Abstract
We have previously demonstrated that interleukin-17A (IL-17) producing Th17 cells are significantly elevated in blood and bone marrow (BM) in multiple myeloma (MM) and IL-17A promotes MM cell growth via the expression of IL-17 receptor. In this study, we evaluated anti-human IL-17A human monoclonal antibody (mAb), AIN457 in MM. We observe significant inhibition of MM cell growth by AIN457 both in the presence and absence of BM stromal cells (BMSC). While IL-17A induces IL-6 production, AIN457 significantly down-regulated IL-6 production and MM cell-adhesion in MM-BMSC co-culture. AIN-457 also significantly inhibited osteoclast cell–differentiation. More importantly, in the SCIDhu model of human myeloma administration of AIN-457 weekly for 4 weeks after the first detection of tumor in mice led to a significant inhibition of tumor growth and reduced bone damage compared to isotype control mice. To understand the mechanism of action of anti-IL-17A mAb, we report here, that MM cells express IL-17A. We also observed that IL-17A knock-down inhibited MM cell growth and their ability to induce IL-6 production in co-cultures with BMSC. These pre-clinical observations suggest efficacy of AIN 457 in myeloma and provide the rationale for its clinical evaluation for anti-myeloma effects and for improvement of bone disease.
Introduction
Bone marrow (BM) micro-environments have been shown to play a critical role in multiple myeloma (MM) pathobiology1. Immune cells form an important component of this micro-environment, and are modulated by the conditions generated in the BM2. We have previously reported dysfunctional regulatory T cells3 and an increased number of IL-17A expressing T helper (Th17) cells in MM4. These immune abnormalities have been considered to favor tumor cell progression, both directly as well as by suppressing anti-MM immune responses. These immune changes also induce associated bone disease and predispose patients to immune-paresis and associated infectious complications5.
T helper cells play an important role in developing a robust and lasting immune response against bacterial, fungal and viral infections as well as against tumor cells. Besides Th1, Th26 and Treg cells3,7–8, Th17cells play an important role in immune protection against pathogens9–11. Furthermore, Th17 cells participate in mediating immuno-pathological manifestations of a number of autoimmune diseases12–15. Interestingly, interactions between MM cells and the BM micro-environment lead to a production of a number of cytokines and chemokines (TGF-β, IL-6, IL-1β and IL23)1 that skew the T helper cell subset differentiation to Th17 cells. The Th17 cells in turn, both directly and via pro-inflammatory cytokines produced by them, modulate tumor cell growth, suppress Th1 immune responses4 and affect other components of tumor micro-environment, especially osteoid elements as in rheumatoid arthritis15–16. Higher proportion of Th17 cells are induced from naïve CD4 T cells in MM compared to healthy donors4. Dendritic cells (DC) also induce a higher number of Th17 cells in BM of MM patients17. Furthermore, serum levels of IL-17 are significantly elevated in MM compared to healthy donors and this increase is stage-dependent18–22. IL-17 has also been shown to play a critical role in the genesis of bone disease in myeloma by mediating osteoclast formation and activation23–24. On the other hand, bisphophonates treatment is shown to decrease serum levels of IL-17, thus reducing the bone damage reported in MM25. IL-17A induces significant increase in proliferation of MM cell lines and primary cells in vitro via IL-17A receptor (IL-17RA)4 expressed on tumor cells and IL-17A pretreatment led to the development of significantly larger tumors compared to the control in murine xenograft model of MM4. Increased frequency of Th17 cells is also observed in a number of other human malignancies including, ovarian, prostate, renal, and pancreatic carcinomas26–28. These studies provided the rationale to pre-clinically evaluate the effects of anti-IL-17A mAb on MM cell-growth both in vitro and in vivo. The results show that MM cell-growth and survival are significantly inhibited by anti-IL-17A mAb both in vitro as well as in animal studies. IL-17A is produced by myeloma cells and its suppression affects myeloma cell growth indicating a possibility of an autocrine loop.
Materials and Methods
Patient samples
Patient BM samples were collected from newly-diagnosed myeloma patients, and from patients without treatment for at least 3 months. These samples were collected after informed consent in accordance with the Declaration of Helsinki and approved by the institutional review board (IRB) from Dana-Farber Cancer Institute. Healthy donor bone marrow samples were obtained from AllCells (Emeryville, CA).
Myeloma cell-proliferation assays
MM cells (MM1S, KMS-12PE, RPMI 8226, KMS-12BM, OPM-1, OPM-2, INA-6, H929, U226, and ARP1), cultured in RPMI 1640 supplemented with 10% FBS and antibiotics for three days in the presence of isotype or anti-IL-17A mAb (10 µg/ml, AIN 457, Novartis). Proliferation was measured by 3H-thymidine incorporation and MTT assay (Life Technologies, Grand Island, NY, USA). Co-culture studies were performed with BMSC in the presence of isotype control antibody or AIN 457. IL-6 with or without AIN 457 and its antibody with or without IL-17A (R & D Systems, Mineapolis, MN, USA)) were used in co-culture to determine the growth and proliferation in these assays. Colony forming assays were performed using MethoCult agar media (Stem Cell Technologies, Vancouver, BC, Canada) in the presence of isotype control antibody or anti-IL-17A mAb for three weeks4.
Measurement of IL-6 production by IL-17A and inhibition by AIN 457 using ELISA assays
BMSC were cultured for three days in the presence or absence of IL-17A (100ng/ml), IL-21, IL-22, IL-23, IL-27 and LPS at 10ng/ml concentration. For antibody inhibitory studies, BMSC in the presence or absence of MM cell-lines was cultured with isotype control antibody, or IL-17A, or anti-IL-17A mAb or in combination with IL-17A + anti-IL-17A mAb. For ex-vivo studies, the fetal bone chips with MM cells were incubated in the presence of isotype control antibody or anti-IL-17A mAb for 2 days. Myeloma cells were co-cultured with BMSC in the presence of IL-17A with or without cell-signaling inhibitors (JAK2, STAT3, JNK, MEK, NFkB and PI3 inhibitors at 10µM from Cell Signaling, Danvers, MA, USA). IL-6 production was measured by standard ELISA (R&D Systems, Minneapolis, MN). The immunohistochemical analysis of myeloma cells in the bones was performed by staining them with anti-CD138 antibodies4.
Adhesion Assay
Cell adhesion assay was performed as previously describe29. In brief, serum-starved MM cells (5 × 106/mL) were first labeled with calcein AM (Molecular Probes, Eugene, OR) and cultured with BMSC in the presence of isotype control antibody or anti-IL-17A mAb. The absorbance of each well was measured at 492/520 nm plate reader, as described4.
Osteoclast formation
As described earlier30, osteoclasts (OCs) were differentiated from BMMCs from healthy volunteers in 6-well plates. Adherent cells were cultured for 21 days in α-MEM containing with (positive control) or without (negative control) 50 ng/mL of macrophage colony-stimulating factor (M-CSF; R&D Systems, Minneapolis, MN) and RANKL (PeproTech, Rocky Hill, NJ, USA) with anti-IL-17A mAb or isotype control antibody at 10µg/ml concentration. Cells stained for tartrate-resistant acid phosphatase (TRAP) using an acid-phosphatase leukocyte staining kit (Sigma Aldrich, St Louis, MO, USAl).
Myeloma murine Xenograft model
Six- to eight week old male Fox Chase CB-17 severe combined immune-deficient (SCID) mice (Charles River Laboratories Intl. Inc., Wilmington, MA, USA) were injected with five million OPM-1 myeloma cells with isotype or AIN 457 ((10µg/ml)by sc route with approvals by the Institutional Animal Care and Use Committee (IACUC) (VA Boston Healthcare System). Tumor volume was measured at indicated time intervals as described previously31. In another set of experiments SCID mice were treated with with weekly sc injections following MM cell-injections. In addition, Balb/C mice were injected with murine plasmacytoma cell-line (ATCC CCL167/MPC11) and treated with isotype control antibody or murine anti-IL-17A mAb (10µg/ml) for a week with sc injections and tumor volumes were measured.
SCIDhu human myeloma model
Human fetal bone grafts were subcutaneously implanted into SCID mice (SCID-hu), as previously described32. Four weeks following bone implantation, 2.5×106 INA-6 MM cells were injected directly into the human bone implant. One group was treated with isotype control antibody and another group of mice was treated weekly subcutaneously with AIN-457 (10µg/ml) for four consecutive weeks following first detection of tumor.. Tumor growth was evaluated by measuring serum level of soluble humans IL-6R with ELISA R & D Systems).
MicroCT analysis of bones
The bone damage caused by myeloma cells from these mice was assessed by micro-computed tomography (microCT) analysis at the end of the study as described33.
Quantitative PCR and western blot analysis
Quantitative PCR was performed for IL-17A using RNA isolated from MM cell-lines and purified MM primary cells. Results are presented as relative expression value in comparison with GAPDH. Cell lysates were probed with anti-sera to IL-17A and GAPDH (Santa Cruz Biotech, Santa Cruz, CA, USA). Myeloma cell lines were stained with isotype control antibody or anti–IL-17A antibody and analyzed by confocal microscopy. One representative cell-line of 4 experiments is shown at 640 magnification4.
siRNA and shRNA transfections
MM cells (U266) were transfected with siRNA or sh RNA or respective controls (Santa Cruz Biotech) for IL-17A and cultured to measure viability, colony formation and IL-6 levels with BMSC.
Statistical analysis
Statistical analyses were performed by student ‘t’ test. P < 0.05 was considered statistically significant.
Results
Growth inhibitory activity of anti-IL-17A antibody on myeloma
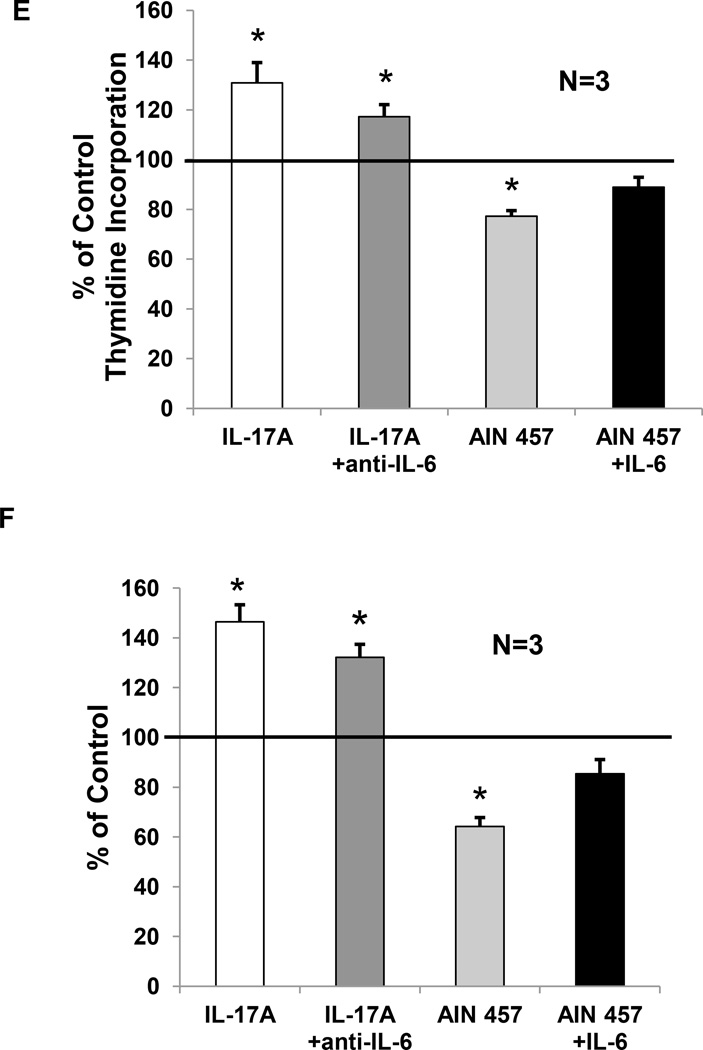
As IL-17A promotes myeloma cell growth and survival4, we investigated the effects of human anti-IL-17A monoclonal antibody (AIN-457) in MM. As seen in Figure 1A, anti-IL-17A mAb significantly inhibited MM cell proliferation measured by 3H-thymidine incorporation (−21.8±2.14%, p<0.05) compared with proliferation of MM cell-lines with isotype control antibody. The inhibitory effect of anti-IL-17A mAb on myeloma cell-growth (−16.2± 2.3, p<0.05) compared with cell-growth of MM cell-lines with isotype control antibody was also observed using MTT assay. (Figure 1 B). We observed that anti-IL-17A mAb significantly inhibited colony formation in methoCult colony assay (40±5, p<0.05, compared to isotype control antibody, 80±4) (N=3) and with primary MM cells (N=4) (31±5, p<0.05, compared to isotype control, 84±7) (Figure 1C and D). These results suggest that anti-IL-17A mAb significantly inhibits growth of MM cell-lines and primary cells in vitro. We also observed anti-IL-6 antibody in the presence of IL-17A significantly reduced growth and proliferation induced by IL-17A in co-culture with BMSC, however, it did not take to control level in both thymidine incorporation (Figure 1E) and in MTT assays (Figure 1F). On the other hand, adding IL-6 in the presence of anti-IL-17A antibody slightly increased growth and proliferation of myeloma cells in co-culture with BMSC and it did not restore the growth and proliferation of MM cells to the control level.
Figure 1. Inhibitory activity of anti-IL-17A antibody on myeloma cell proliferation.
(A) Myeloma cell lines alone (N=8) were incubated with isotype control antibody or anti-IL-17A mAb to measure proliferation by 3H-thymidine incorporation after 3 days. Data is presented as percentage inhibition in proliferation in presence of anti-IL-17A mAb compared with isotype control antibody and showed as mean ± SEM. (B) Myeloma cell lines alone (N=5) were incubated with isotype control antibody or anti-IL-17A mAb to measure metabolic activity by MTT assay after 3 days. Data is presented as percentage inhibition in proliferation in presence of anti-IL-17A mAb compared with isotype control antibody and showed as mean ± SEM. (C) Myeloma cell lines (U266) were cultured in methocult agar plates in the presence of isotype control antibody or anti-IL-17A mAb. Representative photomicrograph and results (N=3) are presented. Photographs were obtained using a Nikon TE200 microscope (40× objective) with attached camera (Nikon) at room temperature (total magnification 200) and analyzed with Metafluor software (Molecular Devices). The number of colonies were counted in unit area and presented as mean ± SEM. (D) Primary MM cells (N=4) were cultured in methocult agar plates in the presence of isotype control antibody or anti-IL-17A mAb. The number of colonies were counted in unit area and presented as mean ± SEM. *P <0.05. Myeloma cells (U266) was cocultured with BMSC for three days in the presence of isotype, IL-17A with or without anti-IL-6 (10 µg/ml) antibody, and anti-IL-17A antibody with or without IL-6 (10ng/ml) and proliferation was measured by thymidine incorporation (Figure E) and MTT (Figure F) assays. Data is presented as percentage control co-culture proliferation and showed as mean ± SEM.
Down regulation of BMSC-mediated MM cell-growth by anti-IL-17A antibody via blockade of IL-6 production
We evaluated the influence of anti-IL-17A mAb on BMSC-mediated MM cell-growth. As seen in Figure 2A, anti-IL-17A mAb significantly inhibited MM cell-proliferation (−22.7±2.9%, p<0.05) in presence of BMSC compared with MM cell-proliferation in presence of BMSC with isotype control antibody in co-culture assays as measured by 3H-thymidine incorporation.
Figure 2. Down regulation of BMSC-mediated MM cell-growth by anti-IL-17A antibody via blockade of IL-6 production.
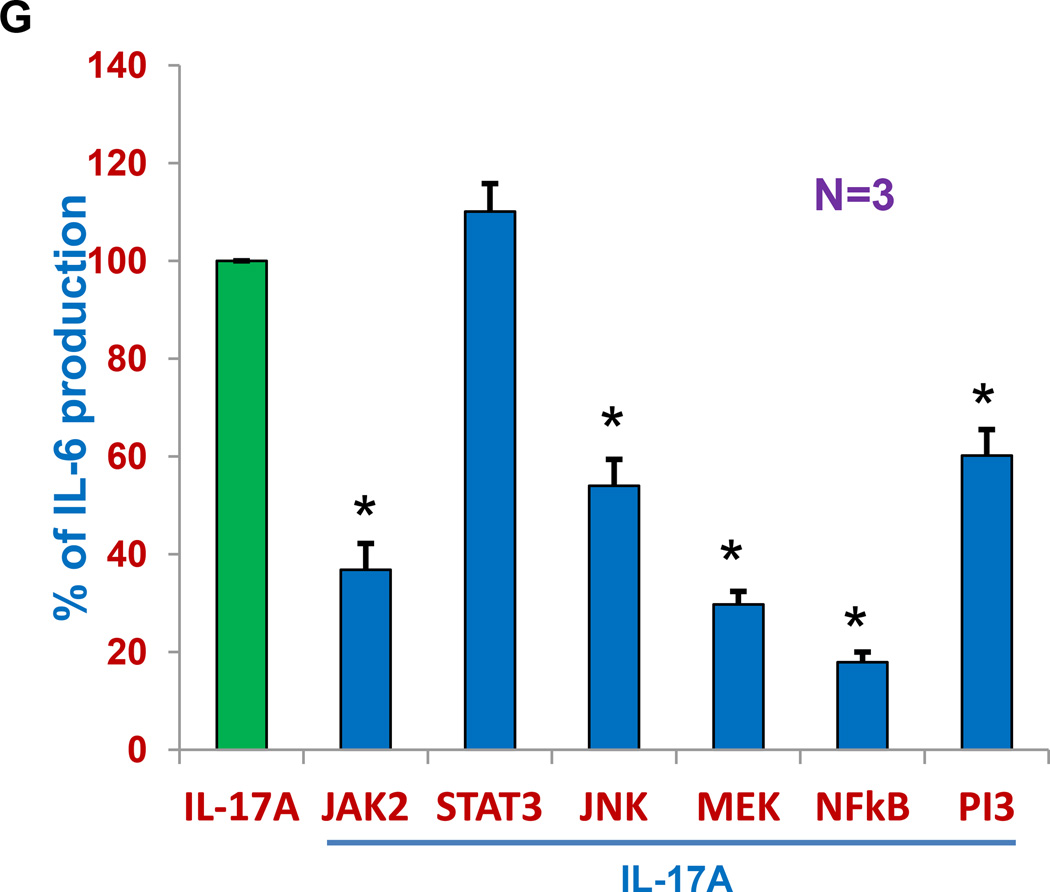
(A) MM cell lines (N=7) were cultured with BMSCs in the presence of isotype control antibody or anti-IL-17A mAb and proliferation measured by 3H-thymidine incorporation after 3 days and presented as percentage of inhibition in proliferation with isotype control antibody. (B) BMSC was cultured for three days in the presence or absence of IL-17A (100ng/ml), IL-21, IL-22, IL-23, IL-27 and LPS at 10ng/ml concentration. IL-6 production was measured by standard ELISA (R&D Systems, Minneapolis, MN). Bar graph represents mean ± SEM of the data (N=4) calculated as percent of increase or decrease in IL-6 levels in culture supernatants compared to IL-6 levels (as endogenous IL-6 production) obtained from BMSC alone without any stimulation. (C) BMSC in the presence (right or absence (left) of MM cell-lines was cultured with isotype control antibody, IL-17A, or anti-IL-17A mAb or in combination with IL-17A + anti-IL-17A mAb and IL-6 levels were measured in culture supernatants by ELISA. Bar graph represents mean ± SEM of the data calculated as percent of increase or decrease in IL-6 levels in culture supernatants compared to IL-6 levels (as endogenous IL-6 production) obtained from BMSC alone with isotype control antibody without any stimulation. (D) Serum-starved MM cells were labeled with calcein AM, washed, and added to BMSC-coated plates in the presence of isotype control antibody or anti-IL-17A mAb for 4 hours and non-adherent cells were removed by washing. Adhesion was measured by measuring the absorbance using 492/520 nm filter set with a fluorescence plate reader. Results represent mean ± SEM of 3 independent experiments performed in triplicate. The absorbance values obtained with isotype control antibody was considered as 100% and percentage of inhibition was calculated for anti-IL-17A treatment. (E) The fetal bone chips with MM cells were incubated in the presence of isotype control antibody or anti-IL-17A mAb for 2 days for ex-vivo for the evaluation of IL-6 production that is measured by ELISA (R & D Systems). Bar graph represents mean ± SEM of the data calculated as percent of IL-6 levels (as endogenous IL-6 production) in culture supernatants from BMSC alone with isotype control antibody. (F) The immunohistochemical analysis of bones was performed by staining them with anti-CD138 antibody. Arrows indicates presence bright CD138+cells. (G) Myeloma cells were co-cultured with BMSC in the presence of IL-17A with or without cell-signaling inhibitors (JAK2, STAT3, JNK, MEK, NFkB and PI3 inhibitors) and IL-6 production was measured with standard ELISA. *P <0.05.
It is well established that IL-6 produced by MM-BMSC interaction mediates MM cell growth. So we further investigated whether IL-17A affects IL-6 production by BMSC alone or in co-culture assays and whether anti-IL-17A mAb mediates part of its activity via its effect on IL-6 produced by BMSC. We evaluated the effect of the number of cytokines associated with Th17 pro-inflammatory pathway (IL-21, IL-22, IL-23, IL-27 and TGF-β) on IL-6 production by BMSCs. As seen in Figure 2B, only IL-17A, in addition to LPS used as a positive control, was able to induce significant increase in IL-6 production by BMSC compared with endogenous or base-line IL-6 production by BMSC without LPS. We further evaluated the influence of anti-IL-17A mAb on IL-17A-induced IL-6 production by BMSC alone or in co-culture systems. As seen in Figure 2C, IL-17A was able to significantly increase IL-6 production by BMSC alone (360±37% increase compared to endogenous IL-6 production by BMSC alone, p<0.05, in the left side of Figure 2C) and in co-culture with MM cells (514±76% increased compared to endogenous levels of IL-6 by BMSC alone and co-culture controls, p<0.05, in the right of Figure 2C). While anti-IL-17A mAb was able to significantly decrease (p<0.05) the IL-6 production by BMSC (77±4.5%, in the left side of Figure 2C) compared with endogenous levels of IL-6 by BMSC alone with isotype control antibody and in co-culture system (107±9% in the right of Figure 2C) compared with endogenous levels of IL-6 in co-cultures with isotype control antibody (212±32%). In addition, when we used anti-IL-17A mAb + IL-17A in combinational studies, we observed that anti-IL-17A mAb was also able to reduce significantly IL-6 production in presence of IL-17A, (142±18% compared with IL-6 production by BMSC with IL-17A with isotype control antibody, 360±37%, in the left side of Figure 2C) and in co-culture (245±33% compared with IL-6 production in co-culture with IL-17A with isotype control antibody, 514±76%, in the right of Figure 2C). We next evaluated the effect of anti-IL-17A mAb on the ability of myeloma cells to bind to BMSC using established adhesion assay. Calcein- labeled MM cells were co-cultured with BMSC for 4 hours and adhesion was measured as described in methods. Presence of anti-IL-17A mAb (N=3) significantly reduced (27±3.8%, p<0.05) MM cell-BMSC adhesion as compared to adhesion of MM cells to BMSC in the presence of isotype control antibody as measured by the absorbance using a fluorescence plate reader (Figure 2D). Finally, when we inject MM cells ex-vivo into human fetal bone chips IL-6 production was significantly reduced in presence of anti-IL-17A mAb, compared to IL-6 production in the presence of isotype control antibody after 48 hours (Figure 2E). Analysis of these bone chips by immune-histochemistry using anti-CD138 mAb showed significant reduction of CD138+ expression (dim or low) of plasma cells by anti-IL-17A mAb compared to CD138+ expression (bright or high) in the presence of isotype control antibody (Figure 2F). When myeloma cells were co-cultured with BMSC in presence of IL-17A with or without different cell-signaling inhibitors (JAK2, STAT3, JNK, MEK, NFkB and PI3 inhibitors), IL-6 production was significantly inhibited by all of these signaling pathway inhibitors except STAT3.
Down-regulation of osteoclast development in vitro by ant-IL-17A mAb
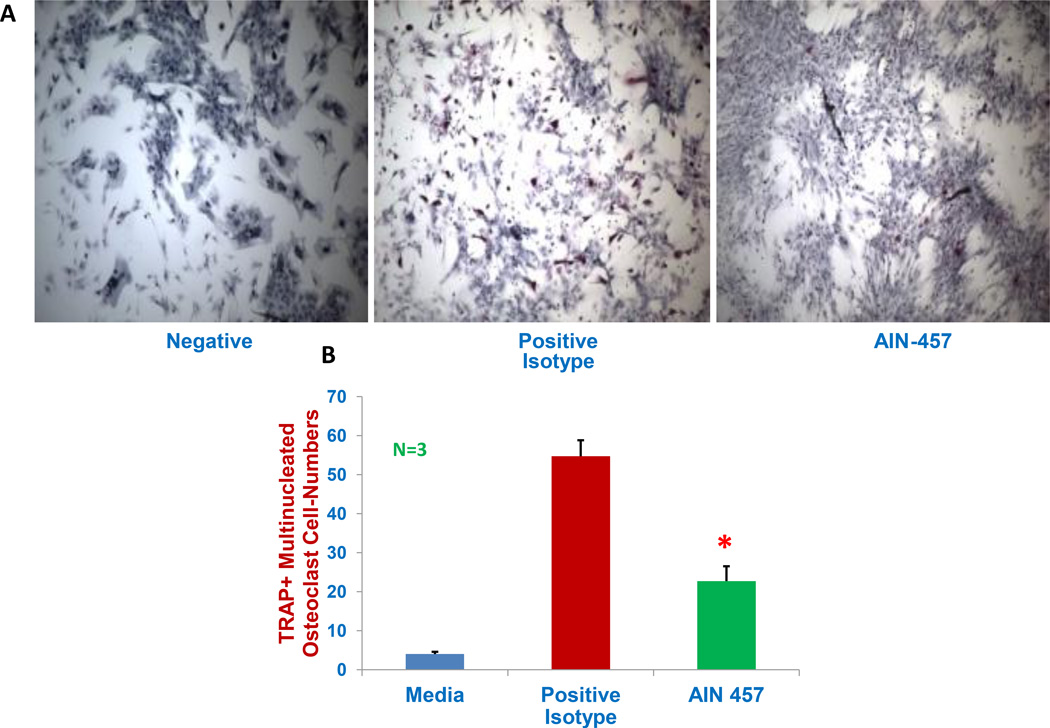
IL-17 has also been shown to play a critical role in the genesis of bone disease in myeloma by mediating osteoclast formation and activation23–24. Normal BMMC were cultured for three weeks in osteoclast-supporting medium with isotype control antibody or anti-IL-17A mAb, and stained for TRAP+ multi-nucleated osteoclast cells. TRAP-positive stained cells containing 5 or more nuclei/cell were enumerated with the aid of microscope and image J software. A representative image was depicted (Figure 3A) showing pink cells as TRAP+multi-nucleated osteoclasts in positive control in the presence of isotype control antibody as compared with anti-IL-17A mAb treated group showing osteoblast-looking growth patterns. As seen in Figure 3B, the osteoclast cell number was significantly decreased (by 42%) by anti-IL-17A mAb treatment (23±4) compared with the positive control with isotype control antibody (55±4) (p<0.05) in osteoclast-supporting media.
Figure 3. Reduced osteoclast cell numbers by the antibody.
Normal BM cells were cultured for three weeks in osteoclast supporting-medium (consisting 25ng/ml of macrophage colony-stimulating factor and 25 ng/ml of receptor activator of nuclear factor kappa-B ligand) with isotype control antibody (as positive control) or anti-IL-17A antibody and the tartrate-resistant acid phosphatage (TRAP+) multinucleated osteoclast cells were stained and cell-numbers were counted. Cells were cultured without osteoclast-supporting-medium as negative control. Images were obtained at 10 magnifications with microscope (Eclipse TS100, Nikon instruments, Melville, NY, USA) with spot insight camera. TRAP-positive cells containing 5 or more nuclei/cell were enumerated using image J 1.45 software (NIH, Bethesda, MD, USA). A representative image was depicted (Figure A) and composite results from three experiments were shown in bar graph Figure B). Star indicates statistical signifies (p<0.05). There were no significant differences observed among three groups in cell-viability measured with alamor blue staining.
Inhibition of human myeloma cell-growth in vivo by systemic administration of anti-IL-17A mAb in murine models of human myeloma
Next, we evaluated the efficacy of anti-IL-17A mAb in animal models using two different animal models of human myeloma. Initially in xenograft model, SCID mice were injected with myeloma cells along with isotype control antibody or AIN 457 subcutaneously. Tumor volumes were measured at 24 and 30 day time intervals after MM cell injections. As seen in Figure 4A in a representative of two experiments, the presence of anti-IL-17A mAb significantly reduced tumor size (37% inhibition at 24 days and 47% inhibition at 30 days, p<0.05) compared to the tumor volumes in the presence of isotype control antibody treatment group at both time points evaluated, as seen in Figure 4A. The reduction in tumor volumes by anti-IL-17A mAb may be due to both either inhibiting tumor growth and/or tumor engraftment in this animal model. In another set of experiments, SCID mice were treated with weekly sc injections following MM cell-injections. Even though we observed that anti-IL-17A mAb reduced (by 35% at 24 days after MM cell injection) myeloma growth in this SCID xenograft mice study and the difference in tumor volumes between isotype control antibody (2007±584mm3) and treated groups (1299±282mm3) is not statistically significant, as seen in Figure 4B. To evaluate the efficacy of anti-IL-17A mAb in immune competent mice, Balb/C mice were injected with murine plasmacytoma cell-line (ATCC CCL167/MPC11) and treated with isotype control antibody (N=13) or murine anti-IL-17A mAb (N=20) (10µg per mice) for a week and their tumor volumes were measured. However, we observed that anti-IL-17A mAb reduced (by 32%) myeloma growth in this immune competent mice study and the difference in tumor volumes between the isotype control antibody (1217±207mm3) and the treated groups (827±113mm3) was not statistically significant by the student “t” test evaluation.
Figure 4. Effect of AIN-457 humanized antibody on myeloma growth using three mouse models without tumor microenvironment.
(A) SCID mice were injected with myeloma cells by sc route. Mice were injected in 2 groups (N=3). One group was injected with myeloma cells with isotype control antibody; and second of mice injected with MM cells in anti-IL-17A mAb (10µg/ml). Tumor volume was measured at indicated time intervals from the MM cell-injections. Representative of two experiments is shown (p<0.05). (B) SCID mice were treated with isotype control antibody or anti-IL-17A mAb (10µg/ml) with weekly sc injections following subcutaneous MM cell-injections (N=4). Tumor volumes (mm3) were measured at indicated time intervals. Representative of two experiments is shown. (C) Balb/C mice were injected with murine plasmacytoma cell-line and treated with isotype control antibody or murine anti-IL-17A mAb (10µg/ml) for a week with sc injections and tumor volumes were measured.
Next, we evaluated the efficacy of anti-IL-17A mAb in the SCIDhu animal model of human myeloma. This model represents human myeloma development in the presence of BMSC. SCID mice were transplanted with human fetal bone-chips, and after four weeks, myeloma cells were injected into the fetal bones. Following the first detection of the tumor, one group of mice was treated with human IgG isotype control antibody and the other group of mice was treated with anti-IL-17A mAb (10µg/mouse/injection) subcutaneously with weekly injections, for four weeks. Serum samples were collected weekly and level of human soluble IL-6R was measured by standard commercially available ELISA as a measure of tumor growth29. As seen in Figure 5A a representative of two experiments, anti-IL-17 mAb significantly inhibited (by 5 times-lower) tumor growth (5.9±2.8 ng/ml of sIL-6R) compared to isotype control antibody (30.9±10.5 ng/ml of sIL-6R) mice (p<0.05). This SCIDhu human myeloma model is very similar to human myeloma conditions with its BM microenvironmental settings. The efficacy of this anti-IL-17A mAb in this SCIDhu human myeloma against myeloma cells is excellent by inhibiting 81% of myeloma cells ability to produce soluble IL-6 receptor while they are growing. We have used this SCIDhu human myeloma model for evaluating pre-clinical efficacy of number of agents including chemotherapeutic agents and antibodies. In our pre-clinical efficacy evaluation studies using the SCIDhu human myeloma model over the past decade, the anti-IL-17A mAb was shown to be the best and have the highest efficacy against myeloma. As we are seeing obvious statistically significant differences between isotype control antibody and anti-IL-17A mAb treated mice, and as results are confirmed in 2 different animal models, we believe it provides adequate evidence for clinical evaluation of anti-IL-17 mAb in MM. Additionally, anti-IL-17A mAb was also able to prevent the bone resorption caused by MM cells. At the termination of the experiment, microCT studies of the human bones harvested from animals reveals protection by anti-IL17A from the bone resorption associated with myeloma tumor growth in the presence isotype control antibody as seen in Figure 5B. The measurements of bone resorption showed that the prevention of resorption is significantly higher by the anti-IL-17A mAb treatment group compared with isotype control antibody treatment group (Figure 5C).
Figure 5. Inhibition of tumor growth and prevention of bone resorption by AIN-457, IL-17A antibody in SCID human myeloma model.
A) SCID mice were transplanted with human fetal bones, and after four weeks, myeloma cells were injected into the bones. One group was treated with vehicle with isotype control antibody and another group of mice were treated subcutaneously with AIN-457 (10µg/ml-/mouse/injection) for four weeks following first detection of tumor by measuring human soluble IL-6R in the serum. Serum samples were collected weekly and level of humans IL-6R was measured by ELISA. Baseline values before treatment were not significantly different among groups. Representative of two experiments is shown (p<0.05). B) Treatment with anti IL-17A protects implanted bones from osteolysis. At the termination of the experiment, implanted human bones were excised and imaged by microcomputed tomography (microCT). Shown are the three-dimensional reconstructions of the bones and sections sliced longitudinally through the midpoint of each specimen injected with MM cells followed by treatment of the animal with either isotype control antibody (excessive bone resorption is seen) or anti IL-17A (no bone resorption is observed, trabecular bone is intact). The areas of increased osteoclastic bone resorption in the isotype control antibody-treated sample are indicated by arrowheads. Scale bar equals 1 mm3. Anti-IL-17A antibody reduced the bone resorption caused by myeloma cells. C) Significant differences in the measurements of bone resorption following anti-IL-17A mAb treatment.
Expression of IL-17A in myeloma cells and functional consequences by knockdown with siRNA and shRNA on growth and survival of myeloma cells
To validate expression of IL-17A by MM cells, we performed quantitative RT-PCR in both MM cell lines and CD138+ primary MM cells from patients. We observed the expression of IL-17A in majority of MM cell-lines as well as primary MM cells (Figure 6A/B). The IL-17A expression was higher in 5 out 7 tested MM cell-lines compared with the expression of IL-17A in PBMC collected from healthy donor. Expression of IL-17A protein was confirmed by western blot in all MM cell-lines and 7 out of 9 purified primary MM cells (Figure 6C/D). The IL-17A protein levels were lower in CD138+ primary MM cells compared with cell-lines and Th17 cells as a positive control. Normal CD138+ cells collected from healthy donors have shown no IL-17A protein. Finally, we also show intra-cellular IL-17A protein in MM cells by confocal microscopy (Figure 6E).
Figure 6. Analysis of IL-17A expression in myeloma cells.
The MM patient samples were collected after informed consent in accordance with the Declaration of Helsinki and approved by the institutional review board (IRB) from Dana-Farber Cancer Institute. Healthy donor bone marrow samples were obtained from AllCells (Emeryville, CA). MM primary cells were purified as described earlier5. RNA was isolated from MM cell-lines (A) and purified MM primary cells (B). Quantitative PCR was performed for IL-17A using 7900HT from Applied Biosystems. Representative experimental results from three different experiments were presented as relative expression value in comparison with GAPDH. For immuno-blot experiments, total cell lysates were prepared from MM cell-lines (C) and purified CD138+ MM primary cells (D), and separated by electrophoresis on 5% to 20% polyacrylamide gradient gels. Samples were probed with anti-sera to IL-17A and GAPDH as indicated. Representative immunoblot of three different experiments was shown. Myeloma cell lines were stained with isotype control antibody or anti–IL-17A antibody and analyzed by confocal microscopy. One representative cell line of 4 experiments was shown at 640 magnifications (E).
In order to investigate the functional consequences of IL-17A expression by MM cell-line (U266), we evaluated impact of IL-17A knock-down on MM cell growth and colony formation. IL-17A Knock-down, using IL-17-specific siRNA (Figure 7A) inhibited MM cell-growth as well as colony formation in MethoCult agar plates (Figure 7C). We also confirmed these results using IL-17A-specifc shRNA (Figure 7B). IL-17A induces IL-6 production by stromal cells in various tissues20 and we have previously reported that IL-17A elevates IL-6 production by BMSC. So, we evaluated impact of IL-17A knock-down on IL-6 production by BMSC. IL-6 production in MM/BMSC co-culture was significantly reduced following IL-17A knock-down in MM cells (Figure 7D). These results show that IL-17A expressed by MM cells have impact on growth of MM cell as well as on the microenvironment.
Figure 7. IL-17A knockdown decreases myeloma cell-number and their ability to produce IL-6 in a co-culture with bone-marrow stromal cells.
Myeloma cell-line (RPMI 8226) cells were used to transduce human IL-17A siRNA according to manufactures recommendations to determine the influence on myeloma cell-proliferation and their ability to produce IL-6 when co-cultured with bone-marrow stromal cells. Cell lysates were analyzed using immuno-blots to assess decrease in intra-cellular protein expression of IL-17A (A). Cell-proliferation was analyzed by counting live cells following transfection. Representative study results of three different experiments were shown. MM cell-line (U266) was used to transduce human IL-17A shRNA according to manufactures recommendations to determine the influence on myeloma cell-expression and –proliferation (B). Representative study results of three different experiments were shown. Colony formation was evaluated using MethoCult agar plates following transfection with siRNA or shRNA (C). Representative study results of five different experiments were shown. IL-6 production was measured with standard ELISA following co-culturing myeloma cells with or without transfected MM cells with BMSC (D). Representative study results of three different experiments were shown.
Discussion
IL-17A, a key pro-inflammatory cytokine, is predominantly produced by T helper cells in addition to other immune cell-types including γδ and CD8+ T cells, and NKT cells34. Although it provides protective effects against intra-cellular bacterial/viral, fungal and parasitic infectious agents9, it plays a deleterious role in immune-pathology of autoimmune13 and inflammatory diseases including asthma35, in addition to cancer26–28,36. The demonstration of elevated levels of Th17 cells and serum IL-17A in myeloma may help explain the reported higher incidence of MM in patients with autoimmune diseases. Our recent data also suggests elevated levels of other Th17-associated pro-inflammatory cytokines including IL-21, IL-22 and IL-23 in both peripheral blood and BM sera samples from MM compared to control samples4. Moreover, IL-17A induces MM cell proliferation with or without BMSC and increases MM cell adhesion to BMSC4. The presence and requirement of IL-17RA on MM cells further supports the role of IL-17A in MM cell growth and survival, and confirms it as a novel therapeutic target. IL-17A effects on immuno-paresis in myeloma with suppressed Th1 responses in the presence of IL-224 and its activating effects on osteoclast with consequent effect on bone disease in myeloma23–24, provide added rationale to the target IL-17A in MM.
Here, we have evaluated the preclinical efficacy of human anti-IL-17A mAb on MM in vitro and in vivo. We observe that anti-IL-17A mAb significantly inhibit the growth of MM cell lines as well as primary cells and are able to overcome protective effects of BMSCs. The observed effects of anti-IL-17A mAb on MM cells in culture as well as in colony assay is intriguing and raises the question about the source of IL-17A in this system. In MM, we and others, have shown that IL-17A supports the MM cell growth and survival4,17,23. Further disease progression was observed with increased IL-17A serum levels18–22. Reduction of IL-17 after bisphosphonate treatment has also been reported25. These studies point out a significant role for IL-17A modulation in myeloma therapeutics.
It has been well established that IL-17A may cause its deleterious pathological effects through the increased production of IL-6, particularly in rheumatoid arthritis37–38. We have shown for the first time that MM IL-17A induces IL-6 production by BMSC alone and in co-culture with MM cells. In addition, these results indicate that the other pro-inflammatory cytokines-associated with Th17 cells including IL-21, IL-22, IL-23 and IL-27 do not induce the IL-6 production by BMSC. We observed that anti-IL-17A mAb was able to significantly down-regulate MM cell-BMSC adhesion and IL-6 production. These results were consistent with previous observations that IL-6 production was elevated in stroma in tissues38. In order to show the importance of IL-17A in the production of IL-6 in co-culture settings, neutralization studies showed that anti-IL-6 antibody did not completely inhibit IL-17A effects and adding IL-6 did not restore AIN 457 inhibition on the growth and proliferation of MM cells. These studies taken together indicate that IL-17A-mediated growth and proliferation MM cells are in part mediated by IL-6 and in part mediated other factors. IL-17A induces number of pro-inflammatory factors in addition to IL-638.
Since elevated levels of IL-6 and MM-BMSC interaction are involved in bone damaging effects during the progression of myeloma disease, inhibition of effects of IL-17A on IL-6 production can help improve bone disease in MM. IL-17A increases osteoclast-differentiation and thereby is partly responsible for associated bone damages observed in rheumatoid arthritis39–40and IL-17 has also been shown to play a critical role in the genesis of bone disease in myeloma by mediating osteoclast formation and activation23–24. Therefore, we evaluated and observed significant inhibition of TRAP+ multinucleated osteoclast cell–differentiation by anti-IL-17A mAb. These results are in agreement with the recent report showing that the elevated levels of IL-17A and IL-1β in the BM plasma correlate with lytic bone disease in MM patients23. We have also observed anti-MM activity of anti-IL-17 mAb in two murine models of myeloma; the subcutaneous MM xenograft model, where a pre-treatment with anti-IL-17 mAb led to significant reduction in tumor volume, and more importantly in the SCIDhu model of human MM cells. In the second model the results are especially interesting as we have a human micro-environment in this setting providing some of the cellular components of the human BM micro-environment. Number of studies has described increased frequency of Th17 cells in other human malignancies including, ovarian, prostate, renal, and pancreatic carcinomas26–28. Moreover, IL-17 has also been linked to poor survival in number of other cancers, including AML, breast, colorectal, liver, lung, and melanoma40–42. We have showed that myeloma cells express IL-17 by number ways including western blot and quantitative PCR assays in addition to confocal microscopy. In human studies, transduction of IL-17 in 3 different non-small cell lung cancer cell lines, also expressing IL-17 receptors, led to significant increase in tumor growth in SCID model compared to control cells suggesting possible role of autocrine loop in these cancer cells43. Similar in vitro and in vivo results have been also reported in cervical cancer suggesting role for IL-17A expression by tumor cells44. Conversely, regardless of the source of IL-17, IL-17 receptor deficiency has been reported to show reduced number of invasive prostate adenocarcinoma with lower rate of cellular proliferation suggesting that IL-17-mediated signaling promotes tumor growth45. These studies collectively suggest that expression of IL-17A and its receptors by tumor cells may affect their growth. In our setting it appears that MM cells inherently express both IL-17R as we have previously reported4, and also IL-17A as reported here. We have also observed the functional impact by IL-17A knock-down on tumor cell growth as well as on supporting BM microenvironment. The impact of IL-17A produced by MM cells on BMSC is also very intriguing. Induction of IL-6 in BMSCs, by MM cell-derived IL-17A, may suggest in part a unique pathway for IL-6 production. A report of IL-17 production by MM cell may provide an explanation of the source IL-17 in this system46. Moreover, we have shown earlier4, using various methods, that MM cells do express IL-17 receptors, suggesting a possible autocrine loop is in play in MM using IL-17A and its receptor pathway. IL-17R-mediated pathways are very complex due to the presence of five different IL-17R and warrant further confirmative studies. The efficacy of neutralization of IL-17A in a variety of disease states, including asthma and lung inflammatory diseases47, rheumatoid arthritis48–49, and multiple sclerosis50 where IL-17A participates in the development and/or patho-physiology disease, has been shown in addition to other cytokine targets in Th17 pathway51–52.
IL-17A is present in synovial fluids in RA and it participates in bone erosion53. Efficacy of anti-IL-17A mAb has been confirmed in three proof-of-concept trials recently; clinically significant improvements were observed in patients with psoriasis, rheumatoid arthritis and uveitis, where IL-17A has been implicated in the disease process without major adverse effects54. The second trial evaluating patients with rheumatoid arthritis where anti-IL-17A mAb, ixekizumab (LY2439821), showed improved signs and symptoms of RA without strong adverse effects55 against psoriasis56. The third study is a phase 2, randomized, double-blind, placebo-controlled dose-ranging study of brodalumab (AMG 827), a human anti-IL17-receptor monoclonal antibody for psoriasis57.
In summary, we show that the anti-IL-17A mAb (AIN 457) is able to inhibit MM growth and survival both in vitro and in vivo studies by blocking in part, positive autocrine feedback loop in addition to inhibiting IL-6 production by BMSC ; it improves bone defects in MM and has the potential to improve immune function in MM. These results confirm the rationale for clinical evaluation of anti-IL-17 mAb in MM.
ACKNOWLEDGMENTS
This work is supported by: Department of Veterans Affairs Merit Review Award1 I01 BX001584-01, NIH grants RO1-124929 and PO1-155258 (NCM) and P50-100707, and PO1-78378, (NCM and KCA). JSG is supported by the Department of Veterans Affairs Office of Research and Development through a Career Development Award-2. This work is also supported by MMRF senior award to RHP.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding MM pathogenesis in the BM to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 2.Munshi NC. Immunoregulatory mechanisms in multiple myeloma. Hematol Oncol Clin North Am. 1997;11(1):51–69. doi: 10.1016/s0889-8588(05)70415-9. [DOI] [PubMed] [Google Scholar]
- 3.Prabhala RH, Neri P, Bae JE, Tassone P, Shammas MA, Allam CK, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107(1):301–304. doi: 10.1182/blood-2005-08-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115(26):5385–5392. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhala RH, Munshi NC. Immune therapies. Hematol Oncol Clin North Am. 2007;21(6):1217–1230. doi: 10.1016/j.hoc.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(6):2348–2357. [PubMed] [Google Scholar]
- 7.Fehérvari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114(9):1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126(2):375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 9.Peck A, Mellins E. Precarious Balance: Th17 cells in host defense. Infect Immun. 2010;78(1):32–38. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32(5):681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, et al. A homozygous CARD9 mutation in a family with susceptibility tofungal infections. N Engl J Med. 2009;361(18):1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opinion Immunol. 2011;23(5):613–619. doi: 10.1016/j.coi.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13(2):139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 14.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 15..Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructivearthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100(10):5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhodapkar KM, Barbuto S, Matthews P, Kukreja A, Mazumder A, Vesole D, et al. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112(7):2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandrakis MG, Pappa CA, Miyakis S, Sfiridaki A, Kafousi M, Alegakis A, et al. Serum IL-17 and its relationship to angiogenic factors in MM. Eur J Intern Med. 2006;17(6):412–416. doi: 10.1016/j.ejim.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Song XN, Yang JZ, Sun LX, Meng JB, Zhang JQ, Lv HY, et al. Expression levels of IL-27 and IL-17 in multiple myeloma patients: a higher ratio of IL-27:IL-17 in bone marrow was associated with a superior progression-free survival. Leuk Res. 2013;37(9):1094–1099. doi: 10.1016/j.leukres.2013.06.022. PubMed PMID: 23849453. [DOI] [PubMed] [Google Scholar]
- 20.Shen CJ, Yuan ZH, Liu YX, Hu GY. Increased numbers of T helper 17 cells and the correlation with clinicopathological characteristics in multiple myeloma. J Int Med Res. 2012;40(2):556–564. doi: 10.1177/147323001204000217. PubMed PMID: 22613416. [DOI] [PubMed] [Google Scholar]
- 21.Lemancewicz D, Bolkun L, Jablonska E, Czeczuga-Semeniuk E, Kostur A, Kloczko J, et al. The role of Interleukin-17A and Interleukin-17E in multiple myeloma patients. Med Sci Monit. 2012 Jan;18(1):BR54–BR59. doi: 10.12659/MSM.882204. PubMed PMID: 22207110; PubMed Central PMCID: PMC3560674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scudla V, Petrova P, Minarik J, Pika T, Bacovsky J. Analysis of the serum levels of selected biological parameters in monoclonal gammopathy of undetermined significance and different stages of multiple myeloma. Neoplasma. 2011;58(6):499–506. PubMed PMID: 21895403. [PubMed] [Google Scholar]
- 23.Noonan K, Marchionni L, Anderson J, Pardoll D, Roodman GD, Borrello I. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116(18):3554–3563. doi: 10.1182/blood-2010-05-283895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucci M, Stucci S, Savonarola A, Ciavarella S, Cafforio P, Dammacco F, et al. Immature dendritic cells in multiple myeloma are prone to osteoclast-like differentiation through interleukin-17A stimulation. Br J Haematol. 2013;161(6):821–831. doi: 10.1111/bjh.12333. PubMed PMID: 23594390. [DOI] [PubMed] [Google Scholar]
- 25.Oteri G, Allegra A, Bellomo G, Alonci A, Nastro E, Penna G, et al. Reduced serum levels of IL-17 in patients with osteonecrosis of the jaw and in MM subjects after bisphosphonates administration. Cytokine. 2008;43(2):103–104. doi: 10.1016/j.cyto.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105(40):15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kottke T, Sanchez-Perez L, Diaz RM, Thompson J, Chong H, Harrington K, et al. Induction of hsp70-mediated Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer Res. 2007;67(24):11970–11979. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 28.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumor incidence and growth. Nature. 2006;442(7101):461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 29.Tai YT, Podar K, Catley L, Tseng YH, Akiyama M, Shringarpure R, et al. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidylinositol 3-kinase/ AKT signaling. Cancer Res. 2003;63(18):5850–5858. [PubMed] [Google Scholar]
- 30.Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S, et al. MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood. 2007;110(10):3744–3752. doi: 10.1182/blood-2007-05-093294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neri P, Tassone P, Shammas M, Yasui H, Schipani E, Batchu RB, et al. Biological pathways and in vivo anti-tumor activity induced by Atiprimod in myeloma. Leukemia. 2007;21:2519–2526. doi: 10.1038/sj.leu.2404912. [DOI] [PubMed] [Google Scholar]
- 32.Tassone P, Neri P, Carrasco DR, Burger R, Goldmacher VS, Fram R, et al. A clinically relevant SCID-hu in vivo model of human multiple myeloma. Blood. 2005;106(2):713–716. doi: 10.1182/blood-2005-01-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruegsegger P, Koller B, Muller R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int. 1996;58:24–29. doi: 10.1007/BF02509542. [DOI] [PubMed] [Google Scholar]
- 34.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of IL-17 family members. Immunity. 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Wang YH, Wills-Karp M. The potential role of interleukin-17 in severe asthma. Curr Allergy Asthma Rep. 2011;11(5):388–394. doi: 10.1007/s11882-011-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, et al. Interleukin 17A promotes hepato-cellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 2011;6(7):e21816. doi: 10.1371/journal.pone.0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161(1):409–414. [PubMed] [Google Scholar]
- 38.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce pro-inflammatory and hematopoieticcytokines. J Exp Med. 1996;183(6):P2593–P2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12(7):1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 40.Xu C, Yu L, Zhan P, Zhang Y. Elevated pleural effusion IL-17 is a diagnostic marker and outcome predictor in lung cancer patients. Eur J Med Res. 2014 May 8;19(1):23. doi: 10.1186/2047-783X-19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Y, Xu J, Su H, et al. Interleukin-17 is a favorable prognostic marker for colorectal cancer. Clin Transl Oncol. 2014 Jul 12; doi: 10.1007/s12094-014-1197-3. [DOI] [PubMed] [Google Scholar]
- 42.Han Y, Ye A, Bi L, Wu J, Yu K, Zhang S. Th17 cells and IL-17 increase with poor prognosis in patients with acute myeloid leukemia. Cancer Sci. 2014 May 29; doi: 10.1111/cas.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175(9):6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 44.Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V, Banchereau J, Fridman WH, Wijdenes J, Lebecque S, Sautès-Fridman C. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59(15):3698–3704. [PubMed] [Google Scholar]
- 45.Zhang Q, Liu S, Ge D, Zhang Q, Xue Y, Xiong Z, Abdel-Mageed AB, Myers L, Hill SM, Rowan BG, Sartor O, Melamed J, Chen Z, You Z. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 2012;72(10):2589–2599. doi: 10.1158/0008-5472.CAN-11-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Peng S, Duan J, Zhou J, Wang L, Wang J. A human B cell line AF10 expressing HIL-17. Biochem Mol Biol Int. 1998;45(6):1113–1119. doi: 10.1080/15216549800203332. [DOI] [PubMed] [Google Scholar]
- 47.Shen N, Wang J, Zhao M, Pei F, He B. Anti-interleukin-17 antibodies attenuate airway inflammation in tobacco-smoke-exposed mice. Inhal Toxicol. 2011;23(4):212–218. doi: 10.3109/08958378.2011.559603. [DOI] [PubMed] [Google Scholar]
- 48.van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 49.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50(2):650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 50.Gensicke H, Leppert D, Yaldizli O, Lindberg RL, Mehling M, Kappos L, et al. Monoclonal antibodies and recombinant immunoglobulins for the treatment of multiple sclerosis. CNS Drugs. 2012;26(1):11–37. doi: 10.2165/11596920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 51.Kurzeja M, Rudnicka L, Olszewska M. New interleukin-23 pathway inhibitors in dermatology: ustekinumab, briakinumab, and secukinumab. Am J Clin Dermatol. 2011;12(2):113–125. doi: 10.2165/11538950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Yago T, Nanke Y, Kawamoto M, Furuya T, Kobashigawa T, Kamatani N, et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res Ther. 2007;9(5):R96. doi: 10.1186/ar2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulatorof osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 55.Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. J Arthritis Rheum. 2010;62(4):929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 56.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, et al. Anti–Interleukin-17 Monoclonal Antibody Ixekizumab in Chronic Plaque Psoriasis. NEJM. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 57.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an Anti–Interleukin-17–Receptor Antibody for Psoriasis. NEJM. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]