Abstract
Objective
To estimate and identify factors associated with incidence of all-cause end-stage renal disease (ESRD) among newly diagnosed systemic lupus erythematosus (SLE) patients.
Methods
Data from a national registry of treated ESRD were linked to data from a lupus registry of SLE patients who were newly diagnosed and living in Atlanta, Georgia, in 2002-2004 (median follow-up, 7.8 years). Cumulative incidence and incidence rates (ESRD treatment initiations per 1000 patient-years) were calculated, and age- and race-adjusted Poisson models were used to calculate incidence rate ratios (IRRs).
Results
Among 344 newly diagnosed SLE patients, 29 initiated ESRD over 2603.8 years of follow-up. Incidence rates were 13.8 (95% CI, 9.4-20.3) and 3.3 (95% CI, 0.8-13.0) per 1000 patient-years among black and white patients, respectively; corresponding 5-year cumulative incidence was 6.4% and 2.5%. Lupus nephritis documented prior to 2005, which occurred in 80% of those who progressed to ESRD, was the strongest risk factor for incident ESRD (IRR=6.7, 95% CI, 2.7-16.8; incidence rate=27.6 per 1000 patient-years). Results suggested that patients who were black vs. white (IRR=3.9, 95% CI, 0.9-16.4) or <18 years (vs. ≥30 years) at diagnosis (IRR=2.1, 95% CI, 0.9-5.3) may be more likely to progress to ESRD, but incidence did not differ by sex or other characteristics.
Conclusion
Incidence of all-cause ESRD among patients with a recent diagnosis of SLE is high in Georgia. Interventions to decrease ESRD incidence among newly diagnosed SLE patients should target young and black patients as well as patients with lupus nephritis.
More than 5000 patients initiated treatment for end-stage renal disease (ESRD) attributed to systemic lupus erythematosus (SLE) in 2007-2011 (1). The population-based incidence of SLE-attributed ESRD has been estimated at 3-4 per million per year (2-5). Substantial disparities have been described in population incidence of SLE-attributed ESRD, by younger age (4-6), female sex (4), black race (2, 4-6), lower socioeconomic status (SES) (7, 8), reduced access to care (9), and residence in the South (4, 6). However, there is little reliable information on the incidence of ESRD among those with SLE. Estimates of lupus nephritis (LN) incidence among SLE patients range widely, from 35% to 60% (10-14). Similarly, estimates of ESRD among those with existing LN range from 10% to 35% (15, 16). The biases inherent in these estimates include survival bias (due to calculation of cumulative incidence rather than incidence rates) and misclassification (due to case definitions that often depend on administrative data to identify SLE and LN). In addition, combining estimates of LN incidence among those with SLE with estimates of ESRD incidence among those with LN—from different studies with varying follow-up times, population demographics, and sample sizes—could introduce even greater error and exclude ESRD cases not related to LN. Population-based ESRD incidence rate estimates among individuals with a validated diagnosis of SLE are important because ESRD remains the strongest risk factor for early mortality in the SLE population (17-20).
Estimates of the incidence rate of ESRD, as well as potential predictors of ESRD, among SLE patients are critical to guide treatment, screening, and management of this population. The Georgia Lupus Registry (GLR) (21, 22), which recently published estimates of population incidence of SLE in metropolitan Atlanta (23), offers a unique opportunity to estimate all-cause ESRD incidence among individuals with a new, validated diagnosis of SLE, free of much of the bias and variability of previous studies. The purpose of this study is to provide estimates of the incidence of ESRD using a population-based registry of newly diagnosed SLE patients in the southeastern United States and identify patient characteristics that might contribute to variation in ESRD incidence among SLE patients.
Patients and Methods
Data Sources and Study Population
Georgia Lupus Registry
The GLR is a population-based registry of validated SLE cases in a large (1.5 million) population with ~50% of individuals at high risk for SLE due to black race(14, 23-28). The primary aim of the GLR was to more accurately estimate the incidence and prevalence of SLE in 2002-2004 in Fulton and DeKalb Counties (Atlanta), Georgia (23). GLR methods are described in detail elsewhere (22, 23). Briefly, Emory investigators served as designated agents of the Georgia Department of Public Health, who used its public health surveillance exemption to the HIPAA Privacy Rule (45 CFR parts 160 and 164) to review medical records and capture protected health information without requiring patient consent [HIPAA 45 CFR 164.512(b)]. Potential SLE cases were identified from multiple sources, including hospitals; rheumatologists, dermatologists, and nephrologists; commercial and hospital-based laboratories; regional pathology laboratories; lupus research databases; and population databases, including the United States Renal Data System (USRDS), Veterans Affairs data, Medicaid claims data, and state mortality and hospital discharge data. The presence of diagnostic codes [International Classification of Diseases, 9th revision (ICD-9)] for SLE (710.0) and related conditions—including discoid lupus (695.4), other specified connective tissue disease (710.8), and other unspecified connective tissue disease (710.9)—in any of these sources flagged patients as potential SLE cases (22). All available medical records were fully abstracted for each potential case with residence in Fulton or DeKalb County in 2002-2004. Trained abstractors abstracted nearly 250 data elements, including clinical data needed to validate the diagnosis of SLE [e.g., American College of Rheumatology (ACR) (29) and Systemic Lupus International collaborating Clinics (SLICC) (30) classification criteria], earliest date of physician-stated diagnosis of SLE in the medical record, demographics, residential address, and date of death. Data were abstracted from the earliest available medical record through 12/31/2004, when ascertainment and validation of incident cases for GLR concluded. Vital status was ascertained by periodic linkages of the GLR to the state mortality database and batch searches in the Social Security Death Index (last available date, 4/30/11). The project was reviewed and approved by the Emory University and Georgia Department of Public Health Institutional Review Boards.
United States Renal Data System
The USRDS provides an ongoing, integrated database for outcomes research on the treated U.S. ESRD population, who are Medicare-eligible regardless of age or disability (1). We used the 2014 USRDS Standard Analytic Files, which contain data through 9/30/12 and originate primarily from the Centers for Medicare & Medicaid Services (CMS), to obtain date of initiation of ESRD treatment.
U.S. Census
Publicly available data on characteristics of U.S. residential neighborhoods, as defined by census tracts, were obtained from the 2000 U.S. Census (www.census.gov) via the Minnesota Population Center (www.nghis.org) (31). Aggregate census tract-level data on education and poverty were extracted.
Data linkage
Identifiers [Social Security number (SSN), date of birth, sex, first name, and surname] on incident SLE patients were sent to the USRDS to identify those who progressed to ESRD from diagnosis of SLE (2002-2004) to the last date of follow-up in the USRDS (9/30/12). Census data were spatially linked to the geocoded data from the GLR identifying the census tract associated with the first patient residential address recorded in Fulton or DeKalb County in 2002-2004. The GLR-USRDS data linkage was approved by the Emory Institutional Review Board.
Study Population
Newly diagnosed SLE cases (n=345) were defined as those with an initial SLE diagnosis date from 1/1/02 through 12/31/04. For this study, SLE cases were defined by a combined case definition (23) to estimate population prevalence and incidence of SLE, as follows: (i) presence of ≥4 ACR criteria for classification of SLE (29) in the medical record (n=267); (ii) presence of 3 ACR criteria plus a treating, board-certified rheumatologist’s diagnosis of SLE (n=69); or (iii) <4 ACR criteria plus SLE kidney disease, as defined by a biopsy consistent with class II-VI LN (n=9) (32, 33).
Study Variables and Definitions
Incident ESRD
Incident ESRD was defined by a first ESRD treatment, for any attributed cause, on or after the date of SLE diagnosis (Figure 1).
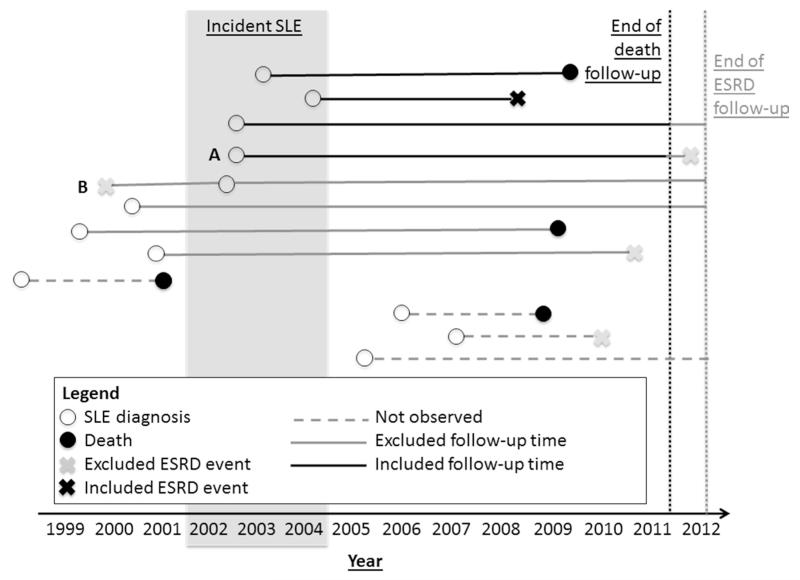
Figure 1.
Examples of included and excluded end-stage renal disease (ESRD) events and follow-up time in the estimation of ESRD incidence among newly diagnosed (2002-2004) SLE patients in the Georgia Lupus Registry. End of death follow-up, 4/30/11; end of ESRD follow-up, 9/30/12. Of a total of 345 patients with 31 events in our data, 1 patient had a censored ESRD event after 4/30/11 (A) and 1 patient was excluded due to ESRD prior to SLE diagnosis (B), leaving 344 patients with 29 events over 2603.8 patient-years of follow-up for the primary incidence estimates.
Patient characteristics
The GLR included information on age, sex, race, and LN, defined as documentation of (i) urine abnormalities (documented at least twice; ≥500 mg protein in 24-hour urine, random urine protein ≥300 mg/dl, spot protein:creatinine ratio of ≥0.5, or positive urine cellular casts), (ii) any renal biopsy consistent with LN classes II-VI (31), or (iii) documentation of LN by a treating rheumatologist or nephrologist. Because GLR data abstraction ended by 2005, LN could only be documented within a maximum of 3 years from SLE diagnosis; thus, LN is hereafter referred to as early LN. Analyses by race were limited to black and white patients, due to low numbers of other races (Asian, n=11, 1 event; missing, n=3, 0 events). We used the term “black” for consistency with the U.S. census classification (23). ESRD incidence by ethnicity was not reported due to low numbers (Hispanic, n=16, 1 event; missing ethnicity, n=10, 0 events). Individual SES was estimated by low-education and poor neighborhoods, defined as residential census tracts with greater than the median percentage of residents aged >25 without a high school degree or equivalent and households living below 100% of the federal poverty threshold, respectively.
Statistical Analysis
Patient characteristics were summarized for newly diagnosed SLE patients, overall and by ESRD status. All-cause ESRD incidence rates were calculated as the total number of patients initiating ESRD treatment over follow-up, divided by the sum of all patients’ contributed follow-up years. Follow-up time was defined as the time from date of SLE diagnosis to the time of death, ESRD, or last date of follow-up. For primary analyses, the last date of follow-up was 4/30/11, which is the last available date of death in GLR (Figure 1), and SLE was defined by the combined case definition. Secondary incidence estimates used (i) incident SLE patients defined by ≥4 ACR criteria only and (ii) point prevalent patients (n=1488) with an existing diagnosis of SLE (combined case definition) and free of ESRD as of 12/31/04 (cumulative incidence only). Confidence intervals for rates were obtained by quadratic approximation based on the Poisson log-likelihood. Incidence rates were stratified by patient characteristics and incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for the associations between patient characteristics were estimated with Poisson models. Stratified crude and adjusted incidence rates were calculated, and IRRs were adjusted for age group (<18 years, 18-30 years, and >30 years) and race (black and white). Clustering by census tract was minimal (n=86, 94, 48, 36, and 35 living in tracts with 1, 2, 3, 4, and 5 patients, respectively) and neighborhood characteristics were assigned as individual-level variables. Stata v. 13 (StataCorp, College Station, TX) was used for all analyses. Statistical significance was assessed at α=0.05.
Results
Characteristics of Newly Diagnosed SLE Patients
Among the 345 Atlanta-area SLE patients newly diagnosed in 2002-2004, 31 (9.0%) were identified in the USRDS as having received treatment for all-cause ESRD through 9/30/12. For primary analyses, one patient was excluded for having initiated ESRD treatment prior to SLE diagnosis. Another patient who initiated ESRD treatment after 4/30/11 (last available date of vital status ascertainment for GLR) was included but the patient’s event was censored in primary analyses, leaving 29 ESRD events over 2603.8 patient-years of follow-up among 344 newly diagnosed SLE patients without prior ESRD in 2002-2004 (Figure 1). Of these 344 patients, 299 (86.9%) had geocoded addresses that were linked successfully to 2000 Census data. In GLR-USRDS linkage, 100% of the 29 patients with included ESRD events matched on Social Security Number and sex and >90% matched on date of birth and name.
Newly diagnosed SLE patients had a mean age of 36 and were 87% female and 76% black (Table 1). They lived in neighborhoods where medians of 13% had not completed high school and 11% lived below 100% the federal poverty threshold. About one-third of these patients had early LN (Table 1), diagnosed by 2005 (79% of those who progressed to ESRD), although this differed by race (20% and 39% of whites and blacks had early LN; P=0.001). Among those with LN and biopsy information (n=112), 66 (59%) had at least one kidney biopsy (53% and 60.0% of whites and blacks, respectively; P=0.8); only 7% had multiple kidney biopsies (13% and 6% of whites and blacks, respectively, P=0.3).
Table 1.
Characteristics of newly diagnosed (2002-2004) SLE patientsa in the Georgia Lupus Registry, overall and by all-cause end-stage renal disease status through 4/30/11
| Overall (n=344) |
ESRD status as of 4/30/11b |
||||
|---|---|---|---|---|---|
| Characteristic | N | Yes (n=29) | No (n=315) | P c | |
| Age at diagnosis of SLE, mean (SD) |
344 | 36.4 (16.4) | 31.4 (17.7) | 36.9 (16.2) | 0.08 |
| Sex, % | 344 | >0.9 | |||
| Male | 45 (13.1%) | 3 (10.3%) | 42 (13.3%) | ||
| Female | 299 (86.9%) | 26 (89.7%) | 273 (86.7%) | ||
| Race,d % | 330 | 0.04 | |||
| White | 79 (23.9%) | 2 (7.1%) | 77 (25.5%) | ||
| Black | 251 (76.1%) | 26 (92.9%) | 225 (74.5%) | ||
| % HS dropouts in census tract, median (IQR) |
299 | 13.2 (6.6- 22.8) |
14.9 (9.5- 31.2) |
11.7 (6.6- 21.9) |
0.06 |
| % poor in census tract, median (IQR) |
299 | 11.1 (5.8- 22.6) |
13.8 (6.7- 25.8 |
10.7 (5.5- 20.5) |
0.29 |
| Early lupus nephritis, % | 344 | <0.001 | |||
| No | 225 (65.4%) | 6 (20.7%) | 219 (69.5%) | ||
| Yes | 119 (34.6%) | 23 (79.3%) | 96 (30.5%) | ||
| No. of ACR criteria, % | 344 | ||||
| ≤3 | 78 (22.7%) | 74 (23.5%) | 4 (13.8%) | 0.03 | |
| 4 | 121 (35.2%) | 115 (36.5%) | 6 (20.7%) | ||
| ≥5 | 145 (42.2%) | 126 (40.0%) | 19 (65.5%) | ||
ESRD, end-stage renal disease; SLE, systemic lupus erythematosus; IQR, inter-quartile range; HS, high school. Early lupus nephritis was defined by urine or biopsy evidence or treatment rheumatologist or nephrologist documentation of lupus nephritis in the medical record, by 2005. Poor was defined as living below 100% federal poverty threshold.
By combined case definition: ≥4 ACR criteria, 3 ACR criteria plus treating rheumatologist’s diagnosis, or renal involvement as indicated by biopsy consistent with class II-VI lupus nephritis or ESRD requiring dialysis or renal transplantation.
Last date of death follow-up in the Georgia Lupus Registry. A total of 30 patients initiated ESRD treatment between date of SLE diagnosis and 9/30/12, the last date of ESRD follow-up.
By Fisher’s exact test or Wilcoxon rank test across ESRD status.
Restricted to white and black patients only, due to small numbers of patients of other races (Asian, n=11, 1 event; missing race, n=2, 0 events).
Those who progressed to ESRD were younger at SLE diagnosis and more likely to be black (vs. white) and to have greater numbers of ACR criteria (Table 1). Among those progressing to ESRD, 79% had early LN (documented before 2005), compared to only 30% of those who did not progress to ESRD (P<0.001; Table 1). There were no statistically significant differences in patient characteristics between those with and without early LN among those who progressed to ESRD (n=29). Of the 26 black SLE patients progressing to ESRD, 20 (77%) had clinical evidence of early LN; while 16 (62%) had had at least one renal biopsy. In contrast, both white SLE patients who progressed to ESRD had early LN and at least one kidney biopsy in the medical record.
Incidence of ESRD among Newly Diagnosed SLE Patients
The overall crude incidence rate of all-cause ESRD in this Atlanta population with newly diagnosed SLE was 11.1 per 1000 patient-years (Table 2). Incidence rates were 13.8 and 3.3 per 1000 patient-years among black and white patients, respectively (Table 3). Overall incidence was slightly higher (12.5 per 1000 patient-years) among those with SLE defined by ≥4 ACR criteria alone (Table 2). The 5-year cumulative incidence of ESRD among newly diagnosed SLE patients in our study was 5.2% (Table 2; 6.4% vs. 2.5% among black vs. white patients). Over these 5 years, 15 (4.4%) died without an ESRD diagnosis. The median time to ESRD among those who progressed to ESRD by 4/30/11 was ~4 years (Table 2) among newly diagnosed SLE patients, and rates remained steady over follow-up in both blacks (Figure 2) and whites. Among 1488 point prevalent SLE patients (combined case definition) who were free of ESRD on 12/31/04, the 5-year cumulative incidence of ESRD was 5.2% (Table 2; 6.6% and 1.4% among black and white patients) and 16/1488 (1.1%) had incident ESRD within 1 year (1.4% and 0.3% among black and white patients).
Table 2.
Incidence of all-cause end-stage renal disease among SLE patients in the Georgia Lupus Registry, through 4/30/11a
| Cohort | No. of patients at risk |
No. of ESRD events |
Total patient- years at risk |
Median (IQR) years to ESRDb |
Incidence rate, per 1000 patient-years (95% CI) |
5-year cumulative incidence,c % |
|---|---|---|---|---|---|---|
| Incident SLE by combined case definitiond |
344 | 29 | 2603.8 | 4.1 (2.0-5.9) | 11.1 (7.7-16.0) | 5.2 |
| Incident SLE by≥4 ACR criteria only |
266 | 25 | 2007.8 | 4.1 (1.3-5.8) | 12.5 (8.4-18.4) | 6.0 |
| Point prevalent SLEe by combined case definitiond |
1488 | 95 | --- | 2.6 (1.6-4.6) | --- | 5.2 |
ACR, American College of Rheumatology; ESRD, end-stage renal disease; IQR, interquartile range; SLE, systemic lupus erythematosus.
Last date of death follow-up in the Georgia Lupus Registry. A total of 30 patients initiated ESRD treatment between date of SLE diagnosis and 9/30/12, the last date of ESRD follow-up.
Among those who progress to ESRD by 4/30/11.
Representing 18, 16, and 78 ESRD events within 5 years for the three cohorts listed.
Combined case definition, ≥4 ACR criteria, 3 ACR criteria plus treating rheumatologist’s diagnosis, or renal involvement as indicated by biopsy consistent with class II-VI lupus nephritis or ESRD requiring dialysis or renal transplantation.
Point prevalent cohort of patients in the GLR alive with an existing diagnosis of SLE (primary combined case definition) and free of ESRD on 12/31/04. Because patients who died with SLE prior to 12/31/04 were at risk for ESRD, patient-years and incidence rates were not calculated for this cohort.
Table 3.
All-cause end-stage renal disease incidence among newly diagnosed (2002-2004) SLEa patients, by patient characteristics, in the Georgia Lupus Registry, through 4/30/11b
| Characteristic | No. events/patient- years at risk |
Crude ESRD incidence, per 1000 patient-years (95% CI) |
Age- and race- adjustedc incidence rate ratio (95% CI) |
|---|---|---|---|
| Age at SLE diagnosis | |||
| <18 | 7/344.2 | 20.3 (9.7-42.7) | 2.14 (0.86-5.33) |
| 18-30 | 8/636.9 | 12.6 (6.3-25.1) | 1.19 (0.48-2.96) |
| >30 | 14/1622.7 | 8.6 (5.1-14.6) | 1.00 (ref) |
| Sex | |||
| Female | 26/2260.0 | 11.5 (7.8-16.9) | 1.00 (ref) |
| Male | 3/343.8 | 8.7 (2.8-27.0) | 0.77 (0.23-2.56) |
| Race | |||
| White | 2/615.1 | 3.3 (0.8-13.0) | 1.00 (ref.) |
| Black | 26/1877.2 | 13.8 (9.4-20.3) | 3.85 (0.91-16.35) |
| Low educationd | |||
| No | 10/1143.2 | 8.7 (4.7-16.3) | 1.00 (ref) |
| Yes | 18/1118.5 | 16.1 (10.1-25.5) | 1.24 (0.55-2.80) |
| Poord | |||
| No | 12/1136.7 | 10.6 (6.0-18.6) | 1.00 (ref) |
| Yes | 16/1124.9 | 14.2 (8.7-23.2) | 1.14 (0.52-2.50) |
| Early lupus nephritis | |||
| No | 6/1753.0 | 3.4 (1.5-7.5) | 1.00 (ref.) |
| Yes | 23/850.8 | 27.6 (18.2-41.5) | 6.72 (2.69-16.82) |
| No. of ACR criteria, % | |||
| 4 | 6/930.7 | 6.4 (2.9-14.3) | 1.00 (ref.) |
| ≤3 | 4/596.1 | 6.7 (2.5-17.9) | 1.01 (0.38-3.60) |
| ≥5 | 19/1077.0 | 17.5 (11.3-27.7) | 2.24 (0.88-5.71) |
CI, confidence interval; ESRD, end-stage renal disease; SLE systemic lupus erythematosus. Early lupus nephritis defined by urine or biopsy evidence or treatment rheumatologist or nephrologist documentation of LN in the medical record, within 3 years of SLE diagnosis. Early lupus nephritis was defined by urine or biopsy evidence or treatment rheumatologist or nephrologist documentation of lupus nephritis in the medical record, by 2005.
By combined case definition: ≥4 ACR criteria, 3 ACR criteria plus treating rheumatologist’s diagnosis, or renal involvement as indicated by biopsy consistent with class II-VI lupus nephritis or ESRD requiring dialysis or renal transplantation.
Last date of death follow-up in the Georgia Lupus Registry. A total of 30 patients initiated ESRD treatment between date of SLE diagnosis and 9/30/12, the last date of ESRD follow-up.
Adjustment for age group (<18, 18-30, >30 years) and race (black and white only).
Defined as living in a census tract with above (yes)/below (no) median values presented in Table 1: % high school dropouts, 13.2%; and % living below 100% federal poverty threshold, 11.1%.
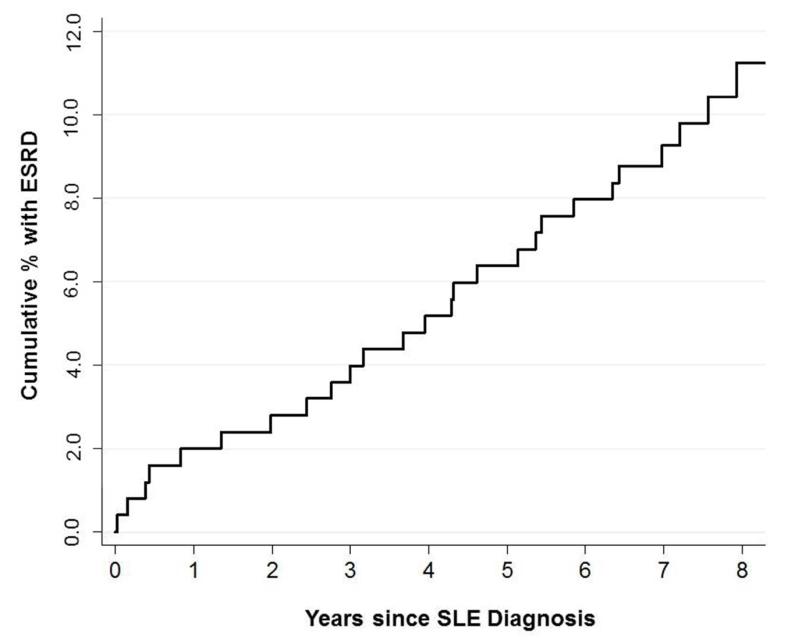
Figure 2.
Cumulative ESRD incidence among 251 black systemic lupus erythematosus patients diagnosed in Fulton and DeKalb Counties, Georgia, in 2002-2004. Note that cumulative incidence among whites is not shown due to small numbers of events (n=2).
Overall ESRD incidence estimates in secondary analyses were similar. With ESRD follow-up extended through 7/31/11, to include all patients who progressed to ESRD, regardless of missing death information after 4/30/11, the incidence rate among those with SLE by the combined case definition was 11.2 per 1000 patient years (cumulative incidence=5.2%). For those defined as having SLE by an intermediate definition (≥4 ACR criteria or 3 ACR criteria plus a treating rheumatologist diagnosis), the incidence rate was 11.1 per 1000 patient-years (cumulative incidence=5.4%).
ESRD Incidence by Newly Diagnosed SLE Patient Characteristics
Those with early LN (diagnosed by 2005) had nearly 7-fold greater rates of ESRD compared to those with no LN diagnosis by 2005, with adjustment for age and race (Table 3). Pediatric (age <18 years) patients at SLE diagnosis were >2-fold more likely than older adults (≥30 years) to progress to ESRD (Table 3). Male sex was not associated with ESRD incidence (Table 3). Blacks were nearly 4 times more likely than whites to progress to ESRD after adjustment for age (Table 3). Only the association with early LN was statistically significant, regardless of chosen referent group. Age- and race-adjusted incidence rates, omitted from Table 3 due to small sample sizes, did not differ substantially from the crude incidence rates presented in Table 3 (data not shown). SLE patients living in low-education and poor neighborhoods had modestly but non-statistically significantly higher rates of incident ESRD (Table 3).
Discussion
We have shown that, among urban Southeastern U.S. SLE patients, ESRD is a relatively common outcome. In a population-based registry of 344 newly diagnosed (2002-2004) Atlanta SLE patients, three of four of whom were black, the incidence rate of subsequent all-cause ESRD was 11.1 per 1000 patient-years. Incidence rates were 13.8 and 3.3 per 1000 patient-years among black and white patients, respectively. We estimated the overall 5-year cumulative incidence to be 5.2%, which is at least twice previous estimates from Taiwan, where 2.5% of newly diagnosed SLE patients developed ESRD over 6-8 years of follow-up (34), and Japan, where 3.1% of SLE patients diagnosed in 1971-1991 progressed to ESRD within 5 years (35). Both populations likely differ from the U.S. population and, particularly, this metropolitan Atlanta population, in terms of race—note that 5-year cumulative incidence was 6.4% among black SLE patients vs. only 2.5% among white SLE patients in our population—as well as environmental and healthcare system factors. Our study is, to our knowledge, the first to report an ESRD incidence rate among a population-based registry of newly diagnosed U.S. SLE patients who were not identified and validated by administrative data alone. Additionally, we were able to take mortality (4.4% of our incident SLE cohort over 5 years) into account by computing incidence rates.
Our results also point to associations of several patient characteristics with higher ESRD incidence among U.S. SLE patients. Early LN was associated with higher ESRD incidence, relative to not having this evidence of early renal disease (27.6 vs. 3.4 per 1000 patient-years), and incidence remained nearly 7-fold higher after adjustment for age and race. Among black SLE patients who did have early LN and progressed to ESRD, 20% did not have evidence of a renal biopsy, suggesting that LN may not always be properly diagnosed, staged, and treated according to ACR guidelines for LN treatment (11), which recommend that all patients with signs of nephritis be biopsied and that all patients with Class III or IV LN be treated aggressively. This potential gap in the care of U.S. LN patients aligns with evidence from the Medicaid population: even among patients with a documented diagnosis of incident LN, only 34% and 56% of these patients were treated with immunosuppressants and renal-protective antihypertensive medications, respectively, to slow the progression of LN (36).
Despite early LN being the strongest risk factor for progression to ESRD that we examined, one in five SLE patients who progressed to ESRD had no early signs of LN in the medical record. Thus, other individual characteristics may be useful to providers and researchers in assessing ESRD risk among SLE patients who have not been screened for renal complications. We found that black and pediatric patients had nearly 4- and 2-fold higher ESRD incidence than white and adult patients. Although not statistically significant, our findings are consistent with other studies showing these characteristics to be associated with development and progression of LN and ESRD in the population (3-6, 15, 37-39).
Further, we showed steady incidence of ESRD among black patients over follow-up, suggesting many have rapid progression. Faster progression among blacks would contribute to disparities by providing a shorter window to identify kidney complications and intervene with aggressive treatments to prevent or delay ESRD. Social and behavioral factors, including those that influence access to care, early diagnosis, and appropriate treatment (e.g., institutional racism, differential availability of subspecialty care, trust in the healthcare system, and adherence) could contribute to this racial disparity. In a recent UK study, black race was not a predictor of poor SLE outcomes among those with equal access to healthcare (40). Although delays in care were actually less likely to occur among black vs. white patients with incident LN in the U.S. Medicaid population (36), additional health system and individual barriers for effective LN treatment among blacks such as differential treatment adherence (41) could potentially explain worse outcomes in this subpopulation. Other contributors to this disparity may be differentially inadequate treatment of comorbid hypertension and diabetes, which are common in SLE (42, 43) and associated ESRD (4) and represent the strongest risk factors for ESRD in the general population (1). Genetic factors may play a role as well: for example, the APOL1 gene, which is more frequent in U.S. blacks than whites, was recently shown to be associated with risk of ESRD among SLE patients in a case-control study (44).
Among children, genetic and family history factors may play an even greater role in ESRD progression risk (45). In our study, crude incidence rates were ~20 per 1000 patient-years among children, more than twice the rate (<9 per 1000 patient-years) among adults aged ≥30 years. However, even among children, sociodemographic factors may play a role: previous studies have shown that nearly 40% of children with SLE have LN (37), that female and non-white children with SLE in the Medicaid population are more likely to have LN (37), that half of children with ESRD due to SLE are on Medicaid insurance (6), and that black children with ESRD due to SLE have increased mortality relative to their white counterparts in the United States (6). Decreasing the incidence of ESRD in this pediatric population is of paramount importance, given additional, age-specific consequences of ESRD, such as decreased growth and school performance (46, 47).
Unlike previous studies in Okinawa (34) and the U.S. multiethnic PROFILE cohort (38), we found that male SLE patients were not at higher risk of ESRD. Our results may reflect differences across populations or simply chance findings due to low numbers of male SLE patients. Confirmation in other U.S. SLE cohorts is needed before male sex can be ruled out as a potential predictor of incident ESRD. Generally, we also found that neighborhood SES factors were not associated with ESRD incidence, although this does not preclude potential individual SES effects.
This study has several limitations. First, the sensitivity of the GLR SLE case-finding approach is unknown. However, the validity of the GLR methods to ascertain SLE cases was supported by results from the capture-recapture analyses, which showed that only 31 incident cases with a validated diagnosis of SLE could have been missed (23). Second, race-stratified estimates may be more appropriate than the overall estimates in generalizing to other U.S. SLE populations with different race/ethnicity distributions than Atlanta, where three-quarters of SLE patients were black and data on Hispanic and Asian SLE patients were lacking. Third, we had limited power to detect modest associations and could not account for potential neighborhood-level effects with multi-level modeling, due to small numbers of events and relatively short follow-up. Fourth, we lacked individual socioeconomic data. Fifth, the effects of potentially protective treatment, such as anti-malarials (48), could not be examined. Sixth, because prospective data capture was not within the scope of the GLR, detailed clinical data were not abstracted after 2004. Particularly for LN, we could not tease apart the potential effects of the presence vs. timing of LN on ESRD incidence. Finally, we were not able to capture any non-Medicare-eligible patients (e.g., undocumented residents) or any patients who moved out of the United States.
This study also has many strengths. Taking advantage of an innovative public health strategy that allowed us to link the GLR with the USRDS, we were able to estimate the first population-based estimates of ESRD incidence among individuals with a recent diagnosis of SLE in the U.S. Southeast. The GLR is one of five CDC National Lupus Registries, the first comprehensive population-based epidemiological study of lupus conducted in the United States (22, 23, 27). Case ascertainment was maximized by the use of multiple sources, and SLE diagnoses were validated by comprehensive clinical data collected from individual records, likely reflecting the full spectrum of SLE. We used a large, representative U.S. Southeastern metropolitan population, and all U.S. patients who were treated for ESRD in were captured. In addition to the cumulative incidence, we calculated the incidence rate of ESRD, providing unbiased estimates that account for mortality and residential mobility within the United States. Thus, our findings overcome many methodological limitations that could lead to survival, migration, and selection biases in the estimates of ESRD incidence in SLE.
In conclusion, this study provides dependable all-cause ESRD incidence estimates in a high-risk population of newly diagnosed SLE patients from the southeastern United States. Additionally, we described SLE populations (patients with early LN and, potentially, pediatric and black patients) who may benefit from earlier identification as higher-risk for ESRD, more aggressive treatment to prevent or delay ESRD, and targeted preventive and quality improvement research efforts. These estimates of the burden of ESRD among U.S. SLE patients inform future research aimed at increasing health care access among those with SLE, facilitating early diagnosis of kidney complications including LN, improving quality of SLE care related to kidney disease, and developing more effective treatments for kidney disease in SLE.
Significance and Innovations.
Using an innovative public health strategy that allowed us to link patient data from a SLE population-based registry with national end-stage renal disease data, we estimated, for the first time to our knowledge, the incidence of subsequent all-cause end-stage renal disease among a high-risk population of newly diagnosed SLE patients
Among 344 metropolitan Atlanta patients diagnosed with SLE in 2002-2004, we found that the incidence of end-stage renal disease was high, with 5-year cumulative incidence of 6.4% vs. 2.5% and incidence rates of 13.8 vs. 3.3 per 1000 patient-years among black vs. white patients
Overcoming many of the biases inherent in previous estimates, via comprehensive SLE case ascertainment, complete capture of all U.S. patients who were treated for end-stage renal disease, and incorporation of available mortality information, these incidence estimates are critical to guide treatment, screening, and management of SLE
The risk for end-stage renal disease is higher in SLE patients with newly diagnosed patients with lupus nephritis and, potentially, those who are black or pediatric; these populations may benefit from earlier diagnosis of kidney complications and more aggressive treatment to prevent or delay end-stage renal disease
Acknowledgements
This research was supported by the Lupus Foundation of America. The Georgia Lupus Registry was supported in part by the CDC, and by cooperative agreement CDC-RFA-DP08-806 and earlier by cooperative agreement PA03022 from the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Financial Support: L.C.P. was supported by the Laney Graduate School, Emory University, and the LFA. S.S.L. and C.D. are supported in part by the NIH (R01AR065493) and the CDC (U01DP005119). R.E.P. was supported in part by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH; ULl TR000454 and KL2TR000455). R.E.P. and S.O.P are both supported in part by 1R24MD008077-01 through the National Institute on Minority Health and Health Disparities.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as an ‘Accepted Article’, doi: 10.1002/acr.22685
References
- 1.United States Renal Data System . 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 2013. [Google Scholar]
- 2.Ward MM. Changes in the incidence of end-stage renal disease due to lupus nephritis, 1982-1995. Arch Intern Med. 2000;160:3136–40. doi: 10.1001/archinte.160.20.3136. [DOI] [PubMed] [Google Scholar]
- 3.Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996-2004. J Rheumatol. 2009;36:63–7. doi: 10.3899/jrheum.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011;63:1681–8. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sexton DJ, Reule S, Solid C, Chen S, Collins AJ, Foley RN. ESRD from lupus nephritis in the United States, 1995-2010. Clin J Am Soc Nephrol. 2015;10:251–9. doi: 10.2215/CJN.02350314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiraki LT, Lu B, Alexander SR, Shaykevich T, Alarcon GS, Solomon DH, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995-2006. Arthritis Rheum. 2011;63:1988–97. doi: 10.1002/art.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward MM. Medical insurance, socioeconomic status, and age of onset of endstage renal disease in patients with lupus nephritis. J Rheumatol. 2007;34:2024–7. [PubMed] [Google Scholar]
- 8.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis. 2008;51:563–72. doi: 10.1053/j.ajkd.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J Rheumatol. 2010;37:1158–63. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imboden JB, Hellmann DB, Stone JH, editors. Current Diagnosis and Treatment: Rheumatology. 3rd ed McGraw-Hill; New York, NY: 2013. [Google Scholar]
- 11.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine. 2006;85(3):147–56. doi: 10.1097/01.md.0000224709.70133.f7. [DOI] [PubMed] [Google Scholar]
- 13.Ward MM, Pyun E, Studenski S. Mortality risks associated with specific clinical manifestations of systemic lupus erythematosus. Arch Intern Med. 1996;156:1337–44. [PubMed] [Google Scholar]
- 14.Alarcon GS, McGwin G, Jr., Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus. 2002;11:95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 15.Contreras G, Pardo V, Cely C, Borja E, Hurtado A, De La Cuesta C, et al. Factors associated with poor outcomes in patients with lupus nephritis. Lupus. 2005;14:890–5. doi: 10.1191/0961203305lu2238oa. [DOI] [PubMed] [Google Scholar]
- 16.Appel GB, Cohen DJ, Pirani CL, Meltzer JI, Estes D. Long-term follow-up of patients with lupus nephritis. A study based on the classification of the World Health Organization. Am J Med. 1987;83:877–85. doi: 10.1016/0002-9343(87)90645-0. [DOI] [PubMed] [Google Scholar]
- 17.Maroz N, Segal MS. Lupus nephritis and end-stage kidney disease. Am J Med Sci. 2013;346:319–23. doi: 10.1097/MAJ.0b013e31827f4ee3. [DOI] [PubMed] [Google Scholar]
- 18.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 19.Sule S, Fivush B, Neu A, Furth S. Increased risk of death in pediatric and adult patients with ESRD secondary to lupus. Pediatr Nephrol. 2011;26:93–8. doi: 10.1007/s00467-010-1640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sule S, Fivush B, Neu A, Furth S. Increased hospitalizations and death in patients with ESRD secondary to lupus. Lupus. 2012;21:1208–13. doi: 10.1177/0961203312451506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim SS, Drenkard C. Epidemiology of systemic lupus erythematosus: capturing the butterfly. Curr Rheumatol Rep. 2008;10:265–72. doi: 10.1007/s11926-008-0043-4. [DOI] [PubMed] [Google Scholar]
- 22.Lim SS, Drenkard C, McCune WJ, Helmick CG, Gordon C, Deguire P, et al. Population-based lupus registries: advancing our epidemiologic understanding. Arthritis Rheum. 2009;61:1462–6. doi: 10.1002/art.24835. [DOI] [PubMed] [Google Scholar]
- 23.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002-2004: The Georgia Lupus Registry. Arthritis Rheumatol. 2014;66:357–68. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastian HM, Roseman JM, McGwin G, Jr., Alarcon GS, Friedman AW, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11:152–60. doi: 10.1191/0961203302lu158oa. [DOI] [PubMed] [Google Scholar]
- 25.Contreras G, Lenz O, Pardo V, Borja E, Cely C, Iqbal K, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 2006;69:1846–51. doi: 10.1038/sj.ki.5000243. [DOI] [PubMed] [Google Scholar]
- 26.Austin HA, 3rd, Boumpas DT, Vaughan EM, Balow JE. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int. 1994;45(2):544–50. doi: 10.1038/ki.1994.70. [DOI] [PubMed] [Google Scholar]
- 27.Somers EC, Marder W, Cagnoli P, Lewis EE, Deguire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: The Michigan Lupus Epidemiology & Surveillance Program. Arthritis Rheum. 2014;66:369–78. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis Rheum. 2013;65:753–63. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 30.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minnesota Population Center . National Historical Geographic Information System: Version 2.0. University of Minnesota; Minneapolis, MN: 2011. Available at: www.nhgis.org. [Google Scholar]
- 32.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–50. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 33.Costenbader KH, Karlson EW, Mandl LA. Defining lupus cases for clinical studies: the Boston weighted criteria for the classification of systemic lupus erythematosus. J Rheumatol. 2002;29:2545–50. [PubMed] [Google Scholar]
- 34.Lin WH, Guo CY, Wang WM, Yang DC, Kuo TH, Liu MF, et al. Incidence of progression from newly diagnosed systemic lupus erythematosus to end stage renal disease and all-cause mortality: a nationwide cohort study in Taiwan. Int J Rheum Dis. 2013;16:747–53. doi: 10.1111/1756-185X.12208. [DOI] [PubMed] [Google Scholar]
- 35.Iseki K, Miyasato F, Oura T, Uehara H, Nishime K, Fukiyama K. An epidemiologic analysis of end-stage lupus nephritis. Am J Kidney Dis. 1994;23:547–54. doi: 10.1016/s0272-6386(12)80377-5. [DOI] [PubMed] [Google Scholar]
- 36.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis Care Res. 2014;66:617–24. doi: 10.1002/acr.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiraki LT, Feldman CH, Liu J, Alarcon GS, Fischer MA, Winkelmayer WC, et al. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum. 2012;64:2669–76. doi: 10.1002/art.34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alarcon GS, McGwin G, Jr., Petri M, Ramsey-Goldman R, Fessler BJ, Vila LM, et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PLoS Med. 2006;3:e396. doi: 10.1371/journal.pmed.0030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adler M, Chambers S, Edwards C, Neild G, Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology. 2006;45(9):1144–7. doi: 10.1093/rheumatology/kel039. [DOI] [PubMed] [Google Scholar]
- 40.Yee CS, Su L, Toescu V, Hickman R, Situnayake D, Bowman S, et al. Birmingham SLE cohort: outcomes of a large inception cohort followed for up to 21 years. Rheumatology. 2015 doi: 10.1093/rheumatology/keu412. in press. [DOI] [PubMed] [Google Scholar]
- 41.Yazdany J, Liu J, Alarcon G, Costenbader KH, Feldman CH. Poor adherence to medications for systemic lupus erythematosus among U.S. Medicaid beneficiaries. Arthritis Rheum. 2013;65:S673. [Google Scholar]
- 42.Molina MJ, Mayor AM, Franco AE, Morell CA, Lopez MA, Vila LM. Prevalence of systemic lupus erythematosus and associated comorbidities in Puerto Rico. J Clin Rheumatol. 2007;13:202–4. doi: 10.1097/RHU.0b013e318124a8af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker B, Urowitz MB, Gladman DD, Lunt M, Bae SC, Sanchez-Guerrero J, et al. Clinical associations of the metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Ann Rheum Dis. 2013;72:1308–14. doi: 10.1136/annrheumdis-2012-202106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 2014;66:390–6. doi: 10.1002/art.38220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apenteng T, Kaplan B, Meyers K. Renal outcomes in children with lupus and a family history of autoimmune disease. Lupus. 2006;15:65–70. doi: 10.1191/0961203306lu2261oa. [DOI] [PubMed] [Google Scholar]
- 46.Janjua HS, Mahan JD. Growth in chronic kidney disease. Adv Chronic Kidney Dis. 2011;18:324–31. doi: 10.1053/j.ackd.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Sethi SK, Bunchman T, Raina R, Kher V. Unique considerations in renal replacement therapy in children: core curriculum 2014. Am J Kidney Dis. 2014;63:329. doi: 10.1053/j.ajkd.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Pons-Estel GJ, Alarcon GS, McGwin G, Jr., Danila MI, Zhang J, Bastian HM, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. 2009;61:830–9. doi: 10.1002/art.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]