Abstract
In this study we investigated whether dysregulation of the fragile X mental retardation protein (FMRP) and mammalian target of rapamycin (mTOR) signaling cascade may have a role in the pathogenesis of encephalopathy of prematurity following perinatal hypoxia-ischemia. We examined the brain tissue of newborns with encephalopathy and compared it to age-matched controls with normal brain development and adults. In normal controls, the FMRP expression in cortical grey matter spiked 4-fold during 36-39 gestational weeks compared to the adult, with a concomitant suppression of p70S6K and S6. In encephalopathy cases, the developmental spike of FMRP was not observed, and FMRP levels remained significantly lower than in normal controls. Importantly, this FMRP downregulation was followed by a significant overexpression of p70S6K and S6. Our novel findings thus suggest that premature hypoxic-ischemic brain injury may affect the FMRP/mTOR pathway, as otherwise observed in inherited syndromes of cognitive disability and autism spectrum disorders.
Keywords: FMRP, mTOR, prematurity, brain injury, hypoxia, autism
Introduction
Brain injury caused by hypoxia-ischemia in premature infants is of enormous public health importance due to the large number of survivors with serious neurocognitive deficits and cerebral palsy [1, 2]. In the USA, approximately 63,000 infants are born annually with a very low birth-weight (≤1500 g). This represents 1.5% of all live-births and the importance of encephalopathy in this large group is indicated by the subsequent occurrence of cognitive, behavioral, attention, or socialization deficits in 25–50% of them, and of major motor deficits (e.g. cerebral palsy) in 5–10% of them [1, 2, 3]. Encephalopathy of prematurity is a form of cerebral injury that occurs in the setting of either primary or secondary hypoxia-ischemia in the premature infant [1, 2]. Previously, it has been shown in the rat model of encephalopathy of prematurity that white matter is selectively vulnerable to hypoxia-ischemia in the 1st postnatal week [4], while similar insults in the 2nd postnatal week cause neocortical strokes and seizures [5]. Hypoxia-ischemia results in excess of extracellular glutamate where excessive activation of receptors can induce “excitotoxicity” via their locations on neurons as well as on glia [4, 5]. Glutamatergic excitotoxicity in white matter oligodendrocytes mediated by AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) glutamate receptors (GluRs) was identified as an important factor in this injury [4, 5]. The neuropathological correlates of encephalopathy of prematurity include various lesions, most importantly periventricular leukomalacia, and accompanying neuronal/axonal deficits that involve the cerebral white matter, thalamus, basal ganglia, cerebral cortex, brainstem, and cerebellum [1]. In addition, premature birth further increases the risk of autism spectrum disorders in patients without known autism-related genetic mutations [3]. A recent epidemiological report singles out hypoxia at birth as the only risk factor significantly more prevalent in older mothers of the children with autism spectrum disorders compared to younger mothers [6].
Given the phenotypic overlap between encephalopathy of prematurity and autism spectrum disorders, we postulated that perinatal hypoxic-ischemic brain injury might affect the control pathway containing the FMR1 gene implicated in Fragile X syndrome. Fragile X syndrome is the most common inherited cause of intellectual disability with concomitant autism spectrum disorders in 30% cases, comprising for up to 6% of all diagnosed cases of autism [3, 7]. It is typically caused by a trinucleotide (CGG) repeat expansion of the FMR1 gene leading to its reduced or absent transcription with consequent decreased reduction in translation of the FMR1 protein (FMRP) [3, 7]. Activity-dependent dendritic mRNA translation is normally modulated by FMRP, which usually acts together with multiple other signaling proteins inhibiting the translation of various genes that affect synaptic plasticity [7, 8]. Not surprisingly, FMRP levels were therefore found to correlate with the level of clinical consequences including structural, functional and cognitive brain involvement [8]. Relevant to encephalopathy of prematurity, FMRP-controlled signaling pathways were shown to be upregulated by hypoxic-ischemic brain injury in wild type rodents, in part via over-activation of ionotropic (iGluRs) and metabotropic glutamate receptors (mGluRs) [9]. The mammalian target of rapamycin (mTOR) signaling cascade, one of FMRP downstream effectors, has been in particular recognized as a central regulator of translation critically needed in developing neurons for establishing synaptic basis for higher order brain functions [9, 10]. Notably, the mTOR substrate p70S6K can regulate mRNA-binding activity of FMRP, while Fmr1 KO mice exhibit enhanced activation of mTOR pathway and display behavioral phenotype similar to patients with autism spectrum disorders [9, 10]. Corresponding molecular and behavioral observations have also been reported by our group in a rodent model of encephalopathy of prematurity [7]. Nonetheless, while oxidative stress and mGluRs have been shown to play a major role in the pathogenesis of encephalopathy of prematurity and other cerebral disorders [11, 12, 13], the role of FMRP expressed in perinatal brain tissue following hypoxic-ischemic injury remains undefined. Aiming to fill that gap in this pilot study, we have evaluated developmental expression of FMRP and mTOR substrates p70S6K and S6 in normal human brain and compared it with the expression patterns in brains of premature patients with encephalopathy of prematurity.
Materials and Methods
Human brain tissue specimens
In this pilot study, we have used snap frozen and formalin fixed/paraffin embedded tissue from newborns with normal brain development at different ages (20-39 gestational weeks) (n=15); age-matched newborns with premature hypoxic-ischemic brain injury (i.e. encephalopathy of prematurity) (n=11) and adult controls (n=4) collected from the Department of Pathology at Children's Hospital Boston, MA; University of Maryland Brain and Tissue Bank, Baltimore, MD and Brigham and Women's Hospital, Boston, MA (adult controls only). The fetuses and infants have died due to prematurity or other disease processes (Table 1). Only tissues with short postmortem intervals (generally < 24 hrs) have been retained and reviewed by a board certified neuropathologist (ML) for diagnostic purposes, and for tissue quality control. Approval of the institutional clinical research committee was obtained. The autopsy permits had also provided parental consent for research. All clinico-pathological information was held strictly confidential. Specimens were identified by a random number (not autopsy or medical record number) throughout their processing and analysis. Effort was made to obtain tissue from both genders and from minorities.
Table 1.
Clinical characteristics of control and EOP sample population.
| Case | GA (weeks) | PNA (days) | PMI (h) | Gender | Postmortem diagnosis |
|---|---|---|---|---|---|
| 1 | 20 | 0 | N/A | N/A | Stillborn |
| 2 | 20 | 0 | 6 | M | Stillborn |
| 3 | 22 | 0 | 8 | F | Chorioamnionitis |
| 4 | 24 | 0 | 11 | F | Extreme prematurity |
| 5 | 26 | 2 | 22 | M | Prematurity/sepsis |
| 6 | 27 | 1 | 18 | M | Stillborn/Renal agenesis |
| 7 | 30 | 0 | 12 | F | Stillborn |
| 8 | 33 | 1 | N/A | F | CHD |
| 9 | 35 | 2 | 19 | M | Stillborn/Renal agenesis |
| 10 | 36 | 0 | N/A | M | Stillborn |
| 11 | 36 | 0 | 21 | F | Stillborn/Umbilical cord abnormality |
| 12 | 37 | 0 | 20 | M | Stillborn/chorioamnionitis |
| 13 | 37 | 2 | 17 | M | CHD |
| 14 | 39 | 0 | 11 | F | Placental abruption |
| 15 | 39 | 0 | 19 | F | Positional asphyxia |
| 16 | 23 | 0 | 12 | F | EOP, Pulmonary hypoplasia |
| 17 | 26 | 2 | 10 | M | EOP, Pneumonia |
| 18 | 28 | 5 | 13 | M | EOP, Hypoplastic left heart syndrome |
| 19 | 32 | 2 | 20 | F | EOP, Necrotizing enterocolitis |
| 20 | 34 | 3 | 21 | F | EOP, Hypoplastic left heart syndrome |
| 21 | 35 | 1 | 14 | F | EOP, MCA |
| 22 | 36 | 0 | 17 | M | EOP, CHD |
| 23 | 38 | 2 | 9 | M | EOP, Sepsis |
| 24 | 39 | 0 | 11 | M | EOP, Pulmonary hypoplasia |
| 25 | 39 | 3 | 12 | F | EOP, Hypoplastic left heart syndrome |
| 26 | 39 | 3 | 12 | M | EOP, CHD |
| 27 | N/A | 47 years | 17 | M | Trauma |
| 28 | N/A | 55 years | 24 | F | Aortic aneurism |
| 29 | N/A | 58 years | 22 | F | Trauma |
| 30 | N/A | 60 years | 11 | M | Pulmonary embolism |
Legend: CHD, congenital heart disease/defect; EOP, encephalopathy of prematurity; F, female; GA, gestational age; GM, germinal matrix; M, male; N/A, information not available; PMI, postmortem interval; PNA, postnatal age; MCA, multiple congenital abnormalities.
Histological analysis and immunohistochemical methods
Standard histological and immunohistochemical procedures, including semiquantitative analyses were performed as previously published [14, 15, 16]. Representative sections of parietal cortex and white matter were stained by hematoxylin and eosin (H&E) and/or immunohistochemical labeling for total FMRP and phospho-S6 (Ser235/236). To avoid subjectivity in the scoring, a pathologist (ML) performed all histological and immunohistochemical evaluations in a blinded fashion.
Antibodies
The following antibodies have been used for immunohistochemical and immunofluorescence procedures: (1) an anti-phospho-S6 ribosomal protein (Ser235/236) rabbit polyclonal antibody (Cat. No. 2211, 1:200, Cell Signaling, Boston, MA) (2) an anti-FMRP rabbit polyclonal antibody (Ab17722, 1:5000, Abcam, Cambridge, MA) (3) an anti-GFAP mouse monoclonal antibody (M0761, prediluted, Dako, An Agilent Technologies, Carpinteria, CA) (4) an anti-OLIG2 mouse monoclonal antibody (MABN50, 1:100, EMD Millipore, Billerica, MA) and (5) an anti-mGluR5 mouse monoclonal antibody (MABN540, 1:100, EMD Millipore, Billerica, MA).
Western blot analyses
Western blot analyses for mTOR downstream targets [p70S6K (Cat. No. 2708, 1:200, Cell Signaling, Boston, MA) and phospho-p70S6K(Thr389) (Cat. No. 9205, 1:250, Cell Signaling, Boston, MA)] and total (Ab17722, 1:1000, Abcam, Cambridge, MA) and phospho-FMRP(Ser499) (Ab48127, 1:500, Abcam, Cambridge, MA); as well as an anti-β-actin mouse monoclonal antibody (A5411, 1:2000, Sigma-Aldrich, St Louis, MO) were performed according to conventional methodology.
Statistical analysis
One-way ANOVA and two-tailed t-tests were used for multiple comparisons across different developmental ages, or between different development and encephalopathy of prematurity groups. Statistical significance was defined as p < 0.05.
Results
Developmental expression of FMRP in normal human brain
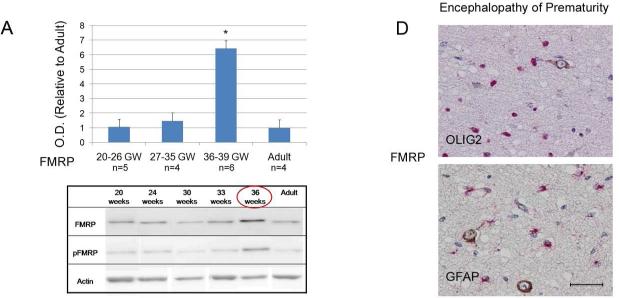
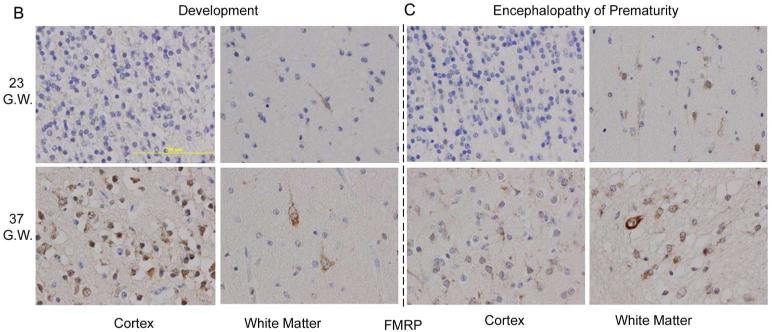
To investigate the role of FMRP in premature hypoxic-ischemic brain injury (i.e. encephalopathy of prematurity), we first had to map its developmental expression in the normal human brain, a research endeavor that to our knowledge has never been done before. For that purpose, we analyzed 15 human postmortem specimens at different stages of normal cortical development, i.e., spanning 20-39 gestational weeks. Western blot analyses show a relatively even baseline expression of the total FMRP and phospho-FMRP over 20-35 gestational weeks, which did not differ from the levels observed in normal adult brains (Figure 1A). As corroborated by semi-quantitative immunohistochemical analysis, baseline cortical levels of the total FMRP in these gestational periods also did not differ from their expression in the white matter (Figure 1B). However, during the period spanning 36-39 gestational weeks (n=6), a transient 4-fold spike in expression of both total (Figure 1A) and phospho-FMRP (quantification not shown) was observed by Western blot analysis in cortical grey matter compared to the adult controls (n=4; p < 0.0001) but not in the white matter where FMRP expression remained low to moderate between 20-39 gestational weeks (Figure 1B).
Figure 1. FMRP expression in the brain during normal development and in encephalopathy of prematurity.
A) Western blots quantification of total FMRP expression during normal human cortical development (n=15). Histograms represent averaged optical density normalized to actin, and expressed relative to the mean adult controls values (n=4). * p < 0.0001 vs. adult control, GW = gestational weeks; OD = optical density. Inset: representative western blot analyses for total and phospho-FMRP at five different developmental time points and in an adult control. B) Representative immunohistochemical stains showing overexpression of FMRP in the human cortex (brown staining) at 37 gestational weeks compared to 23 gestational weeks of normal development. White matter shows no difference in FMRP expression in the same gestational periods. C) Representative immunohistochemical stains showing no change in FMRP expression in the brain of patients with encephalopathy of prematurity during 23rd and 37th gestational weeks. D) FMRP/OLs (OLIG2) and FMRP/astrocytes (GFAP) double-labeling in white matter neurons showing no co-expression (Size bar 50 μm).
Dysregulation of components of FMRP/mTOR signaling cascade in newborns with encephalopathy of prematurity
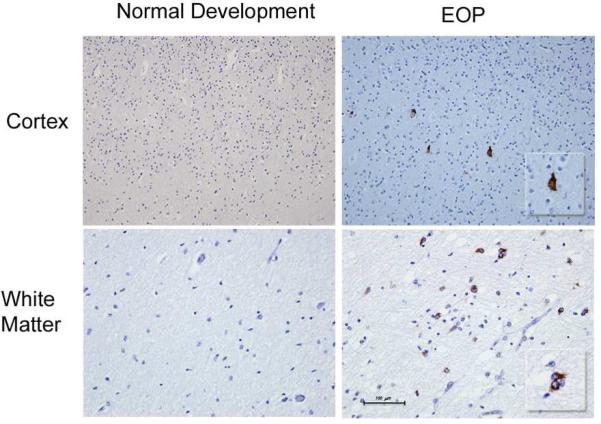
In contrast to newborns with normal human brain development, this developmental spike in the cortical FMRP expression during the 36-39 gestational weeks was not observed in the cases with encephalopathy of prematurity. FMRP levels remained unchanged compared to the adult controls and the earlier gestational periods (23-35 gestational weeks (Figure 1C). A semi-quantitative immunohistochemical scoring in cases with encephalopathy of prematurity also showed a 2-fold increase in FMRP expression predominantly in subplate neurons and the white matter compared to normal human brain development during 23-37 gestational weeks, without co-expression in astrocytes (GFAP) and oligodendrocytes (OLIG2) (Figure 1D). Observed cortical downregulation of the total FMRP and phospho-FMRP in newborns with encephalopathy of prematurity during the 36-39 gestational weeks relative to the newborns with normal human brain development (Figure 2A; p < 0.0001) was followed by an overexpression of p70S6K and phospho-p70S6K compared to the normal age-matched controls (Figure 2B; p < 0.0001). In addition, imunohystochemical analysis for p-S6 expression in four available cases with encephalopathy of prematurity (36-38 gestational weeks) yielded positive staining in subpopulation of cortical neurons and the white matter compared to no expression in non-affected newborns with normal development during the same gestational period (Figure 3).
Figure 2. Expression of components of the FMRP/mTOR signaling cascade in brain tissue of patients with encephalopathy of prematurity.
Western blots quantification of A) FMRP and phospho-FMRP as well as B) mTOR downstream targets p70S6K and phospho-p70S6K during 37-38 gestational weeks in the cortex of patients with encephalopathy of prematurity (n=5) and in normal age-matched controls (n=6). Histograms represent averaged optical density normalized to actin. C) Representative Western blot analyses for total and phospho-p70S6K (n=4) and in an adult control. * p < 0.0001 vs. age-matched control, Ctrl. = control; EOP = encephalopathy of prematurity; GW = gestational weeks; OD = optical density.
Figure 3. phospho-S6 expression in the brain during normal development and in encephalopathy of prematurity.
Representative immunohistochemical stain of phospho-S6 expression in human cortex and the white matter during normal brain development (no expression detected) and a positive staining in a newborn with encephalopathy of prematurity (EOP) during 37th gestational week. Insets: Examples of positive stain for phospho-S6 expression (600x) in a patient with encephalopathy of prematurity.
Co-expression of metabotropic glutamate receptor 5 (mGluR5) with the components of FMRP/mTOR signaling cascade in newborns with encephalopathy of prematurity
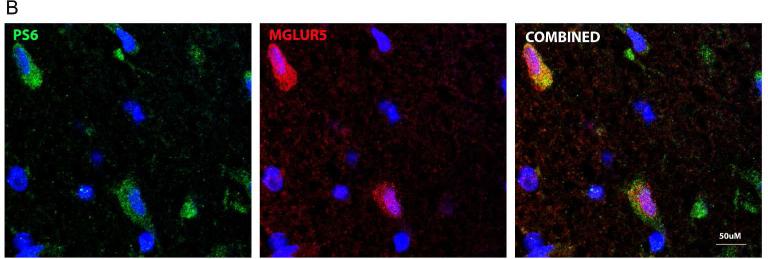
In order to provide some human target validation of the co-expression of the components of the FMRP/mTOR signaling cascade and metabotropic glutamate receptors (mGluRs) we have performed fluorescent double labeling with mGluR5 antibody in four available cases with encephalopathy of prematurity (36-38 gestational weeks). The analysis has confirmed that mGlur5 was indeed invariably co-expressed with both FMRP (Figure 4A) and pS6 (Figure 4B) on neurons of the patients with hypoxic-ischemic brain injury.
Figure 4.
Representative immunohistochemical stains showing A) co-expression of mGluR5 and FMRP and B) mGluR5 and phospho-S6 (PS6) on the neurons of a newborn with encephalopathy of prematurity during 37th gestational week (600x; double staining with DAPI).
Discussion
Results presented here demonstrate overexpression of cortical FMRP in 36-39 gestational weeks developmental window, suggesting that FMRP has a physiological role in translation modulation of genes that affect synaptic plasticity in this particular period [7, 8]. Our results also confirm that 36-39 gestational weeks is a critical period of development for the brain, providing some explanations why even late preterm newborns are still at risk for abnormal neurodevelopment [17, 18].
In contrast, newborns with encephalopathy of prematurity presented downregulation of FMRP and concomitant overexpression of the mTOR downstream targets in this critical developmental period. Observed enhanced activation of all investigated components of the mTOR signaling pathway with concomitant downregulation of FMRP and p-FMRP in patients with encephalopathy of prematurity during the 36-39 gestational weeks closely resembles molecular expressions previously reported in Fmr1 KO mice [10]. The activation of the mTOR signaling cascade is known to contribute to normal neuronal growth by promoting their differentiation, neurite elongation and branching, and synaptic formation during development [19]. However, any interruption in normal physiological processes may have consequences on brain development. As shown previously, excess activation of mTOR signaling causes abnormal development of neurons and glia, leading to brain malformation [19]. In the context of our experiments, these novel observations suggest that one of the mechanisms by which premature hypoxic-ischemic brain injury may cause its long-term sequelae is via dysregulation of signaling pathways otherwise implicated in syndromes of inherited intellectual disability and autism (e.g., fragile X syndrome and tuberous sclerosis) [3, 7].
While the mechanisms of the premature brain injury and encephalopathy of prematurity are multifactorial, hypoxia-ischemia is known to cause increased accumulation of extracellular glutamate, which initiates excitotoxic injury and cell death [11]. Results presented here show co-expression of mGluR5 with both FMRP and pS6 on neurons of patients with encephalopathy of prematurity during the 36-39 gestational weeks, consistent with its reported expression in the brain [20]. In addition, our group recently reported that both mGluR1 and mGluR5 are localized on immature oligodendrocytes throughout human brain development, with mGluR5 being highest in the preterm period [12]. These findings indicate that group I mGluRs are highly expressed on oligodendrocytes during the peak period of vulnerability to hypoxia-ischemia [12]. Combined, these results are consistent with the proposed mGluR theory of pathogenesis in fragile X syndrome suggesting that neurocognitive deficits are related to upregulation of the downstream effectors of the mGluR5 pathway in the cortex and hippocampus [10]. These include phosphatidylinositol-3-kinase (PI3K) / Akt / mTOR signaling components, upregulation of which leads to enhanced long-term depression (LTD) [13].
In addition, we have shown increased FMRP expression predominantly in the white matter and subplate neurons of the newborns with encephalopathy of prematurity, compared to newborns with normal human brain development during 25-37 gestational weeks. This observation may be important because synaptic plasticity in critical periods of development requires intact inhibitory circuitry [21]. Subplate neurons have been reported to regulate the molecular machinery required to establish an adult balance of excitation and inhibition in layer 4 of the white matter [21]. Hence, the link between mGluRs, FMRP and translation in the setting of premature hypoxic-ischemic brain injury is emerging, emphasizing their important roles in synaptogenesis and synaptic plasticity.
Conclusions
Although encephalopathy of prematurity includes various neuronal/axonal deficits, cognitive deficits including elevated rates of hyperactivity and autism spectrum disorders are by far its dominant neurodevelopmental sequelae [3]. Nonetheless, there is still a significant gap in understanding the molecular basis of this complex disorder as well as an unmet need for early identification and treatment of patients at high risk to develop developmental disorders in later life.
Our novel findings suggest that dysregulation of FMRP/mTOR pathways may have a role in pathogenesis of encephalopathy of prematurity, as otherwise observed in the most common inherited syndrome of cognitive disability (e.g. fragile X syndrome). The FMRP/mTOR signaling pathway thus might be a possible new molecular target for treatment of encephalopathy of prematurity and its neurocognitive deficits, a hypothesis that warrants further investigation.
Acknowledgement
Data were presented in part at the 2013 Annual Conference of the American Association of Neuropathologists.
Funding
This work was supported by grants from Harvard Catalyst (KL2 award to ML) and the Baby Alex Foundation (to ML) and by United States National Institutes of Health (NIH) Grants R01 NS 31718 (to FEJ) and DP1 OD003347 (from the Office of the Director) (to FEJ).
Footnotes
Author Contributions
ML designed the study, provided human brain samples, performed neuropathological analyses and wrote the manuscript. PW and LLJ analyzed data, co-wrote and edited the manuscript. KMM and MCJ prepared brain tissue slices and performed immunohistochemical and Western blot experiments of great interpretative value to the manuscript. FEJ coordinated the project, provided clinical input, analyzed data and edited the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The authors declare that they have no conflicting interests.
Ethical Approval
The study was approved by the institutional clinical research committee at the Boston Children's Hospital and Brigham and Women's Hospital, Boston, MA. Parental consent for autopsy was properly obtained in every case.
Resources Sharing
The authors always share our latest discovery and invention with the scientific community through presentations at meetings and publications immediately after any IP has been protected by the respective authors’ academic institution(s), if required. The protocols and compounds used for our experimental methods are available to all researchers by request, free of charge and with no conditions attached.
References
- 1.Volpe JJ. The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16:167–178. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes RL, Sleeper LA, Volpe JJ, Kinney HC. Neuropathologic studies of the encephalopathy of prematurity in the late preterm infant. Clin Perinatol. 2013;40(4):707–22. doi: 10.1016/j.clp.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry. 2004;61:618–627. doi: 10.1001/archpsyc.61.6.618. [DOI] [PubMed] [Google Scholar]
- 4.Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen F. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–20. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen FE. Role of glutamate receptors in periventricular leukomalacia. J Child Neurol. 2005;20:950–59. doi: 10.1177/08830738050200120401. [DOI] [PubMed] [Google Scholar]
- 6.Maramara LA, He W, Ming X. Pre- and perinatal risk factors for autism spectrum disorder in a New Jersey cohort. J Child Neurol. 2014 Jan 10; doi: 10.1177/0883073813512899. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Hagerman RJ, Hoem G, Hagerman P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1:12–26. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10:31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- 9.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen FE. Developmental factors regulating developmental susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–633. doi: 10.1097/MOP.0b013e328010c536. [DOI] [PubMed] [Google Scholar]
- 12.Jantzie LL, Talos DM, Selip DB, An L, Jackson MC, Folkerth RD, Deng W, Jensen FE. Developmental regulation of group I metabotropic glutamate receptors in the premature brain and their protective role in a rodent model of periventricular leukomalacia. Neuron Glia Biol. 2010;6:277–288. doi: 10.1017/S1740925X11000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechpammer M, Manning SM, Samonte F, Nelligan J, Sabo E, Talos DM, Volpe JJ, Jensen FE. Minocycline treatment following hypoxic/ischaemic injury attenuates white matter injury in a rodent model of periventricular leucomalacia. Neuropathol Appl Neurobiol. 2008;34:379–393. doi: 10.1111/j.1365-2990.2007.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wintermark P, Lechpammer M, Warfield SK, Kostaras B, Takeoka M, Poduri A, Madson JR, Bergin AM, Whalen S, Jensen FE. Perfusion imaging of focal cortical dysplasia using arterial spin labeling: Correlation with histopathological vascular density. J Child Neurol. 2013;11:1474–1482. doi: 10.1177/0883073813488666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechpammer M, Clegg MS, Muzar Z, Huebner PA, Jin LY, Gospe SM., Jr Pathology of inherited manganese transporter deficiency. Ann Neurol. 2014;75:608–612. doi: 10.1002/ana.24131. [DOI] [PubMed] [Google Scholar]
- 17.de Jong M, Verhoeven M, van Baar AL. School outcome, cognitive functioning, and behaviour problems in moderate and late preterm children and adults: a review. Semin Fetal Neonatal Med. 2012;17:163–169. doi: 10.1016/j.siny.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. 2013;132:741–751. doi: 10.1542/peds.2013-1131. [DOI] [PubMed] [Google Scholar]
- 19.Takei N, Nawa H. mTOR signaling and its roles in normal and abnormal brain development. Front Mol Neurosci. 2014;7:28. doi: 10.3389/fnmol.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355(3):455–69. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 21.Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Nuron. 2006;51:627–638. doi: 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]