Abstract
Pericardial tuberculosis (TB) is associated with high therapy failure and high mortality rates. Antibiotics have to penetrate to site of infection at sufficient non-protein bound concentrations, and then enter bacteria to inhibit intracellular biochemical processes. The antibiotic concentrations achieved in pericardial fluid in TB pericarditis have never been measured before. We recruited two cohorts of patients with TB pericarditis, and left a pigtail catheter in-situ for serial drug concentration measurements over 24 h. Altogether, 704 drug concentrations were comodeled for pharmacokinetic analyses. The drug concentrations achieved in pericardial fluid were compared to the minimum inhibitory concentrations (MICs) of clinical Mycobacterium tuberculosis isolates. The total rifampicin concentration pericardial-to-serum ratios in 16 paired samples were 0.19 ± 0.33. The protein concentrations of the pericardial fluid in TB pericarditis were observed to be as high as in plasma. The non-protein bound rifampicin concentrations in pericardial fluid were 4-fold lower than rifampicin MICs in the pilot study, and the peak concentration was 0.125 versus 0.208 mg/L in the second (p = 0.001). The rifampicin clearance from pericardial fluid was 9.45 L/h versus 7.82 L/h in plasma (p = 0.002). Ethambutol peak concentrations had a pericardial-to-plasma ratio of 0.55 ± 0.22; free ethambutol peak concentrations were 2.30-lower than MICs (p < 0·001). The pericardial fluid pH was 7.34. The median pyrazinamide peak concentrations were 42.93 mg/L versus a median MIC of 800 mg/L at pH 7.34 (p < 0.0001). There was no significant difference between isoniazid pericardial fluid and plasma concentrations, and isoniazid peak concentrations were above MIC. This is the first study to measure anti-TB drug concentrations, pH and protein in the pericardial TB fluid. Pericardial concentrations of the key sterilizing drugs for TB were below MIC, which could contribute to poor outcomes. A new regimen that overcomes these limitations might need to be crafted.
Keywords: Rifampicin, Ethambutol, Pyrazinamide, Drug penetration, Pericardium, Protein binding
Highlights
-
•
The amounts of antibiotics such as rifampicin, ethambutol, pyrazinamide and isoniazid used to treat TB pericarditis that enter pericardial fluid have up to now been unknown
-
•
The study found that the pH in pericardial fluid was alkaline, which would mean that pyrazinamide effect would be compromised.
-
•
The protein content in pericardial fluid was high, which would lead to low non-protein bound rifampicin and ethambutol concentrations
-
•
The concentrations of rifampicin, ethambutol and pyrazinamide in pericardial were dramatically low and below the MICs of Mycobacterium tuberculosis
1. Introduction
Tuberculous pericarditis is a common medical condition in many countries in Africa and Asia (Mayosi et al., 2005, Mayosi et al., 2006, Cherian, 2004). Despite treatment, one in every four patients is dead within six months, and close to one in two patients with human immunodeficiency virus (HIV) co-infection are dead within six months (Mayosi et al., 2005). We have recently completed the Investigation of the Management of Pericarditis (IMPI) study, a randomized controlled trial examining the effects of either prednisolone or Mycobacterium indicus pranii or both and the standard regimen (isoniazid, rifampin, ethambutol and pyrazinamide) in 1400 patients (Mayosi et al., 2014). The primary composite outcome (death, cardiac tamponade requiring pericardiocentesis, or constrictive pericarditis) rate was approximately 15 events per 100 patient-years of follow-up; the main causes of death were either the pericarditis or disseminated tuberculosis (TB) in greater than 42% of deaths. These outcomes were encountered despite adherence to treatment in about 90% of patients, and are thus attributable to primary failure of standard therapy. The reasons for therapy failure are unclear, but could include high bacterial burden, concurrent immunodeficiency, or treatment factors that could be summarized as antimicrobial pharmacokinetics/pharmacodynamics (PK/PD) (Gumbo et al., 2015a, Craig, 1998). PK/PD science relates drug concentration of antibiotics at site of infection to microbial kill and resistance emergence; non-protein bound concentrations are central to pharmacodynamics effects (Gumbo et al., 2015a, Craig, 1998, Gumbo, 2011, Ambrose et al., 2007, Pasipanodya and Gumbo, 2011, Zeitlinger et al., 2011).
First-line anti-TB drugs exert their effect by binding directly to the bacterial targets inside the Mycobacterium tuberculosis (Mtb) cells. Only non-protein bound drug concentrations enter bacteria. Therefore, in TB pericarditis each antibiotic must penetrate several layers of tissue to achieve adequate free-drug concentrations in the pericardial space. The antibacterial effect of rifampicin, isoniazid, ethambutol and pyrazinamide is closely linked to specific peak concentration, 0–24 h area under the concentration–time curve (AUC0–24), and minimum inhibitory concentration (MIC) (Gumbo et al., 2007a, Gumbo et al., 2007b, Gumbo et al., 2009, Pasipanodya et al., 2013, Srivastava et al., 2010, Chigutsa et al., 2015). Utilizing standard treatment of pulmonary TB, recommended doses barely achieve these target concentrations (Gumbo et al., 2007a, Gumbo et al., 2007b, Gumbo et al., 2009, Pasipanodya et al., 2013, Srivastava et al., 2010, Chigutsa et al., 2015). Moreover, despite administration of recommended doses, between-patient pharmacokinetic variability leads substantial proportions of patients to metabolize some of the drugs more extensively than others, who may end up on effective monotherapy, therapy failure, and acquired drug resistance (Pasipanodya et al., 2013, Srivastava et al., 2011a, Pasipanodya et al., 2012). It is unknown to what extent these drugs penetrate into pericardial space, or the variability thereof. We performed two sets of sub-studies during the ongoing IMPI registry (Mayosi et al., 2006, Mayosi et al., 2008), a pilot study to identify the rifampicin concentrations in paired plasma and pericardial fluid samples, followed by a second more intensive pericardial fluid pharmacokinetic sampling study for all four anti-TB drugs. The findings of these studies are reported herein.
2. Methods
2.1. Ethics
Institutional review board and ethical considerations were published before in publications on the baseline characteristics and outcomes of patients enrolled in the IMPI registry. (Mayosi et al., 2006, Mayosi et al., 2008) For each study, either the registry or clinical trial, approval was received from the appropriate South African regulatory authority and by the University of Cape Town Ethics Committee. Informed consent forms were available in isiXhosa, Afrikaans, and English. The IMPI trial was registered at clinicaltrials.gov (NCT00810849).
2.2. Setting
Both the pilot and intensive sampling pharmacokinetic sub-studies were performed at Groote Schuur Hospital in Observatory, Cape Town, South Africa. The pilot study was performed between June and December 2011. The intensive pharmacokinetic sampling study was performed between October 2012 and June 2014.
2.3. Case Definition
Patients 18 years or older, were enrolled if they had definite or probable pericardial TB, based on a TB pericarditis case definition (Mayosi et al., 2005, Mayosi et al., 2014, Reuter and Burgess, 2006, Pandie et al., 2014). Definite TB pericarditis patients were those with confirmed pericardial TB, based on either the presence of acid and alcohol fast bacilli on microscopy of pericardial fluid, presence of Mtb by culture or by nucleic acid test in pericardial fluid or tissue, or histologic evidence of caseating granulomata. Probable TB pericarditis patients did not have evidence of these microbial, nucleic acid, or histological features, in pericardial fluid or tissue, but had evidence of TB at another anatomical location and/or presence of a lymphocytic pericardial exudate with elevated adenine deaminase or a Tygerberg TB Pericarditis Diagnostic Index Score ≥ 6 (Mayosi et al., 2005, Mayosi et al., 2014, Reuter and Burgess, 2006, Pandie et al., 2014).
2.4. Pilot Study
The pilot study sample size was set based on precedence of some of our prior pharmacokinetic studies of anti-TB agents (Hall et al., 2012). Consecutive patients scheduled to undergo pericardiocentesis as part of their routine care provided informed written consent. Patients had the four-drug standard therapy regimen administered under supervision of a study nurse, after which they underwent pericardiocentesis. At the time of study, most patients were treated with Rifafour®, each containing rifampin 150 mg, isoniazid 75 mg, pyrazinamide 400 mg, and ethambutol 275 mg (fixed dose combination or FDC). Without interfering with patients' scheduled time for pericardiocentesis, the timing of drug therapy was set to allow for sampling time points scattered through the 24 h dosing interval. At each of several time points, simultaneous blood and pericardial fluid samples were drawn, placed on ice, processed, and stored at − 80 °C.
2.5. Intensive Sampling Study
Demographic data, clinical information and laboratory data were collected, including age, sex, weight, HIV status, and concomitant medication. Serum total protein and globulin were taken at the time of enrolment. Echocardiography, followed by fluoroscopy guided pericardiocentesis using the Seldinger technique was performed. The pericardial effusion was drained to “dryness” (to the point of no residual drainage) and a pigtail catheter was left in-situ. Antibiotic therapy was administered under direct supervision, after which simultaneous blood and pericardial fluid samples were collected at 0 (before drug administration), 30 min, 1, 2, 3, 5, 8 and 24 h post-dose in heparinized tubes. The samples were also collected in ethylenediaminetetraacetic acid (EDTA), centrifuged at 2000 relative centrifugal force for 10 min, after which supernatant was collected and stored at − 80 °C.
2.6. Drug Assays
The determination of concentrations in plasma and pericardial fluid was accomplished using validated tandem mass spectrometry high- performance liquid chromatography-tandem mass spectrometry (LC-MS/MS). The methods were validated in plasma and cross validated in pericardial fluid.
For quantification of isoniazid in 20 μL of K3EDTA human plasma, protein precipitation with 0.1% acetic acid in acetonitrile was followed by chromatographic separation on a Kinetex Hilic C18, 2.6 μm column. For quantification of pyrazinamide from 20 μL human plasma, protein precipitation was achieved using a mixture of methanol and acetonitrile, and chromatographic separation on an Atlantis T3 C18, 3 μm column under isocratic conditions. For rifampicin and ethambutol quantification from 20 μL plasma, protein precipitation was achieved with acetonitrile, followed by separation on a Discovery C18, 5 μ, column for rifampicin, and a Phenomenex Onyx Monolith C18 (100 × 3.0 mm) column for ethambutol. An AB Sciex API 3000 mass spectrometer was operated in the multiple reactions monitoring (MRM) mode, monitoring the transitions 138.1 to 79.1 for isoniazid, 124.0 to 81.4 for pyrazinamide, 823.4 to 791.4 for rifampicin, and 205.2 to 116.2 for ethambutol.
The assays were validated over the concentration range of 0.102 μg/mL to 26.0 μg/mL for isoniazid, 0.203 μg/mL to 81.1 μg/mL for pyrazinamide, 0.117 μg/mL to 30 μg/mL for rifampicin, and 0.0844 μg/mL to 5.40 μg/mL for ethambutol. The between-day mean percentage accuracies for isoniazid were 101.0%, 103.2%, and 96.0%, those for pyrazinamide were 96.5%, 95.5% and 98.2%, for rifampicin 102.2%, 99.6% and 101.7%, and those for ethambutol were 98.5%, 101.3% and 99.0% at the high, medium, and low quality control (QC) levels, respectively. The level of precision had a coefficient of variation (% CV) of less than 13% at low, medium, and high QC levels for all analytes.
2.7. Pharmacokinetic Analyses
Our pharmacokinetic studies of several hundred patients in the same Cape Town population as well as from elsewhere in the past have identified rifampicin, isoniazid, and pyrazinamide as one-compartment model drugs, and ethambutol and isoniazid as two compartment drugs (Pasipanodya et al., 2013, Hall et al., 2012). The pharmacokinetics of each of these drugs was modeled and individual drug exposures were estimated in ADAPT software (Biomedical Simulations Resource, University of Southern California) assuming the same number of compartments identified in past studies. To identify clearance and volume of distribution in the pericardial space, the pericardial drug concentrations were modeled separately in ADAPT and the full 24 h concentration–time curve identified from the pharmacokinetic model. These were then used to calculate the full AUC0–24 in the pericardium.
2.8. Comparison of Concentrations Achieved to MICs
We compared drug concentrations achieved in pericardium to their MICs encountered in drug susceptible Mtb isolates in Cape Town, based on our prior publications (Chigutsa et al., 2015, Gumbo et al., 2014a, Gumbo et al., 2014b). Isolates from prior studies were used because a large number is needed in order to accurately identify the correct MIC distribution. Because the non-protein bound peak-to-MIC and AUC-to-MIC ratios of the four first-line drugs determine microbiological and clinical outcomes, and because it is a central tenant of PK/PD science to adjust or calculate the non-protein bound concentrations at site of infection, we calculated the non-bound drug concentrations in pericardial fluid assuming 90% of rifampicin and 30% of ethambutol are protein bound (Gumbo et al., 2015a, Craig, 1998, Gumbo, 2011, Ambrose et al., 2007, Pasipanodya and Gumbo, 2011, Zeitlinger et al., 2011, Gumbo et al., 2007a, Gumbo et al., 2007b, Gumbo et al., 2009, Pasipanodya et al., 2013, Srivastava et al., 2010, Chigutsa et al., 2015, Te Brake et al., 2015, Woo et al., 1996). These drugs are bound to albumin (Woo et al., 1996). In normal pericardial fluid, albumin/total protein ratios are similar to plasma; the albumin ratio is higher in the pericardial fluid exudate during infection (Ben-Horin et al., 2005, Kopcinovic and Culej, 2014). Since isoniazid and pyrazinamide are only 10% protein bound, we used the total concentrations for these drugs (Gumbo, 2011, Woo et al., 1996).
2.9. Statistical Tests
A non-parametric Mann–Whitney test was used to compare peak, AUC and clearances in pericardial fluid and in plasma. Similarly, a non-parametric Mann–Whitney test was used to compare the medians of non-protein bound concentrations and MICs.
2.10. Role of Funding Source
The South African Medical Research Council (MRC), the Lily and Ernst Hausmann Research Trust, and the National Institutes of Health funded the study. These funding agencies were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript.
3. Results
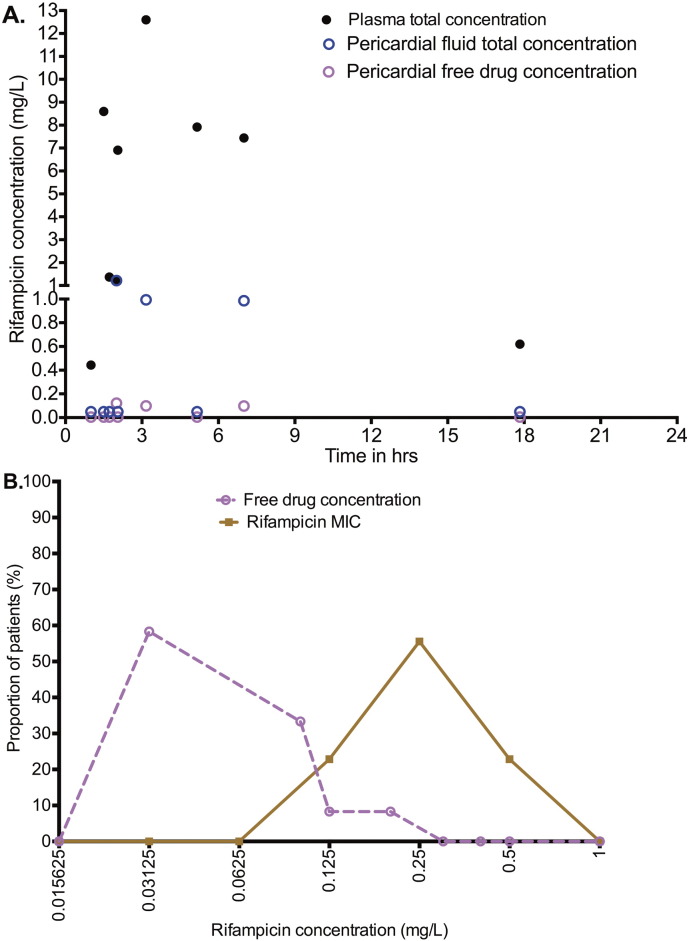
Sixteen patients were enrolled in the pilot study. The clinical and demographic features of these patients are shown in Table 1. Ten (63%) patients were male. Of the 16 patients, 10 were HIV-infected, with the mean CD4 + count shown in Table 1. All patients, apart from one, received four FDC anti-TB tablets (one patient received loose individual tablets), amounting to rifampicin 600 mg a day, isoniazid 300 mg a day, pyrazinamide 1600 mg a day, and ethambutol 1100 mg a day. Blood and pericardial fluid were collected commencing 1 h to 24 h after the dose, as summarized in Table 1. The paired plasma and pericardial rifampicin concentrations are shown in Fig. 1A. The dramatic difference illustrated in the figure, is summarized by ratio of rifampicin pericardial fluid concentration to plasma concentration of 0.19 ± 0.33 for paired samples. Fig. 1B compares the distribution of all the non-protein bound rifampicin concentrations to rifampicin MICs in Western Cape clinical isolates, and shows that none of the pericardial concentrations, at any sampling time-point, was above the MICs. The median concentration was 0.125 mg/L and was lower than the median MIC 0.208 mg/L (p = 0.001). However, in the pilot study the timing of sampling with respect to the antibiotic dose was varied, which could explain some of the inter-individual variability of the results, and thus a more intensive sampling procedure was planned for prior to dose recommendations.
Table 1.
Clinical and demographic characteristics in pilot study patients.
| Variable | Mean | Range |
|---|---|---|
| Age in years | 35.2 | 19.2–48.9 |
| Time from dose to sample collection (hr) | 4.82 | 1–24 |
| Diastolic blood pressure (mmHg) | 69.6 | 60–90 |
| Hemoglobin (g/L) | 9.7 | 5.9–14.3 |
| White cell count × 103cells/μL | 5.73 | 3.6–9.8 |
| CD4 + cells/mL | 307 | 263a |
| Adenosine deaminase (U/L) | 56.9 | 70–133.2 |
| Pericardial fluid total protein (g/L) | 56.9 | 38–75 |
Standard deviation.
Fig. 1.
Rifampin concentrations in plasma and pericardium in pilot study.
A. The figure shows that at all time points the total rifampicin pericardial concentrations were dramatically lower than in the paired plasma. Derived free drug concentrations were even lower.
B. When all the concentrations at any time point were compared to the MICs, virtually all were below the MICs. Thus, no non-protein bound concentration was higher than MIC in pericardial space.
The pilot study results were used to identify the best sampling times to assess rifampicin and companion drugs concentrations over a 24-h period. Eleven patients were enrolled in this more intensive pharmacokinetic sampling study during the IMPI trial. Their clinical and demographic characteristics are shown in Table 2. Five patients were allocated to prednisolone, so that patients were split in the middle between those receiving placebo or immunotherapy, similar to the larger IMPI trial. All eleven patients received four FDC tablets, amounting to rifampin 600 mg, isoniazid 300 mg, ethambutol 1100 mg and pyrazinamide 1600 mg each day. One patient was also on antiretroviral therapy.
Table 2.
Clinical and demographic characteristics in patients who underwent intensive pharmacokinetic sampling.
| Parameter | Mean or proportion | Range or % |
|---|---|---|
| Sex: male | 5 | 45% |
| Age in years | 37.02 | 23.98–58.76 |
| Weight in kg | 58.36 | 40–82 |
| Acid-fast bacilli on microscopy | 9 | 82% |
| HIV-infected | 8 | 73% |
| Duration of symptoms in weeks | 26.77 | 3–60 |
| Blood counts and concentrations | ||
| CD4 + count cells/mL | 251 | 42–874 |
| CD4 + ≤ 200 cells/mL | 7 | 64% |
| Creatinine μmol/L | 87.00 | 20–257 |
| Globulin in g/L | 47.55 | 30–56 |
| Pericardial fluid concentrations | ||
| Total protein (g/L) | 61.18 | 50–70 |
| Adenosine deaminase (U/L) | 77.70 | 25.90–133.00 |
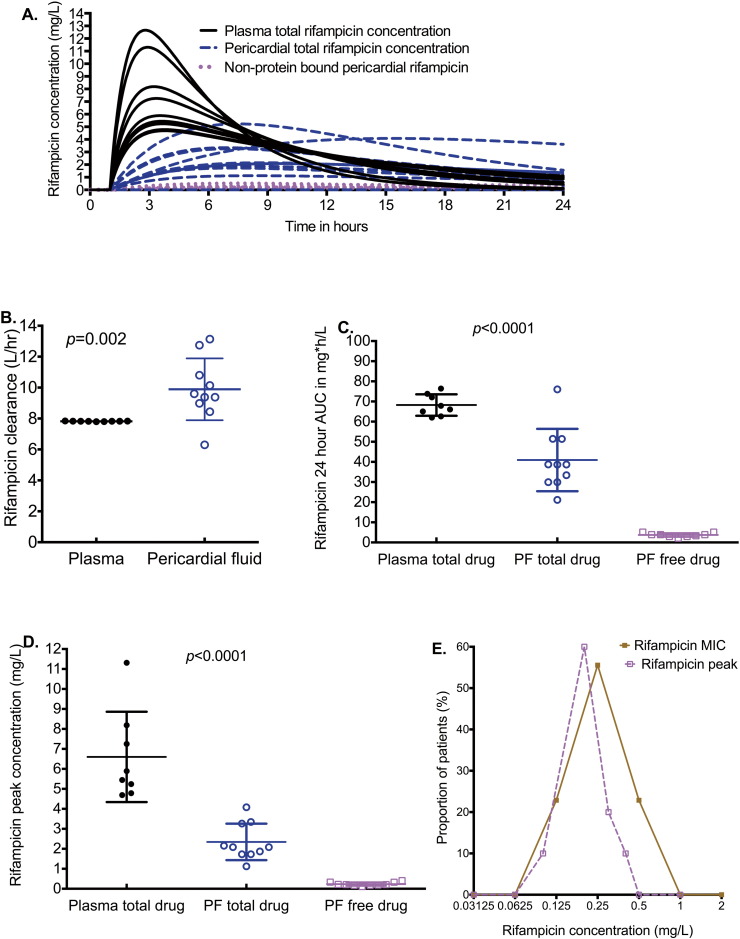
The rifampicin concentrations achieved in patients in both the plasma and pericardium are shown in Fig. 2A. The time to peak concentration in pericardial fluid was a median (range) of 8.18 (6.85–15.40) hours compared to 3.44 (2.76–3.77) hours in blood (p < 0.0001). The shape of the concentration time curves in Fig. 2A is characterized by high plasma peak concentrations and very low and flattened peak concentrations in the pericardium. The shape is due to both delay and decrease in penetration from the blood into pericardial fluid, as well as higher rifampicin clearance from pericardial fluid compared to the blood (Fig. 2A–B). As regards to clearance (Fig. 2B), all patients except one had higher clearances in pericardium than in blood, and the median clearances differed significantly (p = 0.002). The non-protein bound rifampicin concentrations in pericardial fluid are also shown in Fig. 2A–D, which demonstrates very low effective rifampicin concentrations in pericardial fluid. A comparison of free rifampicin peak concentrations to MICs encountered in isolates is shown in Fig. 2E, which shows that the median rifampicin peak concentrations in pericardial fluid, were lower than the median MIC (p = 0.001).
Fig. 2.
Rifampicin concentrations in the blood and pericardial fluid.
The p-values shown throughout are for the Mann–Whitney test, and compared the total drug concentrations in plasma versus pericardial fluid, and not the free drug.
A. The concentration–time profiles of rifampicin in pericardium and in the blood over a 24 h dosing interval. The time to maximum concentration was longer in pericardial fluid, while the peak concentrations were blunted.
B. Clearance of rifampicin from pericardial fluid (PF) and plasma for each patient. The median clearance from the blood was 7.82 (range: 7.79–7.84) L/h while that in pericardial fluid was 9.49 (range: 6.30–13.14) L/h.
C. The 0–24 h AUC for total drug concentrations was lower in pericardial fluid than in plasma; the free drug concentrations were even lower.
D. Total rifampicin peak concentrations achieved in pericardial fluid are lower than in plasma; free concentrations are substantially lower.
E. Comparison of free rifampicin peak concentrations to MICs demonstrates that the maximum concentrations achieved in pericardial fluid are lower than MICs. Thus, as the concentrations are cleared from the pericardium the gap between drug concentration and MIC rapidly widens.
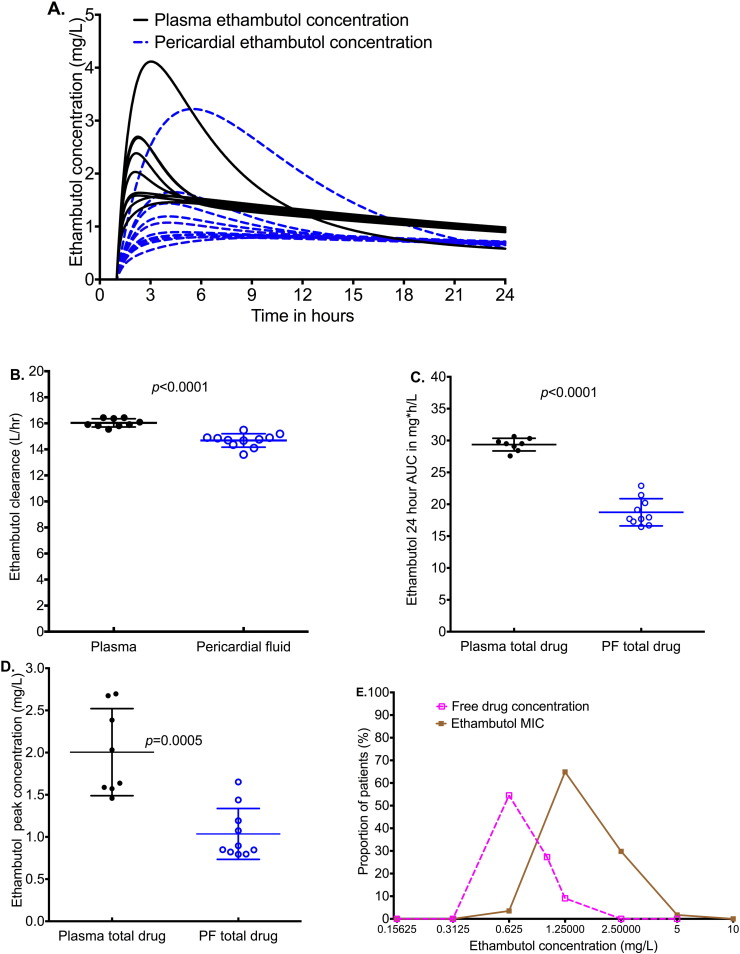
The ethambutol concentrations achieved in pericardial fluid are shown in Fig. 3A. The figure shows that significantly lower ethambutol concentrations were achieved in pericardium compared to plasma (p < 0.001). Fig. 3A shows similar shapes and time to maximum concentration between plasma and pericardial fluid. Despite the lower clearance rates in pericardium (Fig. 3B), the ethambutol AUC0–24 in the pericardium was lower than plasma (Fig. 3C). In addition, peak concentrations were also lower in pericardium (Fig. 3D). The lower peak and AUC0–24 in pericardial fluid compared to plasma, despite the lower clearance in pericardial fluid, mean that the low concentrations were primarily due to poor penetration. As a result, as shown in Fig. 3E, the non-protein bound ethambutol concentrations fell below the MICs encountered in Western Cape isolates. Indeed, even the total ethambutol peak concentrations were below the MICs, without adjusting for protein binding.
Fig. 3.
Ethambutol concentrations in plasma and pericardial fluid (PF).
The concentration time profiles of ethambutol total concentration over 24 h demonstrate reduced penetration of ethambutol.
Clearance from pericardium was slightly lower than plasma (15.91 versus 14.77 L/h).
Despite the lower clearance from pericardial fluid, the median AUC0–24 in pericardial fluid was 17.83 mg ∗ h/L compared to 29.51 mg ∗ h/L in plasma, a 40% reduction.
The median total ethambutol peak concentration was 0.87 mg/L in pericardial fluid compared to 1.84 mg/L in plasma, and was thus 53% lower. Since ethambutol is 30% protein bound, this means the free peak concentration in pericardium is a median of 0.61 mg/L.
The non-protein bound peak ethambutol concentrations were lower than MICs for most patients.
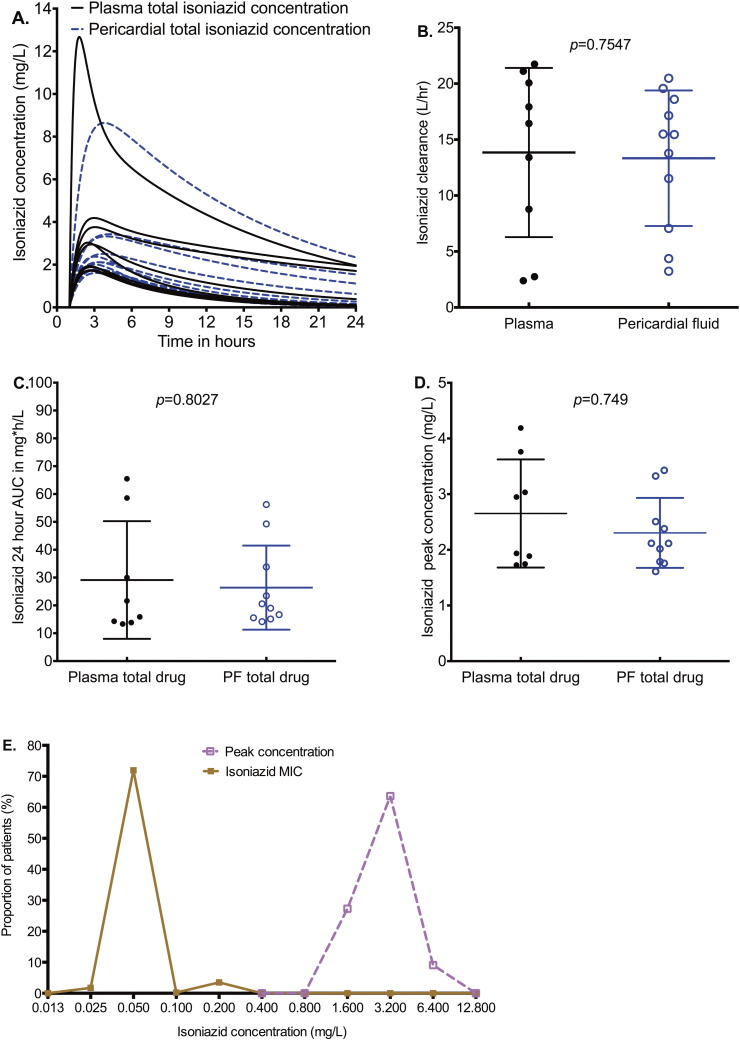
On the other hand, isoniazid concentrations in the pericardial fluid approximated those in the plasma in shape, including time to maximum concentration (Fig. 4A). Indeed, neither the concentration–time curves, nor the peak concentrations, nor the clearances, nor the AUCs differed between pericardial space and plasma (Fig. 4A–D). Since isoniazid is only 10% protein bound, these total concentrations approximate free drug concentrations. A comparison between isoniazid peak concentrations and the MICs encountered in Cape Town, which is shown in Fig. 4E, demonstrates that the isoniazid peak concentrations in pericardial fluid were above MIC. The median peak concentrations were 64-fold higher than the median MIC (p < 0.001).
Fig. 4.
Isoniazid concentrations in plasma and pericardial fluid (PF).
The concentration time profiles of total concentration over 24 h demonstrate matching concentration.
Clearance from pericardium was similar between plasma and pericardial fluid.
The median pericardial fluid AUC0–24 in pericardial fluid was 19.78 mg ∗ h/L compared to 18.75 mg ∗ h/L in plasma.
The median total pericardial peak concentration was 2.12 mg/L in pericardial fluid and 2.44 mg/L in plasma, virtually the same.
Isoniazid penetrated well into pericardial fluid and achieved concentrations of at least 64-fold higher than MICs.
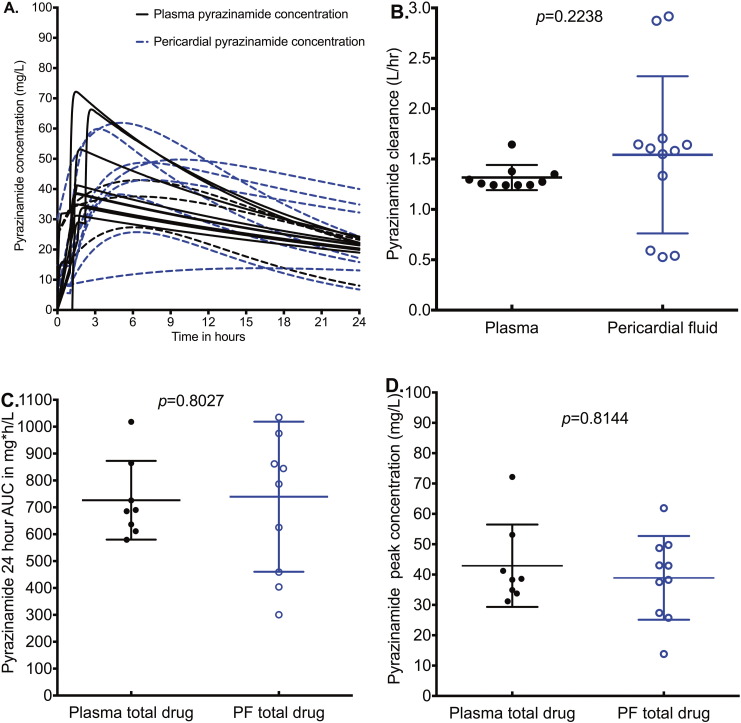
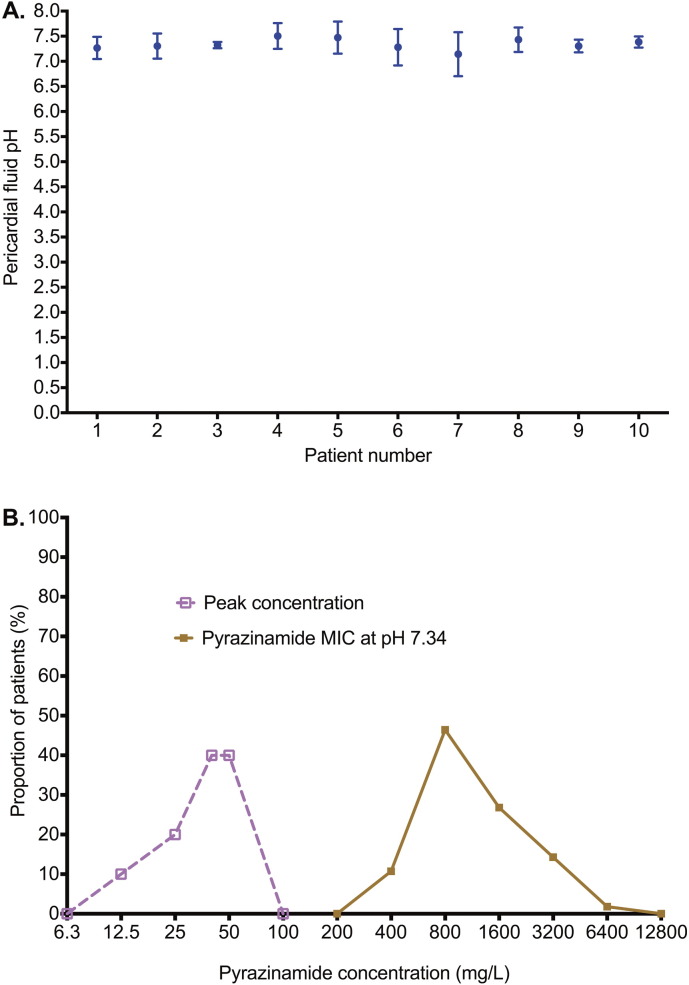
Pyrazinamide concentrations in pericardial fluid also closely mirrored those in plasma. However, Fig. 5A shows that there was a delay to peak concentration in pericardial fluid, compared to plasma. Nevertheless, similar peak concentrations were eventually achieved (even though delayed); the AUCs were similar (Fig. 5B–D). Pyrazinamide requires acidic conditions for activation to kill Mtb and has zero microbial kill at pH ≥ 6.8 (McDermott and Tompsett, 1954, Zhang et al., 2002). Indeed, MIC decreases as pH falls based on the Henderson–Hasselbach relationship (Zhang et al., 2002). We measured the pH in pericardial fluid of 10 of the 11 patients using pH probes. The between-test %CV in pH measurements at 8 different times for the same sample was ± 3.27%, which means that the pH measurements were highly precise. The pH results for the several measurements in each patient's sample are shown in Fig. 6A. The mean (± standard deviation) pericardial fluid pH was 7.34 ± 0.11, exactly the same as physiological pH. Since pyrazinamide MICs are performed at pH 5.9, the actual MICs at pH 7.34 were calculated using the Henderson–Hasselbach equation as described by Zhang et al. (Gumbo et al., 2014a, Zhang et al., 2002, Zhang and Mitchison, 2003). Fig. 6B shows that the pyrazinamide peak concentrations were about 40-times lower than the pH-adjusted MICs, with a median peak of 42·93 mg/L versus a median MIC of 800 mg/L (p < 0.0001).
Fig. 5.
Pyrazinamide concentrations in plasma and pericardial fluid.
While pericardial fluid pyrazinamide peak concentration was somewhat delayed, it nevertheless reached the same level as in plasma.
Pyrazinamide clearance from the pericardium was similar to that from plasma.
The median pericardial fluid AUC0–24 in pericardial fluid was 815.6 mg ∗ h/L compared to 687.90 mg ∗ h/L in plasma.
The median total pericardial peak concentration was 38.45 mg/L in pericardial fluid and 40.59 mg/L in plasma.
Fig. 6.
The pH of pericardial fluid and pyrazinamide MICs.
A. The pH in pericardial fluid in each patient was measured 8 times in triplicate; shown are the mean values and standard deviation. The D'Agostino and Pearson omnibus normality test p-value was 0.991, which means that the pH values in the pericardial fluid were normally distributed. All the pH values were greater than 7.0, and indeed were similar to the pH of 7.365 in the blood, which is slightly alkaline.
B. Pyrazinamide MICs measured at pH 5.9 were used calculate the actual MICs at physiological pH based on the relationship between the active moiety pyrazinoic acid (POA; anion POA−) and HPOA in the Handersen–Hasselbach equation (POA pKa = 2.9), as demonstrated by Zhang et al. (2002). At pH 5.90 the % of the HPOA is 0.1% compared to 0.00363% at pH 7.34, a 27.54-fold change, which is the factor used to calculate the MIC value at pH 7.34 based on that identified at pH 5.9. This leads to an MIC with a median 18.6-fold higher than the median peak concentrations; indeed the highest pyrazinamide peak concentration was 4-fold lower than the lowest MIC.
4. Discussion
We have identified poor penetration and rapid clearance of rifampicin in the infected space of TB pericarditis. There was delayed and decreased penetration from the blood into pericardial fluid, as demonstrated by both the peak concentration achieved and the time to maximum concentration. The reasons for this are unclear, but could include thickened pericardial layers, or fibrosis, among several possibilities. The rapid clearance, compared with plasma is a surprise, given rifampicin's well-known extraction from the blood and metabolism by hepatocytes. We speculate that this is likely due to an active physiological process such as transport out of the pericardium. The consequences, however, are concerning, given that rifampicin, in consort with pyrazinamide, is the primary drug associated with sterilizing effect in the standard TB treatment regimen. We found that the non-protein bound peak concentrations achieved in pericardium were below MICs encountered in Cape Town clinical historical Mtb isolates. AUCs were also very low, which means the AUC-to-MIC ratios were also low. Therefore, there is likely to be little microbial kill from rifampicin at concentrations achieved with current doses in the standard regimen (Gumbo et al., 2015a, Pasipanodya and Gumbo, 2011, Gumbo et al., 2007a, Pasipanodya et al., 2013, Chigutsa et al., 2015). Given the gap between non-protein bound peak and MICs, and the relationship of peak-to-MIC ratios for optimal rifampicin kill and suppression of resistance (Pasipanodya and Gumbo, 2011, Gumbo et al., 2007a, Pasipanodya et al., 2013), it is unlikely that escalating the rifampicin dose alone would be feasible to increase peak-to-MIC ratio to ranges that could provide sterilizing effect in TB pericarditis without causing toxicity, presenting a challenge in treating this condition.
Secondly, the pyrazinamide concentrations were approximately similar to those in plasma. However, this is substantially lower than the concentrations associated with effective microbial kill at the site of infection. In pulmonary epithelial lining fluid, pyrazinamide is concentrated 17–22-fold, compared to plasma, which allows the pyrazinamide to achieve the peak concentrations and AUC-to-MIC ratios associated with optimal sterilizing effect in cavitary TB (Gumbo et al., 2009, Pasipanodya et al., 2013, Conte et al., 1999). Indeed, optimal efficacy is achieved at a pyrazinamide AUC-to-MIC ratio of ~ 210 at site of infection based on hollow fiber system model of TB, which has a quantitative predictive accuracy of 94% of clinical therapeutic values (Gumbo et al., 2009, Pasipanodya et al., 2013, Gumbo et al., 2015b, Gumbo et al., 2015c). Median AUC-to-MIC ratios were ≤ 1 after adjusting for pH. Similarly, the median peak concentration to MIC ratio is unlikely to produce much microbial kill even under optimal pH conditions (Pasipanodya and Gumbo, 2011, Gumbo et al., 2009). These are all the more concerning given that pyrazinamide has no antimicrobial effect at physiological pH and only works at a pH below 6.0 (Gumbo et al., 2009, McDermott and Tompsett, 1954, Zhang et al., 2002, Zhang and Mitchison, 2003). Direct measurement of pericardial fluid pH in the patients revealed that in fact the pH was slightly alkaline and similar to physiologic pH. At this pH, we expect little to no pyrazinamide effect on Mtb in pericardial fluid. Since rifampicin concentrations are also low, and the sterilizing effect of the current regimen is driven by the synergistic effect of these two drugs, this suggests that there may be little to no sterilizing effect during treatment of pericardial TB (Pasipanodya et al., 2013, Chigutsa et al., 2015).
Thirdly, ethambutol concentrations were also lower in pericardial fluid compared to plasma concentrations, due to reduced penetration. Similar to rifampicin, pyrazinamide, and isoniazid, ethambutol penetration from plasma into pericardial fluid varied by as much as 50% between patients. This additional variability needs to be taken into account when designing optimal doses. The result of the low ethambutol concentrations, when added to low rifampicin and pyrazinamide concentrations, means that effectively the drug concentrations of the majority of drugs in the regimen are suboptimal.
Fourthly, isoniazid concentrations in pericardium appear to be the most favorable. Indeed it achieved similar concentrations to those in plasma, to achieve both peak and AUCs in the range associated with positive outcomes in other types of TB. In addition, the clearance was similar to that from plasma; thus isoniazid pharmacokinetics in this compartment can easily be inferred from those in the blood. From the data identified in this pilot study, the doses of isoniazid for the treatment of TB pericarditis likely need no change.
Given the foregoing it means the current rifampicin, isoniazid, ethambutol, and pyrazinamide combination regimen is a “one trick pony”. Only isoniazid seems to penetrate into pericardial fluid to adequate concentrations. However, we have shown elsewhere that at low rifampicin concentrations such as those in pericardial fluid, isoniazid antagonizes rifampicin efficacy in a concentration-dependent fashion (Chigutsa et al., 2015, Conte et al., 1999, Srivastava et al., 2011b). Thus one consequence of a low rifampicin concentration but adequate isoniazid concentration could be drug antagonism, further compromising the regimen. We likely have an anti-TB drug regimen that is of limited utility in TB pericarditis, based on the poor penetration of the key sterilizing effect drugs. Indeed, in the IMPI trial, and IMPI registry studies, there were high rates of mortality from TB pericarditis and disseminated TB, encountered while patients are on therapy. Given that we have now shown that proven TB pericarditis is a high bacillary burden disease, with about 106 colony forming units per milliliter, and that TB pericarditis mortality is higher at CD4 + counts below 200 cells/mL (Pasipanodya et al., nd), it means that we are unlikely to derive benefit from the immune system to sterilize the pericardial space of this large bacterial burden. Thus antibiotics with high efficacy are needed. For drugs such as rifampicin and pyrazinamide, the gap could be too wide to be achieved by further dose optimization. Nevertheless, attempts at dose optimization for these drugs should still be made. We propose that a new regimen specific to TB pericarditis that is developed using the PK/PD aspects specific to TB pericarditis, is needed. Indeed, there is need for drugs that are rapidly bactericidal and can sterilize the Mtb encountered in TB pericarditis within weeks rather than months.
Our study has several limitations. First is the small sample size. It is possible that with larger studies different results could be obtained. However, given the need to leave a pigtail catheter for the intensive pharmacokinetic sampling, it was important to limit the number of patients exposed to this procedure. Thus, our results should be interpreted with caution. However, given the dramatic nature of the results, and that rifampicin was actually studied in a total of 27 patients and showed the same results, it is very likely that the same results will be obtained in larger studies. Second, we did not measure the MICs directly from the patients in the study. However, given the numbers of patients, the number of Mtb isolates would have been too small to identify a good enough distribution, and we would still have had to rely on MICs from larger studies. Third, use of steroids could have altered the ability of antibiotics to penetrate into pericardium. However, there were no apparent differences in antibiotic concentrations between patients who received steroids and those who did not.
In summary, this is the first study of its kind to measure anti-TB drug concentrations in pericardial fluid. The study also related these concentrations to protein binding, and the pH encountered in TB pericardial fluid. Concentrations of rifampicin, ethambutol and pyrazinamide were dramatically lower relative to MICs.
Author Contributions
Conception and design: T. Gumbo, B.M. Mayosi.
Development of methodology: J. Shenje, F. I. Adimora-Nweke, I. L. Ross, L. Wiesner, H. M. McIlleron, T. Gumbo, B. M. Mayosi.
Acquisition of data: J. Shenje, F. I. Adimora-Nweke, I. L. Ross, M. Ntsekhe, L. Wiesner, A. Deffur, H. M. McIlleron, J. Pasipanodya, T. Gumbo, B. M. Mayosi.
Analysis and interpretation of data: J. Shenje, I. L. Ross, T. Gumbo, B. M. Mayosi.
Writing, review and/or revision of the manuscript: J. Shenje, I. L. Ross, M. Ntsekhe, L. Wiesner, H. M. McIlleron, T. Gumbo, B. M. Mayosi.
Study supervision: I. L. Ross, H. M. McIlleron, T. Gumbo, B. M. Mayosi.
Acknowledgments
Funding for this study was obtained from the South African Medical Research Council for self-initiated research from November 2012 to April 2015 and the Lily and Ernst Hausmann Research Trust to Bongani Mayosi, and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI079497) to Tawanda Gumbo.
References
- Ambrose P.G., Bhavnani S.M., Rubino C.M., Louie A., Gumbo T., Forrest A., Drusano G.L. Pharmacokinetics–pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 2007;44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- Ben-Horin S., Shinfeld A., Kachel E. The composition of normal pericardial fluid and its implications for diagnosing pericardial effusions. Am. J. Med. 2005;118:636–640. doi: 10.1016/j.amjmed.2005.01.066. [DOI] [PubMed] [Google Scholar]
- Cherian G. Diagnosis of tuberculous etiology in pericardial effusions. Postgrad. Med. J. 2004;80:262–266. doi: 10.1136/pgmj.2003.013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigutsa E., Pasipanodya J.G., Visser M.E. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob. Agents Chemother. 2015;59:38–45. doi: 10.1128/AAC.03931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte J.E., Jr., Golden J.A., Duncan S. Intrapulmonary concentrations of pyrazinamide. Antimicrob. Agents Chemother. 1999;43:1329–1333. doi: 10.1128/aac.43.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W.A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- Gumbo T. Chemotherapy of Tuberculosis, Mycobacterium avium Complex Disease, and Leprosy. In: Brunton L.L., Chabner B., Knollmann B., editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw Hill Medical; New York, NY: 2011. [Google Scholar]
- Gumbo T., Louie A., Deziel M.R. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 2007;51:3781–3788. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbo T., Louie A., Liu W. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob. Agents Chemother. 2007;51:2329–2336. doi: 10.1128/AAC.00185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbo T., Siyambalapitiyage Dona C.S., Meek C. Pharmacokinetics–pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob. Agents Chemother. 2009;53:3197–3204. doi: 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbo T., Chigutsa E., Pasipanodya J. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J. Antimicrob. Chemother. 2014;69:2420–2425. doi: 10.1093/jac/dku136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbo T., Pasipanodya J.G., Wash P. Redefining multidrug-resistant tuberculosis based on clinical response to combination therapy. Antimicrob. Agents Chemother. 2014;58:6111–6115. doi: 10.1128/AAC.03549-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbo T., Angulo-Barturen I., Ferrer-Bazaga S. Pharmacokinetic–pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J. Infect. Dis. 2015;211(Suppl. 3):S96–S106. doi: 10.1093/infdis/jiu610. [DOI] [PubMed] [Google Scholar]
- Gumbo T., Pasipanodya J.G., Nuermberger E., Romero K., Hanna D. Correlations between the hollow fiber model of tuberculosis and therapeutic events in tuberculosis patients: learn and confirm. Clin. Infect. Dis. 2015;61(Suppl 1):S18–S24. doi: 10.1093/cid/civ426. [DOI] [PubMed] [Google Scholar]
- Gumbo T., Pasipanodya J.G., Romero K., Hanna D., Nuermberger E. Forecasting accuracy of the hollow fiber model of tuberculosis for clinical therapeutic outcomes. Clin. Infect. Dis. 2015;61(Suppl 1):S25–S31. doi: 10.1093/cid/civ427. [DOI] [PubMed] [Google Scholar]
- Hall R.G., Swancutt M.A., Meek C., Leff R., Gumbo T. Ethambutol pharmacokinetic variability is linked to body mass in overweight, obese, and extremely obese people. Antimicrob. Agents Chemother. 2012;56:1502–1507. doi: 10.1128/AAC.05623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcinovic L.M., Culej J. Pleural, peritoneal and pericardial effusions — a biochemical approach. Biochem. Med. (Zagreb) 2014;24:123–137. doi: 10.11613/BM.2014.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayosi B.M., Burgess L.J., Doubell A.F. Tuberculous pericarditis. Circulation. 2005;112:3608–3616. doi: 10.1161/CIRCULATIONAHA.105.543066. [DOI] [PubMed] [Google Scholar]
- Mayosi B.M., Wiysonge C.S., Ntsekhe M. Clinical characteristics and initial management of patients with tuberculous pericarditis in the HIV era: the Investigation of the Management of Pericarditis in Africa (IMPI Africa) registry. BMC Infect. Dis. 2006;6:2. doi: 10.1186/1471-2334-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayosi B.M., Wiysonge C.S., Ntsekhe M. Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. S. Afr. Med. J. 2008;98:36–40. [PubMed] [Google Scholar]
- Mayosi B.M., Ntsekhe M., Bosch J. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N. Engl. J. Med. 2014;371:1121–1130. doi: 10.1056/NEJMoa1407380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott W., Tompsett R. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am. Rev. Tuberc. 1954;70:748–754. doi: 10.1164/art.1954.70.4.748. [DOI] [PubMed] [Google Scholar]
- Pandie S., Peter J.G., Kerbelker Z.S. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-gamma in a high burden setting: a prospective study. BMC Med. 2014;12:101. doi: 10.1186/1741-7015-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasipanodya J., Gumbo T. An oracle: antituberculosis pharmacokinetics–pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob. Agents Chemother. 2011;55:24–34. doi: 10.1128/AAC.00749-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasipanodya J.G., Srivastava S., Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin. Infect. Dis. 2012;55:169–177. doi: 10.1093/cid/cis353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasipanodya J.G., McIlleron H., Burger A. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J. Infect. Dis. 2013;208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasipanodya JG, Mubanga M, Ntsekhe N, Pandie P, Beki T, Magazi BT, Gumedze F, Myer L, Gumbo T, Mayosi BM. Tuberculous pericarditis is multibacillary and bacterial burden drives high mortality. EBiomed. [DOI] [PMC free article] [PubMed]
- Reuter H., Burgess L., van V.W. Diagnosing tuberculous pericarditis. QJM. 2006;99:827–839. doi: 10.1093/qjmed/hcl123. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Musuka S., Sherman C. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J. Infect. Dis. 2010;201:1225–1231. doi: 10.1086/651377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Pasipanodya J.G., Meek C. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J. Infect. Dis. 2011;204:1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Sherman C., Meek C., Leff R., Gumbo T. Pharmacokinetic mismatch does not lead to emergence of isoniazid- or rifampin-resistant Mycobacterium tuberculosis but to better antimicrobial effect: a new paradigm for antituberculosis drug scheduling. Antimicrob. Agents Chemother. 2011;55:5085–5089. doi: 10.1128/AAC.00269-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Brake L.H., Ruslami R., Later-Nijland H. Exposure to total and protein-unbound rifampin is not affected by malnutrition in indonesian tuberculosis patients. Antimicrob. Agents Chemother. 2015;59:3233–3239. doi: 10.1128/AAC.03485-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J., Cheung W., Chan R. In vitro protein binding characteristics of isoniazid, rifampicin, and pyrazinamide to whole plasma, albumin, and alpha-1-acid glycoprotein. Clin. Biochem. 1996;29:175–177. doi: 10.1016/0009-9120(95)02024-1. [DOI] [PubMed] [Google Scholar]
- Zeitlinger M.A., Derendorf H., Mouton J.W. Protein binding: do we ever learn? Antimicrob. Agents Chemother. 2011;55:3067–3074. doi: 10.1128/AAC.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y1., Mitchison D. The curious characteristics of pyrazinamide: a review. Int. J. Tuberc. Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- Zhang Y., Permar S., Sun Z. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J. Med. Microbiol. 2002;51:42–49. doi: 10.1099/0022-1317-51-1-42. [DOI] [PubMed] [Google Scholar]