Abstract
The objective of this study was to investigate the relationship between cardiorespiratory (CR) fitness and the brain’s white matter tract integrity using diffusion tensor imaging (DTI) in the Alzheimer’s disease (AD) population. We recruited older adults in the early stages of AD (n=37; CDR=0.5 and 1) and collected cross-sectional fitness and diffusion imaging data. We examined the association between CR fitness (peak oxygen consumption [VO2peak]) and fractional anisotropy (FA) in AD-related white matter tracts using two processing methodologies: a tract-of-interest approach and tract-based spatial statistic (TBSS). Subsequent diffusivity metrics (radial diffusivity [RD], mean diffusivity [MD], and axial diffusivity [A×D]) were also correlated with VO2peak. The tract-of-interest approach showed that higher VO2peak was associated with preserved white matter integrity as measured by increased FA in the right inferior fronto-occipital fasciculus (p=0.035, r=0.36). We did not find a significant correlation using TBSS, though there was a trend for a positive association between white matter integrity and higher VO2peak measures (p<0.01 uncorrected). Our findings indicate that higher CR fitness levels in early AD participants may be related to preserved white matter integrity. However to draw stronger conclusions, further study on the relationship between fitness and white matter deterioration in AD is necessary.
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease and the most common cause of dementia. AD affects one in nine people over the age of 65 and one in three over the age of 85 (Thies et al., 2013). Post-mortem (Brun and Englund, 1986, Braak and Braak, 1991) and neuroimaging reports detail a specific pattern of brain degeneration in AD throughout disease progression (Thompson et al., 2003, Sexton et al., 2011, Burggren and Brown, 2014). Current contemporary pharmacological treatments have proven largely ineffective in attenuating AD-related brain degeneration. Thus, increasing research is focusing on lifestyle changes that could potentially mitigate the progressive degeneration associated with AD. For instance, aerobic exercise that improves one’s overall cardiorespiratory (CR) fitness level, may support brain health and cognition (Colcombe et al., 2006, Boots et al., 2014, Erickson et al., 2014).
In healthy older adults, higher levels of CR fitness are associated with a variety of benefits in the brain, namely preserved overall brain volume, hippocampal volume, white matter microstructure, and cognition (Colcombe et al., 2003, Marks et al., 2007, Erickson et al., 2009, Johansen-Berg and Rushworth, 2009, Marks et al., 2011, Johnson et al., 2012, Petersen and Posner, 2012). In the AD population, higher levels of CR fitness have been associated with preserved brain volume (Burns et al., 2008a, Petersen and Posner, 2012). More specifically, higher CR fitness levels have been associated with increased gray and white matter volumes in the parietal and medial temporal cortices (Honea et al., 2009, Tian et al., 2014). However to our knowledge there has been little research associating CR fitness levels with diffusion tensor imaging (DTI) metrics of white matter integrity in those with AD.
DTI is a structural magnetic resonance imaging technique that characterizes the diffusivity of water molecules. In white matter, the movement of these molecules is anisotropic mainly along axonal bundles and supportive glial cells (Walhovd et al., 2014). Disruption in these white matter tracts occurs during aging as well as neurodegenerative diseases (e.g. AD) (Salat, 2011). Previous DTI investigations of CR fitness in healthy non-demented cohorts showed that increased CR fitness is associated with preserved white matter integrity in frontal and temporal regions (Voss et al., 2013b, Tian et al., 2014). Moreover, higher levels of aerobic fitness have been related to increased white matter integrity in the cingulum (Marks et al., 2007, Marks et al., 2011), uncinate fasciculus (Marks et al., 2007), and corpus callosum (Johnson et al., 2012). Hence in a cohort of people with early AD and based on previous publications, we hypothesized that higher levels of CR fitness would be associated with increased integrity (i.e. higher anisotropy) in white matter tracts that are more susceptible to AD pathological changes. To characterize this association, we used FA as our primary measure of interest in two DTI methods, an a-priori tract-of-interest approach and a tract-based spatial statistics approach (TBSS) (Smith et al., 2006). The tract-of-interest method allows us to quantify the integrity of specific AD-related white matter tracts while TBSS allows for a whole brain voxel-wise analysis.
METHODS
Sample
This study took place at the University of Kansas Alzheimer’s Disease Center (KU ADC) as part of the Alzheimer’s Disease Exercise Program Trial (ADEPT). For this investigation, we collected baseline data from individuals (n=40) enrolled in an ongoing aerobic exercise trial (Vidoni et al., 2012b). The final sample included 37 sedentary older adults in the earliest stages of AD (CDR 0.5; n= 23, CDR 1; n= 14) after removing individuals due to substantial imaging distortion (n=1) or bad quality acquisition (n=2). Institutionally approved informed consent was obtained before enrollment. Additionally, this trial excluded individuals with significant neurological diseases other than AD, major psychiatric disorders, major depression (Geriatric Depression Scale > 5), clinically-evident stroke or systemic infection, myocardial infarction or significant cardiovascular or respiratory disease, history of cancer in the last 5 years, current or past history of drug or alcohol abuse, insulin-dependent diabetes, or significant pain or musculoskeletal disorder that would limit exercise.
Clinical assessment
A clinician performed physical and neurological examinations using a semi-structured interview of the participant and a collateral source (e.g. participant’s spouse or adult child). Medications, past medical history, family history, education, and demographic information were collected from the collateral source. Diagnostic classification was made at a consensus conference attended by neurologists, neuropsychologists, and nurse practitioners of the University of Kansas Alzheimer’s Disease Center. Diagnostic criteria for AD included the gradual onset and progression of an impairment in memory and at least one other cognitive or functional domain (NINCDS-ADRDA criteria) (McKhann et al., 1984). The Clinical Dementia Rating (CDR) determined the severity of dementia (Morris, 1993). Only participants with a Global CDR of 0.5 (very mild) or 1.0 (mild dementia) and a diagnosis of probable AD or who were classified as having mild cognitive impairment due to probable AD were included in the study. A battery of neuropsychological tests, the uniform data set (UDS), was also administered to each participant by trained psychometricians. Then, these scores were normalized using a method previously described (Shirk et al., 2011) and divided into 5 cognitive domains (memory, attention, speed, executive function, and language) for further analysis.
CR fitness assessment
CR fitness was measured as peak oxygen consumption (VO2peak [ml/kg/min]) during a cardiopulmonary exercise test using a Cornell modified Bruce protocol (Hollenberg et al., 1998, Burns et al., 2008a). Each participant was asked to start walking on a treadmill while the speed and incline increased progressively. Only individuals who achieved a respiratory exchange ratio (RER) ≥ 1.0 were included in the study. Oxygen consumption was averaged over 15-second intervals, and the highest measurement was considered VO2 peak (Anderson et al., 2011, Vidoni et al., 2012b).
Neuroimaging
MRI was collected at baseline within three weeks of the CR fitness assessment. The session included a high-resolution T1 anatomic image (MPRAGE; 1×1×1mm voxels; TR = 2500, TE = 4.38, TI = 1100, FOV 256 × 256 with 18% oversample, 1mm slice thickness, flip angle 8 degrees). In addition, a diffusion-weighted sequence was designed to provide optimal results for this analysis while minimizing scanner duration for the participant. The diffusion weighted acquisition used a Siemens 3.0 Tesla Skyra MRI with a repetition time (TR)= 1000ms and echo time (TE)=90ms. Diffusion gradients were applied in 65 directions (b0= 0 s/mm2 and b1–64= 1000 s/mm2). Seventy-five 2-mm sections were acquired in an in-plane resolution of 128×128 with a 300mm field of view (FOV).
Imaging Analysis
We processed the diffusion-weighted images using the FMRIB Software Library (FSL 5.0.4) (Smith et al., 2004). The remaining 37 images were eddy-current corrected for small distortions and simple head motion by alignment of the diffusion weighted images to the b0 image. Next, a brain extraction tool (BET2) was applied to strip the brain from the skull. Diffusivity measures were calculated using DTIFIT and FSLMATHS, part of the FSL toolbox. Fractional anisotropy (FA) provides a measure of degree of anisotropic diffusion ranging from 0 (perfectly isotropic) to 1 (perfectly anisotropic) and is related to an overall measurement of white matter microstructural integrity (Alexander et al., 2011). Radial diffusivity (RD) is a measure of perpendicular diffusivity and reflects changes in axonal diameter and myelination (Song et al., 2005). Axial diffusivity (A×D) measures the magnitude of diffusion in the principal diffusion direction (Alexander et al., 2007). Mean diffusivity (MD) measures overall diffusion by averaging the three orthogonal components.
For the a-priori tract-of-interest method, each diffusivity measure was non-linearly registered, aligned, and transformed into a common 1×1×1mm standard MNI space template (FMRIB58), following the initial steps of the TBSS processing pipeline, while omitting the skeletonizing step (Smith et al., 2006). Instead, we smoothed the image with a 2mm kernel using FSLMATHS. To protect against Type I error we identified tracts previously reported to be associated with AD (Figure 1) and divided them by hemisphere: the cingulum (CCG) (Xie et al., 2005, Zhang et al., 2007, Burzynska et al., 2010, Liu et al., 2011, Zhang et al., 2014), the inferior fronto-occipital fasciculus (IFOF) (Gold et al., 2010, Teipel et al., 2010, Alves et al., 2012), the superior longitudinal fasciculus (SLF) (Liu et al., 2011, Sexton et al., 2011, Alves et al., 2012, Bosch et al., 2012), and the uncinate fasciculus (UF) (Liu et al., 2011, Sexton et al., 2011, Bosch et al., 2012). We created our white matter binary tract masks from the Johns Hopkins University (JHU) probabilistic atlas registered to the common MNI space (Mori et al., 2005, Hua et al., 2008). For every subject’s FA, our primary diffusivity metric, we then “masked in” every tract, included only voxels thresholded at FA values higher than 0.2, and calculated an averaged FA value on every tract. Similarly averaged values for our subsequent diffusivity metrics (e.g. RD, MD, or A×D) were calculated. Hence, every participant had an overall diffusivity value for every tract, which was fed into a statistical program for further statistical analysis. To determine the relationship between cognition and white matter integrity, we also performed partial correlations (correcting for age and gender) of the primary diffusivity measure (FA) in the a-priori white matter tracts from our tract-of-interest analysis with mean z-scores of the 5 cognitive domains (attention, language, verbal memory, processing speed, and executive function).
Figure 1.
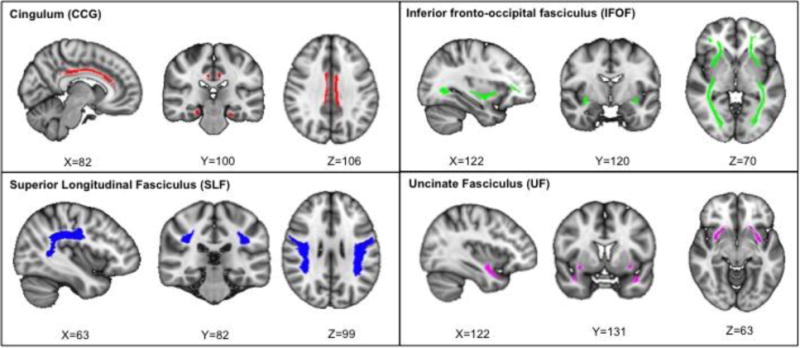
AD-related white matter tracts: the cingulum (red, top left), the inferior fronto-occipital fasciculus (green, top right), the superior longitudinal fasciculus (blue, bottom blue), and the uncinate fasciculus (pink, bottom right). Tract representation is shown using the standard MNI brain in radiological orientation.
In addition to the tract-of-interest method, we also performed TBSS analyses (Smith et al., 2006). First, we created FA images by fitting the tensor model to the raw diffusion data using FDT, part of FSL. After brain extraction, all subject’s FA data were aligned into a common MNI space using nonlinear transformations (Andersson et al., 2007). Next, the FA data were thinned out to create a mean FA skeleton representing the center of all the tracts common to the group. Then, each subject’s diffusivity metrics were projected into this skeleton and thresholded to include only voxels with FA values higher than 0.2. The resulting data was statistically analyzed using RANDOMISE, a tool for non-parametric permutation inference on neuroimaging data (Winkler et al., 2014). Similar to the tract-of-interest approach, FA was the primary diffusivity metric with subsequent measures of RD, MD, and A×D.
Statistical analysis
For the tract-of-interest approach, statistical analyses were conducted using SPSS 22.0 (IBM Corp., Armonk, NY). As our primary diffusivity measure, we tested partial correlations of FA values with VO2peak in every tract-of-interest, controlling for age and gender. A total of 8 correlation comparisons were performed, splitting our tracts by hemisphere (left and right). Because these planned comparisons were carefully selected based on prior reports and FA was designated our primary outcome measure, set our alpha to 0.05 uncorrected and treated each non-overlapping tract-of-interest as an independent analysis, as previously suggested (Keppel and Wickens, 2004).
For whole brain TBSS analyses, we performed non-parametric analyses using permutation based statistical inference. We assessed linear correlations between VO2peak and FA across the white matter skeleton controlling for age and gender, and set the number of permutations to 5000 using threshold-free-cluster-enhancement. We set our alpha to be 0.05 corrected. Subsequent analyses were performed in the other diffusivity metrics (RD, MD, and A×D).
RESULTS
Demographics
Table 1 summarizes the demographics, physical, and CR fitness characteristics of the 37 participants included in the final analysis.
Table 1.
Participant demographics and fitness characteristics
| Demographics | Mean (SD) |
|---|---|
|
| |
| Age (n=37) | 72.35 (7.9) |
| Female (#,%) | 14 (37.8) |
| MMSE | 25.6 (3.3) |
| CDR Global = 0.5 (#,%) | 25 (67.6) |
| CDR Sum of Boxes | 3.4 (1.5) |
| Education (years) | 15.4 (3.6) |
|
| |
| Fitness and Body measures | |
|
| |
| BMI | 26.9 (4.1) |
| Lean Mass (kg) | 47.6 (10.2) |
| VO2peak (ml/kg/min) | 21.6 (5.1) |
SD, Standard deviation; BMI, body mass index; CDR, Clinical Dementia Rating; MMSE, Mini Mental Status Exam
CR fitness and WM integrity
In our tract-of-interest analysis, we found a significant correlation between increased FA and higher VO2peak lateralized to the right inferior fronto-occipital fasciculus (r=0.358, p=0.035), after controlling for age and gender (Table 2, Figure 2). We did not find significant correlations with VO2peak and MD, RD, or A×D. However there was a trend for decreased RD with higher VO2peak in the right cingulum (r=−0.315, p=0.065) and the right inferior fronto-occipital fasciculus (r=−0.313, p=0.067) (Supplementary Table 1).
Table 2.
Tract of Interest FA Results
| White matter tract: | Fractional Anisotropy |
|---|---|
|
| |
| Cingulum | |
| Left | 0.48 (0.04) |
| Right | 0.42 (0.04) |
|
| |
| Inferior Fronto-Occipital Fasciculus | |
| Left | 0.41 (0.03) |
| Right | 0.41 (0.03)* |
|
| |
| Superior Longitudinal Fasciculus | |
| Left | 0.41 (0.03) |
| Right | 0.41 (0.03) |
|
| |
| Uncinate Fasciculus | |
| Left | 0.39 (0.03) |
| Right | 0.40 (0.03) |
Mean fractional anisotropy (standard deviation) on every tract-of-interest.
denotes significance at p<0.05 in the partial correlation of FA with VO2peak analysis.
Figure 2.
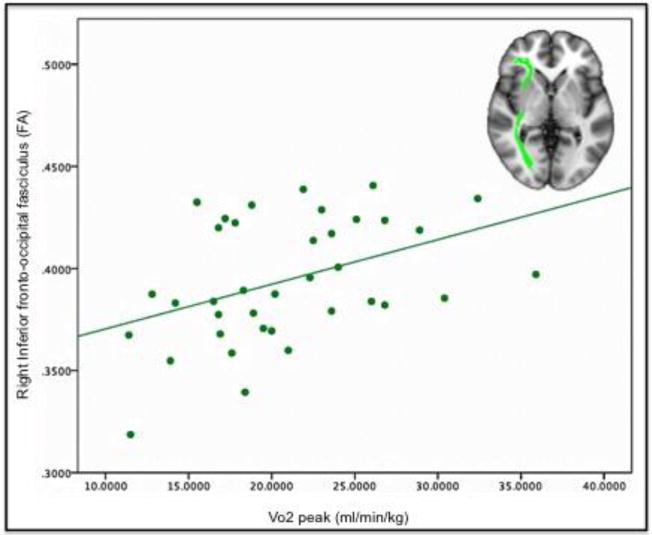
Linear fit plot for VO2peak and fractional anisotropy (FA) in the right inferior fronto-occipital fasciculus (green). The tract-of-interest is overlaid on a T1 MNI template, and orientation is radiological (left is right).
In our TBSS analysis, we did not find any significant correlations of the diffusivity metrics (FA, RD, MD, or A×D) with VO2peak at a statistical threshold of p<0.05 FWE corrected after correcting for age and gender.
However, as an exploratory measure at an uncorrected threshold of p<0.01, there was a positive association of FA with VO2peak in several tracts in the right hemisphere (primarily the inferior fronto-occipital fasciculus and cingulum), with sparse regions on the left side (Figure 3).
Figure 3.
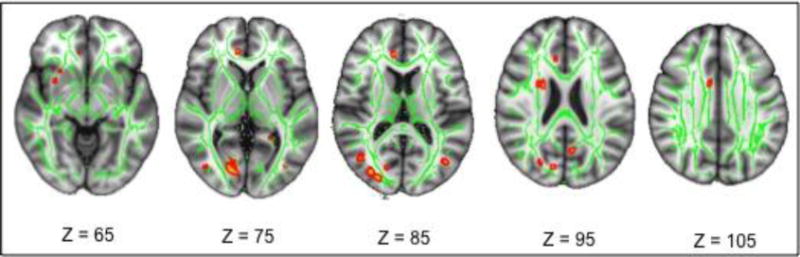
TBSS results from a partial correlation analysis associating FA with VO2peak. Red regions depict positive associations between FA and VO2peak p<0.01 uncorrected overlapped in the green skeleton and the MNI template, and orientation is radiological (left is right).
Cognitive Measures
None of our partial correlations associating FA with mean scores from 5 cognitive domains yielded significant results. However there was a trend for a positive relationship of increased processing speed with higher FA in the IFOF on the left hemisphere (p=.092, r=.293).
DISCUSSION
We found that higher levels of CR fitness were associated with increased white matter integrity in the right inferior fronto-occipital fasciculus (IFOF), a tract that travels from the occipital region to the temporal lobe, Our findings support and extend previous associations of white matter volume and fitness to more specifically address white matter tracts in older adults in the early stages of AD.
Cardiorespiratory fitness impacts brain health in a variety of ways. In animal models, previous studies showed that aerobic exercise attenuated age-related decreases in hippocampal neurogenesis, potentially delayed the onset of AD, reduced amyloid beta deposition and pro-inflammatory cytokines, and enhanced levels of brain-derived neurotropic factors (for a review see (Voss et al., 2013a)). In humans, higher CR fitness level is associated with better cognition, decreased risk for early AD and preserved brain volumes (Hayes et al., 2013). We have previously identified a relationship between CR fitness and larger brain volumes in individuals with early AD (Burns et al., 2008a), specifically in the parietal and medial temporal lobes (Honea et al., 2009). Furthermore, in a longitudinal analysis we found that increased CR fitness over two years was related to lower rates of medial temporal atrophy (Vidoni et al., 2012a). In the present study, our sample of previously sedentary individuals with AD reflects the larger population of older adults, in which less than half are meeting aerobic exercise recommendations (http://www.health.gov/paguidelines/report/pdf/committeereport.pdf). Individuals with AD may even have lower levels of CR fitness than typically aging adults (Burns et al., 2008b). Within this sedentary population, genetics, age, leisure activity and light to moderate physical activity contribute to measured aerobic capacity VO2peak (Talbot et al., Fleg et al., 2005, Bouchard, 2012). A broader age range of fitness may have provided greater insight into the association of white matter integrity and fitness but would likely be less generalizable to the population.
Recent diffusion imaging studies in non-demented individuals found positive associations with higher VO2peak levels and preserved white matter integrity in the cingulum (Marks et al., 2007, Marks et al., 2011), the uncinate fasciculus (Marks et al., 2007), and across the temporal and frontal regions of the brain (Voss et al., 2013b). Moreover a recent study in overweight children found amount of exercise was associated with increased FA in the superior longitudinal fasciculus, a key tract connecting the parietal to the frontal cortices (Krafft et al., 2014). However, to our knowledge none of these reports identified a relationship of higher CR fitness levels and preserved white matter integrity specifically in the IFOF, although this tract could have been included in more global temporal or frontal regions of interest. This tract is known to be one of the longest white matter bundles in the brain and connects parts of the occipital, temporal, and frontal lobes, thus making it difficult to isolate when conducting whole brain white matter analyses. The exact role of the IFOF is still under debate, but previous studies have shown that it is susceptible to AD-related deterioration (Alves et al., 2012, Bosch et al., 2012, Yu et al., 2014), perhaps later in the disease process. In contrast, tracts such as the superior longitudinal fasciculus, the cingulum, and the uncinate fasciculus exhibit earlier decline in AD (Sexton et al., 2011). Thus, a possible interpretation for our findings is that higher levels of CR fitness might be associated with preserved integrity in white matter tracts that have not been already compromised by the disease process. However, in healthy non-demented subjects, higher CR fitness levels may be also associated with preserved white matter integrity in early deteriorating tracts (e.g. the superior longitudinal fasciculus, the cingulum, and the uncinate fasciculus) (Marks et al., 2007, Marks et al., 2011, Tseng et al., 2013, Voss et al., 2013b).
We found a right-lateralized association between fitness and the inferior fronto-occipital fasciculus. One other report has specified a left lateralized relationship with CR fitness, in the cingulum bundle (Marks et al., 2011). Another study also suggested that cortical degeneration in AD occurs faster in the left hemisphere, but mainly in gray matter (Thompson et al., 2003). However, a meta-analysis of DTI studies in across AD individuals indicated that there may not be hemispheric differences in white matter integrity between nondemented, MCI, AD groups (Sexton et al., 2011). Another study in healthy older adults found a relationship between increased FA in the right splenium and genu and cognition (Madden et al., 2009). We did not find any significant relationships between cognition and white matter integrity in our tracts-of interest. Thus more studies are needed to explore hemispheric dominance further in regards to the relationship between CR fitness, white matter, and the possible correlations with cognition.
In this study we performed TBSS analyses to compare our results with previous DTI studies in non-demented participants (Johnson et al., 2012, Gons et al., 2013, Voss et al., 2013b, Hayes et al., 2015). Johnson et al. found a relationship between CR Fitness and FA in the corpus callosum (Johnson et al., 2012), while Gons et al. found relationships between fitness and other diffusion metrics across multiple white matter tracts (Gons et al., 2013). Hayes et al. identified white matter regions where VO2peak was associated with increased FA in older adults, namely the right temporal pole, right fornix, left sagittal striatum, right posterior corona radiate, and the corpus callosum(Hayes et al., 2015). While TBSS is the most commonly used analysis technique, its method is limited to a skeletonized white matter evaluation, which represents only the highest and most perpendicular FA voxel intensities projected along each voxel within the skeleton (Bach et al., 2014). These and other considerations with the TBSS methodology have been noted elsewhere (Zalesky, 2011, Keihaninejad et al., 2013, Bach et al., 2014). Hence, we also performed a-priori tract-of-interest analyses, which allowed us to quantify diffusivity metrics on specific tracts previously implicated in AD (Sexton et al., 2011, Alves et al., 2012, Bosch et al., 2012, Zhang et al., 2014). The tract-of-interest analysis may also be susceptible to partial volume effects, given enlarged ventricles and atrophy, which are common in older adults with early AD. To overcome these limitations, we only included voxels with higher anisotropy values (FA > 0.2) and reduced our tract masks to include only voxels with a higher probability of existence based of the white matter probabilistic atlas (Hua et al., 2008).
Other limitations included the lack of a non-demented control group because this investigation was conducted with preliminary baseline data from AD individuals enrolled in an ongoing aerobic exercise trial. Non-demented controls could have helped identify the effects of CR fitness on brain’s white matter integrity independent of the AD pathology. We also did not have individuals with a broader range of dementia severity, thus we could not test for a relationship of disease progression or severity with fitness-related diffusion change. We are also aware that is might be prematurely calling our participants early AD without an in vivo biomarker measure. However, participants were well characterized clinically and were diagnosed with a suspected underlying etiology of AD as the cause of their cognitive impairment. A marker of AD pathology would improve the specificity of our findings (Berg et al., 1998) (Morris et al., 2001). Additionally, we only recruited participants who were sedentary as determined by the Telephone Assessment of Physical Activity (Mayer et al., 2008, Vidoni et al., 2012b). A wider range of participants (sedentary and active) would have ideally added a better statistical estimation of the AD population. Another limitation is that we did not control for white matter lesions (WMLs) because we did not collect high contrast FLAIR images. We inspected every image for noticeable WMLs and performed a threshold criterion to only include voxels with FA values higher than 0.2. This criterion would ideally exclude highly isotropic voxels, which may be contaminated due to unperceived WMLs. Finally, we acknowledge that during the a-priori tract-of-interest approach we did not correct for multiple comparisons. We feel the increased risk of Type I error was offset by careful execution of a hypothesis driven experiment rooted in existing literature.
CONCLUSION
We assessed the relationship of CR fitness with white matter integrity in individuals with early-stage AD. We found a positive association between CR fitness levels and white matter integrity in the right IFOF even after controlling for age and gender. These results suggest that increased CR fitness might be positively associated with white matter integrity, even in individual with Alzheimer’s disease. This initial cross-sectional study should be followed-up with longitudinal studies on exercise in AD specifically powered to test for changes in white matter tract integrity.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Charles Henry and Michael Hulet and the rest of the Information and Telecommunication Technology Center (ITTC) staff at The University of Kansas for their support with our high performance computing.
Funding: Drs. Burns, Vidoni, Morris, Honea, and Rasinio Graves are supported by the University of Kansas Alzheimer’s Disease Center (P30AG035982). Supported by the National Institute on Aging (NIA) R01AG033673. Dr. Burns was also supported by grants from the NIA and NINDS (R01AG034614 & U10NS077356). Dr. Vidoni was supported in part by Frontiers: The Heartland Institute for Clinical and Translational Research (University of Kansas Medical Center’s CTSA (KL2TR000119). Dr. Honea and Rodrigo Perea are supported by a grant from NIA (K01AG035042). Work conducted in the project is supported by the National Center for Research Resources (M01RR023940), and is now at the National Center for Advancing Translational Sciences (UL1TR000001). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.” The KU Grayhawk Database provided contact information for potential participants.
Footnotes
Author Contributions: All authors contributed to analysis design, results interpretation and manuscript preparation.
Disclosures: None of the authors have relevant disclosures to mention.
Contributor Information
RD. Perea, Email: rperea@kumc.edu.
ED. Vidoni, Email: evidoni@kumc.edu.
JK. Morris, Email: jmorris2@kumc.edu.
RS. Graves, Email: rgraves@kumc.edu.
JM. Burns, Email: jburns2@kumc.edu.
RA. Honea, Email: rhonea@kumc.edu.
References
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Tromp do PM, Zakszewski E, Field AS. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain connectivity. 2011;1:423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves GS, O’Dwyer L, Jurcoane A, Oertel-Knochel V, Knochel C, Prvulovic D, Sudo F, Alves CE, Valente L, Moreira D, Fubetaer F, Karakaya T, Pantel J, Engelhardt E, Laks J. Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PloS one. 2012;7:e52859. doi: 10.1371/journal.pone.0052859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HS, Kluding PM, Gajewski BJ, Donnelly JE, Burns JM. Reliability of peak treadmill exercise tests in mild Alzheimer disease. The International journal of neuroscience. 2011;121:450–456. doi: 10.3109/00207454.2011.574762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. (FMRIB Analysis Group Technical Reports: TR07JA02).Non-linear optimisation. 2007 from www fmrib ox ac uk/analysis/techrep. [Google Scholar]
- Bach M, Laun FB, Leemans A, Tax CM, Biessels GJ, Stieltjes B, Maier-Hein KH. Methodological considerations on tract-based spatial statistics (TBSS) Neuroimage. 2014;100:358–369. doi: 10.1016/j.neuroimage.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik RL, Dowling MN, Gallagher CL, Carlsson CM, Rowley HA, Bendlin BB, LaRue A, Asthana S, Hermann BP, Sager MA, Johnson SC, Okonkwo OC. Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain imaging and behavior. 2014 doi: 10.1007/s11682-014-9325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junque C, Sole-Padulles C, Pena-Gomez C, Bargallo N, Molinuevo JL, Bartres-Faz D. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiology of aging. 2012;33:61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Bouchard C. Genomic predictors of trainability. Experimental physiology. 2012;97:347–352. doi: 10.1113/expphysiol.2011.058735. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Burggren A, Brown J. Imaging markers of structural and functional brain changes that precede cognitive symptoms in risk for Alzheimer’s disease. Brain imaging and behavior. 2014;8:251–261. doi: 10.1007/s11682-013-9278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008a;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Mayo MS, Anderson HS, Smith HJ, Donnelly JE. Cardiorespiratory fitness in early-stage Alzheimer disease. Alzheimer disease and associated disorders. 2008b;22:39–46. doi: 10.1097/WAD.0b013e31815a9ddc. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Backman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiology of aging. 2014;35(Suppl 2):S20–28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Andersen AH, Smith CD. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer’s disease. Neuroimage. 2010;52:1487–1494. doi: 10.1016/j.neuroimage.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gons RA, Tuladhar AM, de Laat KF, van Norden AG, van Dijk EJ, Norris DG, Zwiers MP, de Leeuw FE. Physical activity is related to the structural integrity of cerebral white matter. Neurology. 2013 doi: 10.1212/WNL.0b013e3182a43e33. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Hayes JP, Cadden M, Verfaellie M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Frontiers in aging neuroscience. 2013;5:31. doi: 10.3389/fnagi.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Salat DH, Forman DE, Sperling RA, Verfaellie M. Cardiorespiratory fitness is associated with white matter integrity in aging. Annals of clinical and translational neurology. 2015;2:688–698. doi: 10.1002/acn3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill exercise testing in an epidemiologic study of elderly subjects. J Gerontol A Biol Sci Med Sci. 1998;53:B259–267. doi: 10.1093/gerona/53a.4.b259. [DOI] [PubMed] [Google Scholar]
- Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer disease and associated disorders. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF. Using diffusion imaging to study human connectional anatomy. Annual review of neuroscience. 2009;32:75–94. doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59:1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keihaninejad S, Zhang H, Ryan NS, Malone IB, Modat M, Cardoso MJ, Cash DM, Fox NC, Ourselin S. An unbiased longitudinal analysis framework for tracking white matter changes using diffusion tensor imaging with application to Alzheimer’s disease. Neuroimage. 2013;72:153–163. doi: 10.1016/j.neuroimage.2013.01.044. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis : a researcher’s handbook. Upper Saddle River N.J.: Pearson Prentice Hall; 2004. [Google Scholar]
- Liu Y, Spulber G, Lehtimaki KK, Kononen M, Hallikainen I, Grohn H, Kivipelto M, Hallikainen M, Vanninen R, Soininen H. Diffusion tensor imaging and tract-based spatial statistics in Alzheimer’s disease and mild cognitive impairment. Neurobiology of aging. 2011;32:1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. Journal of cognitive neuroscience. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks BL, Katz LM, Styner M, Smith JK. Aerobic fitness and obesity: relationship to cerebral white matter integrity in the brain of active and sedentary older adults. British journal of sports medicine. 2011;45:1208–1215. doi: 10.1136/bjsm.2009.068114. [DOI] [PubMed] [Google Scholar]
- Marks BL, Madden DJ, Bucur B, Provenzale JM, White LE, Cabeza R, Huettel SA. Role of aerobic fitness and aging on cerebral white matter integrity. Annals of the New York Academy of Sciences. 2007;1097:171–174. doi: 10.1196/annals.1379.022. [DOI] [PubMed] [Google Scholar]
- Mayer CJ, Steinman L, Williams B, Topolski TD, LoGerfo J. Developing a Telephone Assessment of Physical Activity (TAPA) questionnaire for older adults. Prev Chronic Dis. 2008;5:A24. [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PC, Nagae-Poetscher L. MRI atlas of human white matter. 2005 doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412b–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Archives of neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annual review of neuroscience. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH. The declining infrastructure of the aging brain. Brain connectivity. 2011;1:279–293. doi: 10.1089/brain.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiology of aging. 2011;32:2322e2325–2318. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, Atri A. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimer’s research & therapy. 2011;3:32. doi: 10.1186/alzrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Talbot LA, Metter EJ, Fleg JL. Leisure-time physical activities and their relationship to cardiorespiratory fitness in healthy men and women 18–95 years old. Med Sci Sports Exerc. 2000;32:417–425. doi: 10.1097/00005768-200002000-00024. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein KH, Filippi M, Ernemann U, Reiser MF, Hampel H. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis. 2010;22:507–522. doi: 10.3233/JAD-2010-100234. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L, Alzheimer’s A. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, Herman D, Hong MS, Dittmer SS, Doddrell DM, Toga AW. Dynamics of gray matter loss in Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Erickson KI, Simonsick EM, Aizenstein HJ, Glynn NW, Boudreau RM, Newman AB, Kritchevsky SB, Yaffe K, Harris TB, Rosano C. Physical activity predicts microstructural integrity in memory-related networks in very old adults. J Gerontol A Biol Sci Med Sci. 2014;69:1284–1290. doi: 10.1093/gerona/glt287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, Huang H, Zhang R. White Matter Integrity in Physically Fit Older Adults. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM. Cardiorespiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiology of aging. 2012a;33:1624–1632. doi: 10.1016/j.neurobiolaging.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Van Sciver A, Johnson DK, He J, Honea R, Haines B, Goodwin J, Laubinger MP, Anderson HS, Kluding PM, Donnelly JE, Billinger SA, Burns JM. A community-based approach to trials of aerobic exercise in aging and Alzheimer’s disease. Contemp Clin Trials. 2012b;33:1105–1116. doi: 10.1016/j.cct.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Szabo A, Phillips SM, Wojcicki TR, Mailey EL, Olson EA, Gothe N, Vieira-Potter VJ, Martin SA, Pence BD, Cook MD, Woods JA, McAuley E, Kramer AF. Neurobiological markers of exercise-related brain plasticity in older adults. Brain, behavior, and immunity. 2013a;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL, Wojcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2013b;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Johansen-Berg H, Karadottir RT. Unraveling the secrets of white matter - Bridging the gap between cellular, animal and human imaging studies. Neuroscience. 2014;276C:2–13. doi: 10.1016/j.neuroscience.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Xiao JX, Wang YH, Wu HK, Gong GL, Jiang XX. Evaluation of bilateral cingulum with tractography in patients with Alzheimer’s disease. Neuroreport. 2005;16:1275–1278. doi: 10.1097/01.wnr.0000174061.41897.ee. [DOI] [PubMed] [Google Scholar]
- Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer’s disease: an update. Annual review of neuroscience. 2014;37:79–100. doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- Zalesky A. Moderating registration misalignment in voxelwise comparisons of DTI data: a performance evaluation of skeleton projection. Magnetic resonance imaging. 2011;29:111–125. doi: 10.1016/j.mri.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Zhang B, Xu Y, Zhu B, Kantarci K. The role of diffusion tensor imaging in detecting microstructural changes in prodromal Alzheimer’s disease. CNS neuroscience & therapeutics. 2014;20:3–9. doi: 10.1111/cns.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


