, but typically PaO2 ≥ 60 mmHg is accepted as long as there is clinical stability. Inability to wean FIO2 prompts PEEP escalation and subsequent efforts to wean FIO2, attempting to maintain peak inspiratory pressures (PIP) ≤ 35 cmH2O. We exclusively utilize decelerating flow during conventional ventilation (either pressure control or pressure-regulated volume control). Persistently elevated PIP (≥ 35 cmH2O), ongoing hypercarbia (PaCO2 ≥ 80), or oxygenation difficulties (inability to wean FIO2 ≤ 0.60 despite increasing PEEP) prompted consideration for changing mode of ventilation, or escalating to extracorporeal membrane oxygenation (ECMO). There was no standardization of ancillary therapies (inhaled nitric oxide [iNO], surfactant, neuromuscular blockade, prone positioning, corticosteroids), which was left to the discretion of the attending physician.
Calculation of AVDSf
AVDSf was retrospectively abstracted from the medical records at PARDS onset and 24 hours after. We used the first qualifying arterial blood gas from which the initial PaO2/FIO2 and OI at PARDS diagnosis were calculated, and the blood gas 24 hours after PARDS diagnosis, to extract PaCO2. The PETCO2 value immediately preceding these blood gases was used for the following equation: AVDSf = (PaCO2 − PETCO2)/PaCO2, with the requirement that the PETCO2 value used preceded the PaCO2 by no more than 30 minutes and that the endotracheal tube leak was < 20%. This protocol has been used before to assess AVDSf with good reproducibility (15, 18).
Other Equations and Definitions
Metrics of oxygenation utilized were PaO2/FIO2 and OI ((mean airway pressure [mPaw] × FIO2 × 100)/PaO2). The vasopressor score (19, 20) was calculated by: dopamine (μg/kg/min) × 1 + dobutamine (μg/kg/min) × 1 + epinephrine (μg/kg/min) × 100 + norepinephrine (μg/kg/min) × 100 + phenylephrine (μg/kg/min) × 100 + milrinone (μg/kg/min) × 10. Non-pulmonary organ failures at time of PARDS diagnosis were identified using accepted definitions in children (21). The designation of “immunocompromised” required presence of an immunocompromising diagnosis (oncologic, immunologic, rheumatologic, or transplant) and active immunosuppressive chemotherapy, or a congenital immunodeficiency (17, 22). Severity of illness score used was the Pediatric Risk of Mortality (PRISM) III at 12 hours.
The primary reported outcome was PICU mortality. Ventilator-free days (VFD) at 28 days and duration of mechanical ventilation were also recorded. All mention of “mechanical ventilation” in this study implies invasive ventilation, and non-invasive support was not counted toward VFD or total ventilator days. For VFD and duration of mechanical ventilation, the first day was initiation of invasive ventilation. Liberation from invasive ventilation for > 24 hours defined duration of mechanical ventilation. Patients requiring re-initiation of invasive ventilation after 24 hours of extubation had the extra days counted towards total ventilator days. VFD was determined by subtracting total ventilator days from 28 in survivors. All patients with total ventilator days ≥ 28 days, and all PICU non-survivors were assigned VFD = 0. In some analyses, VFD was dichotomized to ≤ 14 days or > 14 days.
Statistical Analysis
All data were analyzed using Stata 10.0 (StataCorp, LP, College Station, TX). Data are expressed as percentages or as median [interquartile range, IQR]. All variables were found to be non-normally distributed by Shapiro–Wilk. Differences between distributions of categorical variables were analyzed by Fisher’s exact test. Continuous variables were compared using Wilcoxon rank sum. Area under the receiver operating characteristic (AUROC) curve was determined for testing the discriminative ability of AVDSf.
Multivariate logistic regression was performed to test for an independent association between AVDSf and mortality, separately for AVDSf at PARDS onset and at 24 hours. Variables with a univariate association with mortality (p < 0.10 in Table 1) were entered into a backward stepwise regression model, with a p < 0.10 for retention in the model. Because of significant co-linearity and overlap between the respiratory variables (PaO2/FIO2, OI, PIP), only OI was modeled. Because of our primary hypothesis, AVDSf was always included in the model. After the first run, each variable was reintroduced into the model one at a time and retained in the final model if p < 0.10. Model fit was assessed using Hosmer-Lemeshow statistics.
Table 1.
Characteristics of PARDS cohort associated with mortality
| All patients (n = 266) | Survived (n = 230) | Died (n = 36) | p value | |
|---|---|---|---|---|
| Age (years) | 4.2 [1.4, 12.8] | 3.8 [1.4, 11.9] | 6.8 [2.1, 15.2] | 0.059 |
| PRISM III at 12 hours | 10 [5, 17] | 9 [5, 15] | 18 [11.5, 30] | < 0.001 |
| Cause of ARDS | ||||
| Infectious pneumonia | 153 (57.5) | 135 (59) | 18 (50) | |
| Aspiration pneumonia | 24 (9) | 20 (9) | 4 (11) | 0.599 |
| Sepsis | 50 (19) | 44 (19) | 6 (17) | |
| Trauma | 21 (8) | 16 (7) | 5 (14) | |
| Other | 18 (6.5) | 15 (6) | 3 (8) | |
| Immunocompromised (%) | 52 (20) | 33 (14) | 19 (53) | < 0.001 |
| Non-pulmonary organ dysfunctions at ARDS onset | 1.5 [1, 3] | 1 [1, 2] | 3 [2, 4] | < 0.001 |
| PARDS onset | ||||
| PaO2/FIO2 | 158 [112, 210] | 160 [118, 210] | 130 [80, 198] | 0.030 |
| OI | 10 [7, 15.4] | 9.7 [7.1, 14.2] | 12.8 [6.5, 24.8] | 0.049 |
| PEEP (cmH2O) | 10 [8, 12] | 10 [8, 12] | 10 [8, 11] | 0.989 |
| Peak inflating pressure (cmH2O) | 30 [25, 35] | 30 [25, 35] | 31 [28, 38] | 0.089 |
| Tidal volume (mL/kg) | 7.5 [6.7, 7.3] | 7.5 [6.7, 8.3] | 7.7 [6.7, 8.3] | 0.566 |
| AVDSf | 0.16 [0.07, 0.25] | 0.13 [0.06, 0.23] | 0.31 [0.19, 0.42] | < 0.001 |
| 24h hours after (n = 216, 27 died) | ||||
| PaO2/FIO2 | 231 [175, 282] | 236 [184, 285] | 175 [131, 233] | < 0.001 |
| OI | 6.3 [4.8, 9] | 6.1 [4.7, 8.3] | 8.9 [6.2, 12.4] | < 0.001 |
| PEEP (cmH2O) | 10 [8, 10] | 10 [8, 10] | 10 [8, 12] | 0.178 |
| Peak inflating pressure (cmH2O) | 27 [24, 32] | 27 [24, 31] | 30 [26, 35] | 0.025 |
| Tidal volume (mL/kg) | 7.3 [6.5, 8.1] | 7.4 [6.6, 8.2] | 7.1 [6.3, 7.9] | 0.480 |
| AVDSf | 0.12 [0.06, 0.18] | 0.12 [0.06, 0.18] | 0.14 [0.06, 0.25] | 0.430 |
| Use of iNO (%) | 91 (34) | 72 (31) | 19 (53) | 0.012 |
| First 72 hours of PARDS | ||||
| Maximum vasopressor score | 10 [3, 19] | 8 [3, 17] | 14 [6, 27.5] | 0.029 |
| Fluid balance (mL/kg) | 95 [37, 179] | 93 [37, 172] | 120 [56, 264] | 0.044 |
Since AVDSf may be modified by iNO use (selective pulmonary vasodilator which may alter dead space), we explored the relationship between AVDSf at 24 hours, iNO exposure, and mortality testing an interaction term as additional independent variable in the backward stepwise regression model. Additionally, we constructed a propensity score for iNO use (see Supplementary Text for details).
RESULTS
AVDSf at PARDS onset is associated with mortality
Of the 283 children in the cohort, 266 (Table 1, Supplementary Table 1) were on conventional ventilation with continuous PETCO2 monitoring allowing for calculation of AVDSf at PARDS onset (11 on high frequency oscillatory ventilation, HFOV; 6 on high frequency percussive ventilation, HFPV). By 24 hours after PARDS onset, this was reduced to 216 children (16 HFOV, 26 HFPV, 4 airway pressure release ventilation, 3 ECMO, 1 dead). The PETCO2 value used was recorded 9 [3, 12] minutes before the PaCO2 utilized. All patients had an endotracheal tube leak < 15% (median 0 [1, 1] %), with 95% of patients having a leak ≤ 5%. PARDS onset occurred 10 [2, 12] hours after intubation. Of the 36 deaths from the cohort of 266 children, 17 had withdrawal of care for poor neurologic prognosis, 11 had multisystem organ failure, and 8 had refractory hypoxemia.
AVDSf was higher in non-survivors at PARDS onset (p < 0.001), but not 24 hours after PARDS onset (p = 0.430; Table 1, pFigure 1A). AVDSf at PARDS onset discriminated survivors from non-survivors (Figure 1B), with an AUROC of 0.76 (95% CI 0.66 to 0.85, < 0.001). AVDSf at PARDS onset discriminated survival better than either OI or PaO2/FIO2 at PARDS onset, although the combination of AVDSf at PARDS onset with either OI or PaO2/FIO2 was not better than AVDSf alone (Supplementary Table 2).
Figure 1.
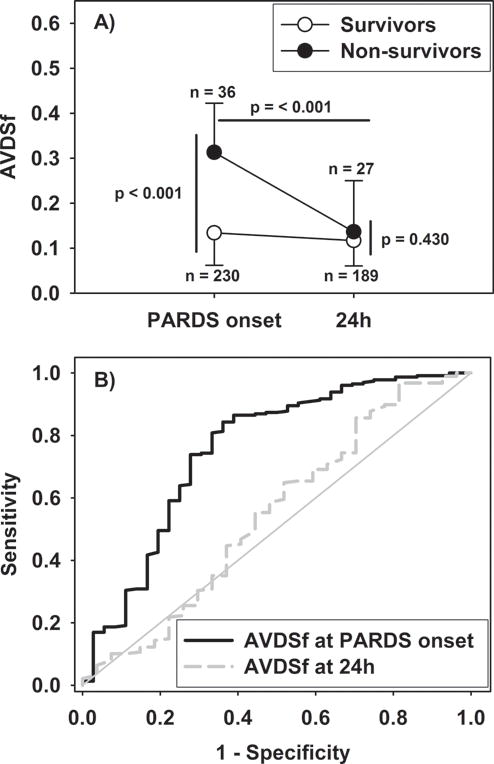
(A) AVDSf at PARDS onset and at 24 hours in survivors and non-survivors. Data is presented as medians and IQR. P-value represents Wilcoxon rank sum tests. (B) ROC curves for AVDSf at PARDS onset (AUROC 0.76, 95% CI 0.66 to 0.85, p < 0.001) and 24 hours after PARDS onset (AUROC 0.55, 95% CI 0.42 to 0.68, p = 0.429).
AVDSf at PARDS onset correlated (all p < 0.05; Supplementary Table 3) with severity of illness (PRISM III), cardiovascular status (non-pulmonary organ dysfunction and vasopressor score), and degree of hypoxemia (OI at PARDS onset and 24 hours after). Mortality was increased in the highest quartile of AVDSf at PARDS onset (Figure 2, p < 0.001). Patients with AVDSf ≥ 0.25 at PARDS onset had higher PRISM III scores, more non-pulmonary organ dysfunctions, worse respiratory indexes, higher vasopressor scores, more frequent use of iNO, longer duration of ventilation, and increased mortality (all p < 0.05; Supplementary Table 4). Patients with AVDSf ≥ 0.25 at PARDS onset had worse mortality in every PALICC oxygenation category (Supplementary Figure 1). A multivariate logistic regression model demonstrated that AVDSf at PARDS onset was independently associated with increased non-survival (adjusted OR 1.10, 95% CI 1.05 to 1.14, p < 0.001; Table 2). OI at PARDS onset was not independently associated with mortality after adjusting for other variables.
Figure 2.
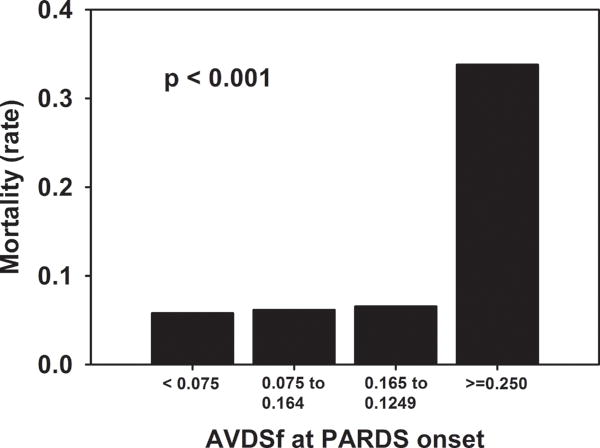
Mortality stratified by quartiles of AVDSf at PARDS onset. P-value represents Fisher exact test.
Table 2.
Multivariate logistic regression of variables associated with mortality (testing initial AVDSf)
| Variable | Adjusted odds ratio | 95% confidence interval | p Value |
|---|---|---|---|
| PRISM III | 1.05 | 1.00 to 1.10 | 0.045 |
| Immunocompromised (yes) | 5.77 | 2.03 to 16.38 | 0.001 |
| Non-pulmonary organ dysfunctions | 1.94 | 1.25 to 3.01 | 0.003 |
| AVDSf at PARDS onset (per every increase by 0.01) | 1.10 | 1.05 to 1.14 | <<0.001 |
Effect of Transition to Alternative Ventilator Modes by 24 hours
By 24 hours after PARDS onset, only 216 children of the original 266 remained on conventional ventilation, allowing calculation of AVDSf at this time-point. The 50 children without calculable 24-hour AVDSf had a higher initial AVDSf (0.22 [0.10, 0.33]) relative to the 216 who remained alive on conventional ventilation at 24 hours (0.15 [0.06, 0.23], p = 0.002). The mortality rate in these 50 patients (18%) was not significantly different relative to the 216 remaining on conventional ventilation (12.5%, Fisher exact p = 0.358).
Effect of Inhaled Nitric Oxide
To address whether the use of iNO impacted the performance of AVDSf 24 hours after PARDS onset, we separately examined the relationship between AVDSf, iNO use, and mortality. Of the initial cohort of 266 children, 91 (34%) were exposed to iNO (Supplementary Table 5); of the 216 children on conventional ventilation 24 hours after PARDS onset, 56 (26%) had been exposed to iNO. The iNO cohort had more organ failures, worse respiratory indexes, higher vasopressor scores, longer duration of ventilation, and increased mortality. After propensity score-adjustment for iNO use (Supplementary Text and Supplementary Table 6), use of iNO was no longer associated with mortality (adjusted OR for mortality 1.17, 95% CI 0.48 to 2.85, p = 0.731).
For non-survivors, AVDSf at 24 hours was lower than at PARDS onset in both cohorts exposed and not-exposed to iNO (Figure 3). In neither cohort did AVDSf at 24 hours discriminate between survivors and non-survivors in univariate analysis (both p > 0.05, Figure 4). Because 50 patients (35 of whom were exposed to iNO) did not have AVDSf available 24 hours after PARDS (because of transition to alternative ventilator modes), repeating the above analyses while excluding these 50 patients produced similar results (Supplementary Figure 2).
Figure 3.
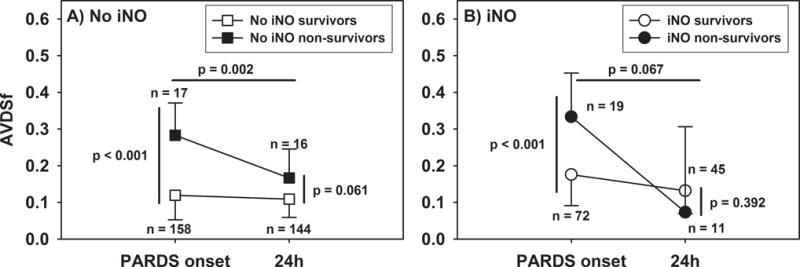
AVDSf at PARDS onset and at 24 hours in patients (A) not exposed and (B) exposed to iNO.
Figure 4.
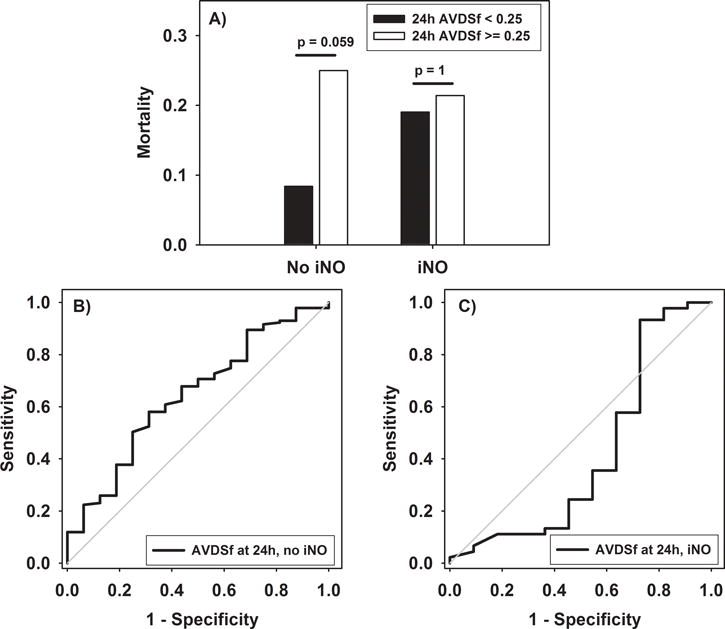
(A) AVDSf at 24 hours in patients not exposed and exposed to iNO. (B) ROC curve for discriminating mortality in patients not exposed to iNO (AUROC 0.64 (95% CI 0.50 to 0.78, p = 0.060). (C) ROC curve for discriminating mortality in patients exposed to iNO (AUROC 0.42 (95% CI 0.19 to 0.64, p = 0.386).
When repeating the multivariate regression testing for independent associations with mortality (Table 3), AVDSf at 24 hours retained an association only when the interaction term between AVDSf at 24 hours and use of iNO was modeled. This suggests that the association between mortality and AVDSf at 24 hours was modified by use of iNO, and that AVDSf at 24 hours was independently associated with mortality only in the cohort not exposed to iNO (adjusted OR 1.07, 95% CI 1.00 to 1.14, p = 0.040).
Table 3.
Multivariate logistic regression of variables associated with mortality (testing AVDSf 24 hours after PARDS onset)
| Variable | Adjusted odds ratio | 95% confidence interval | p Value |
|---|---|---|---|
| PRISM III | 1.08 | 1.02 to 1.15 | 0.014 |
| Immunocompromised (yes) | 3.25 | 1.07 to 9.84 | 0.037 |
| OI 24 hours after PARDS onset | 1.13 | 1.03 to 1.25 | 0.013 |
| Interaction (AVDSf at 24h * Use of iNO) | 0.93 | 0.87 to 0.99 | 0.050 |
| AVDSf 24 hours after PARDS onset (per every increase by 0.01) | |||
| iNO use (no) | 1.07 | 1.00 to 1.14 | 0.049 |
| iNO use (yes) | 0.99 | 0.92 to 1.07 | 0.830 |
DISCUSSION
AVDSf at PARDS onset was independently associated with mortality, and discriminated mortality better than initial OI or PaO2/FIO2. AVDSf at 24 hours retained independent association only in those not exposed to iNO. AVDSf likely reflects decreased pulmonary blood flow associated with low cardiac output, explaining the correlation with PRISM III, number of non-pulmonary organ failures, and vasopressor score. It also reflects degree of pulmonary injury, adding information to oxygenation metrics. Lack of utility of AVDSf 24 hours after PARDS onset seems explained by both transition of the sickest children to alternative modes of ventilation (in which AVDSf could not be calculated) and high iNO utilization. As AVDSf is readily obtained and clinically relevant, it may assist with risk stratification and prognostication in both clinical and research settings.
Metrics of oxygenation have an inconsistent relationship with mortality in adults with ARDS (4–6), and are dependent on ventilator settings (6, 23), degree of venous admixture (24), and FIO2 (25, 26). While children have a somewhat more reproducible relationship between oxygenation defect and mortality (17, 27, 28), several other variables not included in definitions of PARDS, such as non-pulmonary organ failures and immunocompromised status (17, 29), substantially affect mortality and length of ventilation. The imprecision of the definition makes risk stratification and prognostication of ARDS problematic. In children, this is compounded by the fact that neither the 1994 AECC (2) nor the 2012 Berlin (1) definitions included a discussion on pediatrics. Absent specific considerations, children have typically been diagnosed according to adult definitions of ARDS. To address the distinct epidemiology and outcomes of children (17, 27–29), PALICC was convened to propose pediatric-specific definitions for PARDS (3). The PALICC group determined that the primary risk stratification mechanism of the 2015 PARDS definitions should remain oxygenation, with severity categories defined by OI rather than by PaO2/FIO2. As there was only a single report of children with heterogeneous respiratory failure associating AVDSf with mortality (15), AVDSf was considered but ultimately not included in the final PALICC definition. Our data support using dead space measurements in future definitions of PARDS.
Increased physiologic dead space, derived from metabolic monitoring and using the Enghoff modification of the Bohr equation, was an independent predictor of mortality in adult ARDS (9). Dead space fraction was considered when developing the 2012 Berlin definition but ultimately not included, in part because of infrequency of measurement (1). Accurate measurements of expired CO2 have historically been performed using a Douglas bag, collecting up to 60 liters of exhaled gas to determine expired CO2. More recent approximations include calculations derived from metabolic carts and volumetric capnographs. All of these techniques are limited by requiring specialized equipment, and thus are not consistently performed during routine PICU care. This may be addressed by a more easily obtained surrogate of alveolar dead space, such as AVDSf. Furthermore, it is likely that fluctuations in alveolar dead space, rather than anatomic, are responsible for the association between dead space and mortality. As such, alveolar dead space as measured by AVDSf may actually be more sensitive to perturbations in physiology (14, 30).
AVDSf has been associated with mortality in a cohort of 95 mechanically ventilated children with acute hypoxemic respiratory failure (15), and in a larger cohort of intubated children (16), both from the same institution. The AVDSf value reported in those studies used the first 24-hour period of ventilation in which all necessary variables were available, and demonstrated an AUROC for mortality of 0.74 in both studies. We confirm this finding in this larger cohort meeting both the Berlin 2012 definition of ARDS and the PALICC 2015 definition of PARDS, but limited to the initial AVDSf at PARDS onset, and report a comparable AUROC of 0.76. We also demonstrate the association of elevated initial AVDSf with markers of both pulmonary (as reflected by OI) and non-pulmonary (non-pulmonary organ failure, vasopressor score) morbidity. As alveolar dead space is increased with low cardiac output, it is not surprising that AVDSf correlates with other surrogates of low cardiac output states, including vasopressor score and non-pulmonary organ dysfunction. However, the independent association of AVDSf after controlling for surrogates of intrapulmonary shunt (OI) and cardiac output (vasopressor score) suggests that AVDSf may be characterizing additional risk, possibly related to aberrations in pulmonary microcirculation or pulmonary microthrombi.
The lack of utility after 24 hours in the cohort of children exposed to iNO is consistent with AVDSf as a reflection of pulmonary microcirculation. As such, the utility of AVDSf for risk stratification may not lie in its specificity to pulmonary physiology; rather, AVDSf at PARDS onset is a single, useful, readily obtained clinical biomarker reflective of multiple pulmonary and non-pulmonary variables associated with mortality, including oxygenation, cardiovascular status, and organ failures. In our study, it is notable that initial AVDSf ≥ 0.25 identified children at higher risk of mortality, even if the initial OI stratified them as mild or moderate PARDS (Supplementary Figure 1). Early identification and risk stratification of PARDS patients at high risk for mortality, whether for pulmonary or non-pulmonary organ failure, may allow for earlier application of interventions which have not proven successful in heterogenous PARDS cohorts, with lower overall mortality risk. Future clinical trials stratifying by AVDSf may successfully identify a detectable signal by limiting interventions to sicker patients with higher baseline mortality risk. This concept is especially appealing for interventions which may favorably impact ventilation/perfusion ratios, such as prone positioning or iNO.
Kallet et al (31) demonstrated that physiologic dead space retains prognostic significance up to 6 days after ARDS onset in 59 adults. In our cohort, AVDSf at PARDS onset was able to discriminate mortality and was independently associated with non-survival. However, AVDSf 24 hours after did not discriminate mortality, and was only independently associated with non-survival in the cohort of children not exposed to iNO. We speculate that iNO, as has been demonstrated before in a sheep acid aspiration model (14), improved alveolar dead space by passively redistributing pulmonary blood flow towards areas with excess ventilation and deficient perfusion. As iNO therapy directly acted to reduce alveolar dead space, the 24 hour AVDSf value was improved irrespective of mortality risk, and thus prognostic value was lost.
Alternatively, it is possible that our analysis 24 hours after PARDS onset is underpowered for mortality, limited by the unavailability of PETCO2 in 50 patients with a higher mortality rate (18%) relative to the 216 remaining on conventional ventilation (12.5%, p = 0.358). Presumably, the patients who remained on conventional ventilation at 24 hours did not escalate to alternative ventilator modes because they were less ill or because they had a favorable response to ongoing therapy, including iNO. Therefore, the patients exposed to iNO for whom AVDSf was available at 24 hours may have selected for a population with a favorable iNO response on conventional ventilation, and lower mortality. However, the independent association of 24-hour AVDSf with mortality in the cohort not exposed to iNO (Table 3) suggests direct modification of the prognostic utility of AVDSf by iNO.
AVDSf appears to be more consistent with a marker for severity of injury, rather than a therapeutic target. In both children exposed and not exposed to iNO, there was no evident relationship between degree of AVDSf improvement and mortality. Furthermore, in the cohort exposed to iNO, the AVDSf improved more than did the AVDSf in the cohort not exposed to iNO; nevertheless, mortality was nearly double in the cohort exposed to iNO. However, given the observational nature of this study, we are limited in the inferences we can draw. A future prospective physiologic study may clarify whether targeting specific AVDSf values can improve outcomes in PARDS. Echocardiographic monitoring and extrapolated pulmonary artery pressures in such a study may allow dissection of the underlying mechanism underlying AVDSf, including the role of pulmonary vascular resistance.
Our study has limitations. The study was conducted at a single center, and while demographics and severity of illness are comparable to other PARDS cohorts, mortality rate, ventilator practices, and utilization of ancillary therapies (including iNO) may not allow translation to other PICUs. Also, as we restricted enrollment to patients undergoing invasive ventilation with arterial access, and required 2 consecutive PaO2/FIO2 ≤ 300, we selected only a fraction of patients with respiratory failure, likely biased towards those with greater disease severity. However, our eligibility criteria were similar to many pediatric ARDS trials. We did not assess AVDSf beyond 24 hours after PARDS onset in patients, and the utility of AVDSf over time in PARDS deserves further study. The heterogeneity of our population and the low mortality rate may have affected the utility of AVDSf at 24 hours. The AVDSf was derived retrospectively, and may not be an accurate calculation, as ETCO2 may have changed by the time the corresponding blood gas was drawn. However, the ETCO2 value used was only 9 minutes before the PaCO2 utilized for calculation, and this method has been used to derive AVDSf in previous studies (15, 18). These concerns are best addressed via a prospective study, with alveolar dead space assessed by both AVDSf and volumetric capnography.
CONCLUSION
AVDSf at PARDS onset discriminates mortality, and is independently associated with non-survival. For those who remained on conventional ventilation at 24 hours, the independent association with mortality is only evident in the cohort unexposed to iNO. AVDSf represents a single, useful, readily obtained clinical biomarker reflective of multiple pulmonary and non-pulmonary variables associated with mortality, including oxygenation, cardiovascular status, and organ failures.
Supplementary Material
Supplementary figure 1: Mortality stratified by initial PALICC oxygenation categories and initial AVDSf. P values represent Fisher exact tests.
Supplementary figure 2: (A) AVDSf at PARDS onset and at 24 hours in survivors and non-survivors, restricted to only the 216 patients with available AVDSf at PARDS onset and 24 hours after (i.e., remaining on conventional ventilation 24 hours after). (B and C) AVDSf in these 216 patients at PARDS onset and 24 hours after, dichotomized by exposure to iNO. (D) ROC curves for AVDSf at PARDS onset (AUROC 0.75, 95% CI 0.64 to 0.87, p < 0.001) and 24 hours after PARDS onset (AUROC 0.55, 95% CI 0.42 to 0.68, p = 0.429). (E and F) ROC curves for 24-hour AVDSf discriminating mortality in these 216 patients dichotomized by exposure to iNO. For patients not exposed to iNO (E), AUROC 0.64, 95% CI 0.50 to 0.78, p = 0.060); for patients not exposed to iNO (F), AUROC 0.42, 95% CI 0.19 to 0.64, p = 0.614).
Acknowledgments
Financial support: Russell C. Raphaely Endowed Chair in Critical Care Medicine, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital of Philadelphia
References
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 3.Pediatric Acute Lung Injury Consensus Conference G. Pediatric acute respiratory distress syndrome: consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16(5):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Seeley E, McAuley DF, Eisner M, et al. Predictors of mortality in acute lung injury during the era of lung protective ventilation. Thorax. 2008;63(11):994–998. doi: 10.1136/thx.2007.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villar J, Perez-Mendez L, Blanco J, et al. A universal definition of ARDS: the PaO2/FIO2 ratio under a standard ventilatory setting–a prospective, multicenter validation study. Intensive Care Med. 2013;39(4):583–592. doi: 10.1007/s00134-012-2803-x. [DOI] [PubMed] [Google Scholar]
- 7.Greene R, Zapol WM, Snider MT, et al. Early bedside detection of pulmonary vascular occlusion during acute respiratory failure. Am Rev Respir Dis. 1981;124(5):593–601. doi: 10.1164/arrd.1981.124.5.593. [DOI] [PubMed] [Google Scholar]
- 8.Tomashefski JF, Jr, Davies P, Boggis C, et al. The pulmonary vascular lesions of the adult respiratory distress syndrome. The American journal of pathology. 1983;112(1):112–126. [PMC free article] [PubMed] [Google Scholar]
- 9.Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346(17):1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 10.Beitler JR, Thompson BT, Matthay MA, et al. Estimating dead-space fraction for secondary analyses of acute respiratory distress syndrome clinical trials. Crit Care Med. 2015;43(5):1026–1035. doi: 10.1097/CCM.0000000000000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankenfield DC, Alam S, Bekteshi E, et al. Predicting dead space ventilation in critically ill patients using clinically available data. Crit Care Med. 2010;38(1):288–291. doi: 10.1097/CCM.0b013e3181b42e13. [DOI] [PubMed] [Google Scholar]
- 12.Hardman JG, Aitkenhead AR. Estimating alveolar dead space from the arterial to end-tidal CO(2) gradient: a modeling analysis. Anesthesia and analgesia. 2003;97(6):1846–1851. doi: 10.1213/01.ANE.0000090316.46604.89. [DOI] [PubMed] [Google Scholar]
- 13.McSwain SD, Hamel DS, Smith PB, et al. End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respir Care. 2010;55(3):288–293. [PMC free article] [PubMed] [Google Scholar]
- 14.Skimming JW, Banner MJ, Spalding HK, et al. Nitric oxide inhalation increases alveolar gas exchange by decreasing deadspace volume. Crit Care Med. 2001;29(6):1195–1200. doi: 10.1097/00003246-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Ghuman AK, Newth CJ, Khemani RG. The association between the end tidal alveolar dead space fraction and mortality in pediatric acute hypoxemic respiratory failure. Pediatr Crit Care Med. 2012;13(1):11–15. doi: 10.1097/PCC.0b013e3182192c42. [DOI] [PubMed] [Google Scholar]
- 16.Bhalla AK, Belani S, Leung D, et al. Higher Dead Space Is Associated With Increased Mortality in Critically Ill Children. Crit Care Med. 2015 doi: 10.1097/CCM.0000000000001199. [DOI] [PubMed] [Google Scholar]
- 17.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med. 2015;43(5):937–946. doi: 10.1097/CCM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 18.Riou Y, Leclerc F, Neve V, et al. Reproducibility of the respiratory dead space measurements in mechanically ventilated children using the CO2SMO monitor. Intensive Care Med. 2004;30(7):1461–1467. doi: 10.1007/s00134-004-2288-3. [DOI] [PubMed] [Google Scholar]
- 19.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92(8):2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 20.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 22.Yehya N, Topjian AA, Thomas NJ, et al. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome*. Pediatr Crit Care Med. 2014;15(4):e147–156. doi: 10.1097/PCC.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villar J, Perez-Mendez L, Lopez J, et al. An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;176(8):795–804. doi: 10.1164/rccm.200610-1534OC. [DOI] [PubMed] [Google Scholar]
- 24.Gowda MS, Klocke RA. Variability of indices of hypoxemia in adult respiratory distress syndrome. Crit Care Med. 1997;25(1):41–45. doi: 10.1097/00003246-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Douglas ME, Downs JB, Dannemiller FJ, et al. Change in pulmonary venous admixture with varying inspired oxygen. Anesthesia and analgesia. 1976;55(5):688–695. doi: 10.1213/00000539-197609000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Karbing DS, Kjaergaard S, Smith BW, et al. Variation in the PaO2/FIO2 ratio with FIO2: mathematical and experimental description, and clinical relevance. Crit Care. 2007;11(6):R118. doi: 10.1186/cc6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172(2):206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 28.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Fernandez Y, Azagra AM, de la Oliva P, et al. Pediatric Acute Lung Injury Epidemiology and Natural History study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med. 2012;40(12):3238–3245. doi: 10.1097/CCM.0b013e318260caa3. [DOI] [PubMed] [Google Scholar]
- 30.Bhalla AK, Rubin S, Newth CJ, et al. Monitoring Dead Space in Mechanically Ventilated Children: Volumetric Capnography Versus Time-Based Capnography. Respir Care. 2015 doi: 10.4187/respcare.03892. [DOI] [PubMed] [Google Scholar]
- 31.Kallet RH, Alonso JA, Pittet JF, et al. Prognostic value of the pulmonary dead-space fraction during the first 6 days of acute respiratory distress syndrome. Respir Care. 2004;49(9):1008–1014. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Mortality stratified by initial PALICC oxygenation categories and initial AVDSf. P values represent Fisher exact tests.
Supplementary figure 2: (A) AVDSf at PARDS onset and at 24 hours in survivors and non-survivors, restricted to only the 216 patients with available AVDSf at PARDS onset and 24 hours after (i.e., remaining on conventional ventilation 24 hours after). (B and C) AVDSf in these 216 patients at PARDS onset and 24 hours after, dichotomized by exposure to iNO. (D) ROC curves for AVDSf at PARDS onset (AUROC 0.75, 95% CI 0.64 to 0.87, p < 0.001) and 24 hours after PARDS onset (AUROC 0.55, 95% CI 0.42 to 0.68, p = 0.429). (E and F) ROC curves for 24-hour AVDSf discriminating mortality in these 216 patients dichotomized by exposure to iNO. For patients not exposed to iNO (E), AUROC 0.64, 95% CI 0.50 to 0.78, p = 0.060); for patients not exposed to iNO (F), AUROC 0.42, 95% CI 0.19 to 0.64, p = 0.614).


