Abstract
Background
Although medical management of patients with coronary artery disease (CAD) is often based on scientific guidelines, a number of everyday clinical situations are not specifically covered by recommendations or the level of evidence is low. The aim of this study was to assess practice patterns regarding routine management of patients with stable CAD.
Methods
A survey comprising six questions on two clinical scenarios regarding stable CAD management was sent to 345 cardiologists from the Nord-Pas-de-Calais Region (France). We first assessed practice patterns globally and then searched for associations with physician characteristics (age, gender, sub-specialty, and type of practice).
Findings
The response rate was 92%. Regarding management of asymptomatic CAD, 86% of the cardiologists performed routine exercise testing, before which, 69% withdrew β-blockers. After a positive exercise test, 26% immediately performed coronary angiography and 67%, further imaging tests. In the absence of left ventricular dysfunction or history of myocardial infarction, routine β-blocker prescription for stable CAD was selected by 43%. When anticoagulation was needed for atrial fibrillation, 41% initiated direct oral anticoagulants rather than vitamin-K antagonists and 50% combined aspirin with anticoagulants. For recurrent stable angina in patients with known CAD, 24% performed coronary angiography directly, 49% requested a stress test, and 27% opted for medical therapy without further diagnostic testing. Age, gender of the cardiologist, academic environment, and practice of interventional cardiology were associated with certain management patterns.
Interpretation
When not guided by high-level recommendations, practice patterns for routine clinical situations in stable CAD vary considerably. Future clinical trials should address these clinical interrogations.
Keywords: Coronary Artery Disease, Angina, Exercise Testing, Coronary Angiography, Beta-blockers, Anticoagulation
Highlights
-
•
When not guided by clear recommendations, routine decisions for CAD patients vary considerably among cardiologists
-
•
Although the final decision is partly associated with cardiologists' profiles, it remains largely unexplained
In an attempt to assess practice patterns regarding routine management of patients with stable coronary artery disease, a survey was sent to all cardiologists of a geographical area in France. The response rate was high (92%). Our results show that practice patterns vary considerably when not guided by high-level recommendations. Overall, cardiologists were more likely to use diagnostic tests or therapies despite the absence of a rational scientific basis, than to adopt more ‘conservative’ attitudes. Although certain physician characteristics (age, gender, sub-specialty, and type of practice) were associated with management patterns, the final decision remains largely unexplained.
1. Introduction
Coronary artery disease (CAD) is a leading cause of mortality and morbidity worldwide (Roth et al., 2015). The medical management of CAD patients is increasingly dictated by clinical practice guidelines issued by experts and scientific authorities (Fihn et al., 2012, Montalescot et al., 2013, Steg et al., 2012, O'Gara et al., 2013, Windecker et al., 2014). However, certain clinical decisions, including routine decisions, are not specifically covered by guidelines or the level of evidence is low (Tricoci et al., 2009). Examples of these everyday clinical situations are: routine exercise testing in asymptomatic patients with known CAD, the management of asymptomatic CAD patients with positive exercise tests, the management of CAD patients with recurrent chest pain, or the management of such patients with atrial fibrillation requiring anticoagulation. In these cases, the medical choice may depend primarily on the physician's opinion, personal experience and preferences. There is currently a paucity of literature addressing these issues, and little is known about the variability in practice among cardiologists. We therefore designed the present study to: (1) assess practice patterns regarding the routine management of patients with stable CAD; and (2) analyze whether several variables (age and gender of the cardiologist, type of cardiology practice, etc.) are associated with specific medical decisions. To this end, the cardiologists of a geographical region of France, where the prevalence of cardiovascular disease is high (Tunstall-Pedoe et al., 1994), were all invited to take part in a case vignette-based survey (Tunstall-Pedoe et al., 1994).
2. Methods
2.1. Survey Design
We conducted a cross-sectional survey of cardiologists in the Nord-Pas-de-Calais Region (population, 4.05 million) of France. A mailing list of cardiologists actively working in the area was built up from the list of physicians certified in cardiology (N = 414) and cross-checked with professional directories. Physicians working solely as pediatric cardiologists and those working solely as vascular physicians were not included in the survey. Physicians with a cardiology certification but for whom clinical cardiology was not the primary activity were also excluded, as were those who had recently retired. We ended up with a list of 345 cardiologists who were the target population of the present study. The survey was sent out in December 2014 using the French Postal Service. A reminder notice was sent in February 2015. The project was approved by the local Institutional Review Board of the University Hospital of Lille (France).
2.2. Questionnaire
The survey tool was developed by cardiologists routinely involved in the clinical care of CAD patients. To maximize the response rate, we developed a very simple, anonymous questionnaire that could be answered quickly. The survey consisted of six questions pertaining to two clinical scenarios regarding stable CAD patients (Table 1, Table 2). Demographic information (age and gender of the cardiologist, private vs. hospital practice, academic vs. non-academic cardiologist, interventional [coronary] vs. non-interventional cardiologist) was recorded to further define the participating population of physicians.
Table 1.
Case #1.
| A 62-year-old man has sustained an inferior ST segment-elevation MI. He has undergone successful primary angioplasty with implantation of a drug-eluting stent for acute occlusion of the right coronary artery. There were no other significant coronary lesions, and the left ventricular ejection fraction at hospital discharge was 55%. Smoking was the sole cardiovascular risk factor and was stopped at time of MI. Six months after MI, an exercise test was performed (80% of maximum predicted heart rate; negative). At present, two years post-MI, the patient is asymptomatic and is receiving optimal medical therapy for secondary prevention. |
Assuming that the patient is still asymptomatic:
|
Assuming that you send the patient for a treadmill exercise test and that he has been on β-blockers since his MI:
|
Assuming that you send the patient for a treadmill exercise test and that the results are as follows: exercise duration on BRUCE protocol: 11 min; test stopped for fatigue at 90% of maximum predicted heart rate; no chest pain; no arrhythmia; significant downsloping ST-segment depression at 9 min (reaching 1.4 mm at peak exercise).
|
MI, myocardial infarction.
Table 2.
Case #2.
| A 76-year-old diabetic woman underwent coronary angiography because of exercise-induced angina (no history of MI). Three-vessel coronary disease was documented. Firstly, a right coronary stenosis, which appeared as the more severe lesion, was treated by implantation of a drug-eluting stent. However, the patient remained symptomatic, and a CABG (left anterior descending artery, left marginal artery) was performed. After surgery, the patient joined a cardiac rehabilitation program. At the end of the program, the exercise test was submaximal but showed no sign of ischemia. Left ventricular ejection fraction was 60%. |
| Three months after CABG, the patient is doing well. She has no angina. Blood pressure is 130/80 mm Hg, and heart rate is 67 beats/min (in sinus rhythm). Medical treatment includes low-dose aspirin, a statin, an ACE inhibitor, and treatment for diabetes. LDL-cholesterol and glycosylated hemoglobin are well controlled. Renal function is normal. Would you add a β-blocker?
|
Two years later, atrial fibrillation is diagnosed. The patient still has no symptoms of angina. Which anticoagulation treatment would you choose?
|
The patient is now 84 years old (CABG was performed 8 years ago). She has osteoarthritis and is no longer very active. During a routine outpatient visit, the patient reported recurrent angina that began approximately 6 months previously. The chest pain is similar to what she experienced prior to CABG, although less severe (only 1 or 2 episodes per month, when she goes shopping, and with rapid [≤ 1 min] spontaneous cessation at rest).
|
MI, myocardial infarction. CABG, coronary artery bypass graft. ACE, angiotensin-converting enzyme.
2.3. Statistical Analysis
Statistical analyses were performed with the SPSS 20.0.0 IBM software. Continuous variables are presented as means ± standard deviation (SD). Categorical variables are presented as absolute numbers and/or percentages. Univariate analysis using the χ2 test was performed to search for associations between answers and physician characteristics (age tertiles, gender, private vs. hospital practice, academic vs. non-academic, interventional vs. non-interventional cardiologist). Logistic regression analysis was used to determine which physician characteristics were independently correlated with management patterns. Odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated. Statistical significance was assumed at P-value < 0.05.
3. Results
The response rate to the survey was very high (318 out of 345; 92%). Mean age of the participating cardiologists was 49.2 ± 11.4 years (range, 27 to 73 years); 250 (79%) were men. One hundred seventy three (54%) cardiologists were working in private practice and the others (145; 46%) in public hospitals; 34 (11%) were academic physicians. Seventy nine (25%) of the responding physicians were (at least part-time) interventional cardiologists.
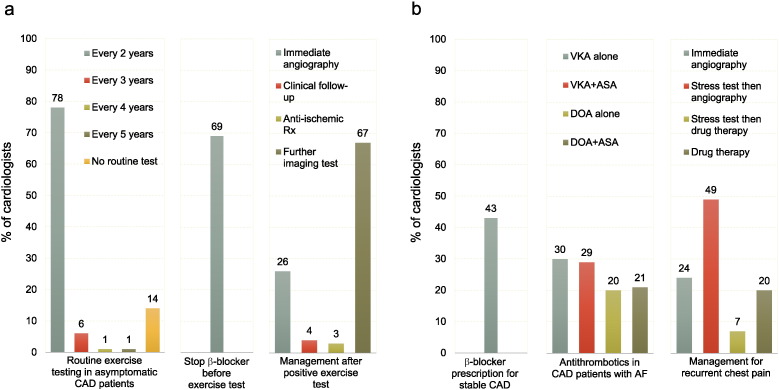
Case #1 (Table 1) focuses on a 62-year-old man who is asymptomatic two years after a myocardial infarction (MI) for which he underwent primary percutaneous coronary intervention (PCI) with complete revascularization. The answers to the questions are shown in Fig. 1a. The majority of cardiologists (86%) stated that they would perform a routine stress test in this situation, even if the patient was asymptomatic, and in most instances, at the time of this visit (two years after MI). Of note, 43 cardiologists (14%) answered that they would not prescribe a stress test as long as the patient remains asymptomatic. Most physicians (69%) would advise patients on β-blockers to discontinue the drug prior to exercise testing. The last question pertains to patient management if the exercise test suggests silent ischemia with preserved exercise capacity. In this instance, the vast majority of cardiologists would ask for additional tests (either coronary angiography directly [26%], or further imaging tests, followed by coronary angiography if ischemia is confirmed [67%]); the proportion of physicians who would simply continue with clinical follow-up (with or without increasing anti-ischemic medication) was low (7%).
Fig. 1.
Responses to the survey.
Panel a: Responses to questions of case #1. Panel b: Responses to questions of case #2.
The 2 clinical scenarios are presented in Table 1, Table 2, respectively.
CAD, coronary artery disease; VKA, vitamin-K antagonist, DOA, direct oral anticoagulant; ASA, aspirin and AF, atrial fibrillation.
Case #2 (Table 2) focuses on a 76-year-old woman with 3-vessel disease who has undergone coronary artery bypass graft (CABG) combined with drug-eluting stent (DES) implantation. She is currently asymptomatic, has no history of MI, and has normal left ventricular ejection fraction. The answers to the questions are shown in Fig. 1b. Forty three percent of the cardiologists stated that they would prescribe a β-blocker for secondary prevention in such a patient. In the event of atrial fibrillation two years later requiring chronic oral anticoagulation, 59% would use a vitamin-K antagonist (VKA) and 41%, a direct oral anticoagulant (DOA); in both groups, half the cardiologists would combine low-dose aspirin with anticoagulation, while the other half would use anticoagulation alone. The last question concerns patient management should recurrent, non-severe stable angina occur eight years after revascularization, at a time when the patient is no longer physically very active. In this situation, most physicians would ask for additional tests (coronary angiography [24%], or a non-invasive stress test followed by coronary angiography if ischemia is confirmed [49%]) but a substantial proportion (27%) would first opt to increase anti-ischemic medication.
We then investigated whether physician characteristics (age, gender, academic vs. non-academic, private vs. hospital practice, interventional vs. non interventional cardiologist) were associated with answers to the questions (Fig. 2). Although globally, practice patterns appeared relatively similar among subgroups, there were several differences that reached statistical significance. After adjustment for all physician characteristics, older physicians were more likely to discontinue β-blockers before performing exercise testing (OR = 2.38 [1.21–4.68] for physicians ≥ 55 years of age vs. < 45 years), more likely to prescribe a combination of aspirin and anticoagulants (OR = 2.40 [1.35–4.26] for physicians ≥ 55 years of age vs. < 45 years) and to request coronary angiography directly in the event of recurrent angina (OR = 2.23 [1.17–4.24]). Male cardiologists were less likely to prescribe β-blockers for stable CAD (OR = 0.51 [0.30–0.87]), and more likely to prescribe DOAs if an anticoagulant was indicated (OR = 2.76 [1.47–5.17]). Interestingly, cardiologists working in an academic environment were less likely to prescribe routine exercise tests in asymptomatic stable CAD patients (OR = 0.22 [0.10–0.50]), less likely to discontinue β-blockers before exercise testing (OR = 0.24 [0.11–0.53]), less likely to combine aspirin with anticoagulants (OR = 0.37 [0.16–0.86]), and had a greater tendency to initiate treatment with DOAs (OR = 2.19 [0.97–4.92]). Lastly, the type of practice (private fee-for-service vs. employment by public institutions) had little influence on management patterns, while interventional cardiologists were less likely to discontinue treatment with β-blockers before performing exercise testing (OR = 0.48 [0.27–0.84]) and more likely to prescribe immediate coronary angiography in the event of recurrent angina (OR = 2.33 [1.31–4.14]).
Fig. 2.
Associations between physician characteristics and survey responses.
Panel a: age. Panel b: gender. Panel c: academic vs. non-academic practice. Panel d: private vs. hospital practice. Panel e: interventional vs. non interventional cardiologist. The P value on the bar graph is for univariate analysis. The odds ratio (OR) below the graph is adjusted for other physician characteristics. The 2 clinical scenarios are presented in Table 1, Table 2, respectively.
CI, confidence interval and CAD, coronary artery disease.
4. Discussion
Nowadays, the expected long-term survival of the vast majority of patients with CAD is excellent, with few recurrent cardiovascular events (Bauters et al., 2014a). During this prolonged period of outpatient care, diagnostic and therapeutic decisions by the referring cardiologist are major drivers of patient management. As highlighted above, recommendations based on high levels of evidence are often lacking for routine decisions relating to stable CAD outpatients. Although intentionally restricted in scope to ensure quick answers from participating physicians, the present survey nevertheless addresses several diagnostic and therapeutic issues that are extremely common for most cardiologists in everyday practice and for which the level of evidence is limited.
Case #1 concerns the use of non-invasive stress tests in an asymptomatic post-MI patient. It is not currently known whether routine tests in this situation are beneficial, as no trial has assessed the impact of routine exercise testing on the clinical outcome of stable CAD patients. According to the European Guidelines (Montalescot et al., 2013), repetition of an exercise ECG in stable CAD patients may only be considered at least two years after the previous test (a class IIb level C-recommendation). According to the American Appropriate Use Criteria (Wolk et al., 2014), non-invasive stress tests in asymptomatic patients with known CAD are rated ‘rarely appropriate’ if performed < 5 years after CABG or < 2 years after PCI, and ‘may be appropriate’ ≥ 5 years after CABG or ≥ 2 years after PCI. Our results show that, despite these ‘neutral’ recommendations, the use of routine stress tests is a very firmly established practice among cardiologists. It is however noteworthy that variability exists with regard to this observation, and that about 15% of the physicians considered that routine stress testing is not useful as long as the patient remains asymptomatic. Beta adrenergic blocking agents can reduce or delay the onset of angina pectoris and ST segment depression on exercise testing (Ho et al., 1985). We observed that about 2/3 of cardiologists would advise patients on β-blockers to discontinue this treatment prior to exercise testing. There are no clear guidelines on this question, and there would appear to be two different standpoints: (i) β-blockers should be stopped because this approach will maximize the ability of the test to detect ischemia; (ii) β-blockers can be maintained since there is no point in ‘detecting’ medically controlled ischemia in patients with documented CAD. Importantly, the approach based on practice of routine non-invasive stress tests cannot be separated from its consequences when the results are known. Although a normal test in this context is simple to manage and will be seen as reassuring by both patient and physician, it is important to determine the extent to which a test suggestive of ischemia is likely to impact on patient management. We intentionally chose a scenario in which exercise testing suggests ischemia without any markers of high-risk (preserved exercise capacity, moderate ST deviation, no angina during exercise), i.e., a situation where revascularization is unlikely to lead to improved event-free survival (Boden et al., 2007). In spite of this, our results show that a positive exercise test will almost inevitably lead to coronary angiography being performed, if ischemia is not ruled out by additional tests.
Case #2 addresses a sequential approach to three different therapeutic and diagnostic decisions pertaining to the stable CAD patient. (i) Although there is no discussion about the fact that patients with systolic heart failure should receive a β-blocker (McMurray et al., 2012, Yancy et al., 2013), there is no evidence from randomized evaluations that β-blockers improve survival in the rest of the stable CAD population (excluding the early post-myocardial infarction period), and observational studies with propensity score adjustments have provided discordant results (Bangalore et al., 2012, Bauters et al., 2014b). Our data, showing that approximately 50% of the participating cardiologists would opt for a β-blocker in such patients, are thus concordant with the current literature. (ii) When atrial fibrillation develops in an otherwise stable CAD patient, it can prove difficult to find the best antithrombotic regimen with the most favorable risk-benefit ratio. International guidelines on atrial fibrillation have suggested that, in patients with stable vascular disease (i.e., > 1 year with no acute events), oral anticoagulation monotherapy may be considered (Camm et al., 2010, Fuster et al., 2011). It was however acknowledged that the level of evidence was low (class IIb level C-recommendation for both European and American guidelines) (Camm et al., 2010, Fuster et al., 2011), and data from recent registries have shown that in the real world, physicians are still reluctant to discontinue all antiplatelet therapy in CAD patients who need oral anticoagulation (Hamon et al., 2014, Lamberts et al., 2014). Again, the results of our survey show a balanced figure with 50% of cardiologists who would continue aspirin and 50% who would withdraw it (irrespective of the choice of anticoagulant). The fact that our fictitious patient had undergone DES implantation, even though it was over two years beforehand, may have affected the decision to pursue with aspirin. (iii) The last question relates to the management of recurrent angina in an elderly patient with a past history of PCI and CABG. Although a quarter of the cardiologists replied that pharmacological treatment would be their first-line choice, most physicians appeared to have a more invasive approach, and would recommend coronary angiography (either immediately, or once ischemia has been confirmed by a non-invasive test).
Our second objective was to analyze whether certain physician characteristics may play a role in these diagnostic and therapeutic decisions. In contrast to what might have been expected, younger cardiologists were not more aggressive than older ones. In fact, if anything, the reverse trend was observed, with older cardiologists more likely to stop β-blockers before performing exercise testing, more likely to combine antiplatelet and anticoagulant agents, and more likely to prescribe coronary angiography directly in the cases of recurrent angina in elderly patients with known CAD. One possible explanation for this latter finding might be the fact that this strategy was more prevalent in the past, at a time when non-invasive tests were not so widely used, but possibly also when it was believed (before the results of randomized clinical trials; Boden et al., 2007) that myocardial revascularization might improve survival in stable patients. Female physicians appeared more reluctant to use DOAs, and more prone to prescribe β-blockers as a routine preventive medication in asymptomatic patients after CABG; this might be interpreted overall as a more cautious attitude. In contrast, academic cardiologists were more likely to adopt new drug approaches, such as DOAs, or newer attitudes such as prescribing anticoagulants alone rather than dual antithrombotic therapy when anticoagulants were needed. The results of trials comparing DOAs and vitamin K antagonists are relatively recent, as are the guidelines (2010–2011) suggesting that oral anticoagulation alone may be a safe option in stable CAD (Camm et al., 2010, Fuster et al., 2011), and these conclusions may well have been more readily adopted by the academic community. One could have hypothesized that cardiologists in private practice may have specific practice patterns, related to the personal financial impact of test prescription. This was however not so clear-cut; in virtually all respects, private cardiologists gave similar answers to those of cardiologists working in hospitals. Similarly, one might have expected to observe a more aggressive approach (tendency toward using more tests and coronary angiographies) by interventional cardiologists. Again there was no indication for this, with the one possible exception of their more frequent decision to go directly to coronary angiography in the cases of recurrent angina in patients with known CAD; on the other hand, interventional cardiologists were less likely to discontinue β-blockers before prescribing an exercise test. Overall, the type of medical practice had relatively little influence on management patterns. Other than the fact that continuous medical education programs for both private and public sector cardiologists are similar, this could be partly explained by the new system of funding of public hospitals in France (“tarification à l'activité”), which has been in place since 2007 and actually encourages physicians to use tests or procedures (typically, coronary angiography) that are highly valued in terms of state funding to their institutions. In other words, the current health system in France encourages physicians to use tests/procedures rather than simply provide clinical care to their patients: private cardiologists earn more if they perform such tests, while the institutions employing public-sector cardiologists benefit directly from the use of these tests.
4.1. Strengths and Limitations
The main strength of our survey is the very high response rate (> 90%) among a population comprising all the practicing cardiologists in a specific region of France known to have a high prevalence of CAD (Tunstall-Pedoe et al., 1994), together with a large sample size (> 300 cardiologists). A response rate such as this is unusually high for this type of survey, and the results can therefore be considered as representative of the physicians' opinions. Conversely, the main limitation is that the results only reflect the medical practice in one geographical region comprising approximately 4 million inhabitants, and therefore may not be adequately representative of the rest of the country, or indeed other countries. In particular, regional academic leadership may affect medical practice differently from one region to another. In the Nord-Pas-de-Calais Region, the regional academic leadership is based on an annual 2-day regional meeting, and on smaller thematic workshops. In addition, biannual 2-day regional seminars are part of the cardiology fellowship program. Because of the increasing development of national or European continuous medical education programs, and the constant flow of information from internet-based medical sites, regional specificities are however less likely to exist than in the past. The degree of financial support–reimbursement regarding medical care is also likely to be a determinant of practice patterns. In France, social coverage and health insurance are uniform over the whole territory, and chronic CAD is one of the long-term conditions entitled to full reimbursement of all costs, including diagnostic procedures and medications. Comparing practice patterns in different countries with other medical care systems would be of interest. Finally, answers to case-vignettes may differ from actual practice when physicians are confronted with similar real-life clinical cases. Although comparison with real-world practice is difficult because of the paucity of data available, it is noteworthy that the 24% rate of physicians going directly to coronary angiography in CAD patients with recurrent stable angina is perfectly in line with the results of the large French registry on coronary angiography and PCI. This observational study conducted by the French Society of Cardiology assessed treatment strategies in a population of 298,105 patients and showed that 25% of those with stable symptoms had undergone direct angiography without prior exercise or imaging stress testing (Puymirat et al., 2013).
4.2. Conclusion
Diagnostic and therapeutic practice for extremely common, everyday clinical situations vary considerably among physicians when management attitudes are not guided by clear recommendations. Although several drivers of management behavior can be identified from the cardiologists' profiles, the final clinical decision remains largely unexplained. Overall, cardiologists in our survey were seen to be more likely to have ‘protective’ attitudes and use diagnostic tests or therapies despite the absence of a rational scientific basis, than to adopt more ‘conservative’ attitudes. Beyond the uncertain consequences of such attitudes with regards to patient health, they are likely to have a considerable economic cost for the society. There is therefore an urgent need for novel clinical trials to address these interrogations and to “close” gaps in current recommendations.
Author Contributions
CB: literature search, study design, data collection, data analysis, data interpretation, writing, figures and final approval of the manuscript. GL: study design, data interpretation and final approval of the manuscript. NL: study design, data interpretation and final approval of the manuscript. ND: data analysis, data interpretation, writing, figures and final approval of the manuscript.
Conflicts of Interest
CB: travel grants from MSD-Schering, Boehringer-Ingelheim, and Servier.
GL: fees for lectures or consulting from Astra-Zeneca, Bristol-Myers Squibb, Daiichi-Sankyo, and Eli-Lilly.
NL: research grant from Pfizer and fees for lectures or consulting from Actelion, Astra-Zeneca, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, MSD-Schering, Novartis, Pfizer, Sanofi-Aventis and Servier.
ND: research grants from Amgen, Astra-Zeneca, Bayer, Daiichi-Sankyo, Eli-Lilly, GSK, Merck, Novartis, Pfizer, Sanofi-Aventis, Servier, and The Medicines Company and fees for lectures or consulting from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, Eli-Lilly, GlaxoSmithKline, MSD-Schering, Novartis, Novo-Nordisk, Pfizer, Roche, Sanofi-Aventis, Servier and The Medicines Company.
Acknowledgments
The authors wish to thank Moyra Barbier for the editorial assistance.
References
- Bangalore S., Steg G., Deedwania P. Beta-blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–1349. doi: 10.1001/jama.2012.12559. [DOI] [PubMed] [Google Scholar]
- Bauters C., Deneve M., Tricot O., Meurice T., Lamblin N. Prognosis of patients with stable coronary artery disease (from the CORONOR study) Am. J. Cardiol. 2014;113:1142–1145. [Google Scholar]
- Bauters C., Lemesle G., Meurice T., Tricot O., de Groote P., Lamblin N. Prognostic impact of β-blocker use in patients with stable coronary artery disease. Heart. 2014;100:1757–1761. doi: 10.1136/heartjnl-2014-305719. [DOI] [PubMed] [Google Scholar]
- Boden W.E., O'Rourke R.A., Teo K.K. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- Camm A.J., Kirchhof P., Lip G.Y. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Eur. Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- Fihn S.D., Gardin J.M., Abrams J. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Fuster V., Ryden L.E., Cannom D.S. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2011;57:e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Hamon M., Lemesle G., Tricot O. Incidence, source, determinants, and prognostic impact of major bleeding in outpatients with stable coronary artery disease. J. Am. Coll. Cardiol. 2014;64:1430–1436. doi: 10.1016/j.jacc.2014.07.957. [DOI] [PubMed] [Google Scholar]
- Ho S.W., McComish M.J., Taylor R.R. Effect of beta-adrenergic blockade on the results of exercise testing related to the extent of coronary artery disease. Am. J. Cardiol. 1985;55:258–262. doi: 10.1016/0002-9149(85)90356-x. [DOI] [PubMed] [Google Scholar]
- Lamberts M., Gislason G.H., Lip G.Y. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation. 2014;129:1577–1585. doi: 10.1161/CIRCULATIONAHA.113.004834. [DOI] [PubMed] [Google Scholar]
- McMurray J.J., Adamopoulos S., Anker S.D. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- Montalescot G., Sechtem U., Achenbach S. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- O'Gara P.T., Kushner F.G., Ascheim D.D. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- Puymirat E., Blanchard D., Perier M.C. Study design and baseline characteristics of the national observational study of diagnostic and interventional cardiac catheterization by the French Society of Cardiology. Am. J. Cardiol. 2013;112:336–342. doi: 10.1016/j.amjcard.2013.03.030. [DOI] [PubMed] [Google Scholar]
- Roth G.A., Forouzanfar M.H., Moran A.E. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg P.G., James S.K., Atar D. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- Tricoci P., Allen J.M., Kramer J.M., Califf R.M., Smith S.C., Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 2009;301:831–841. doi: 10.1001/jama.2009.205. [DOI] [PubMed] [Google Scholar]
- Tunstall-Pedoe H., Kuulasmaa K., Amouyel P., Arveiler D., Rajakangas A.M., Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- Windecker S., Kolh P., Alfonso F. 2014 ESC/EACTS Guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur. Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- Wolk M.J., Bailey S.R., Doherty J.U. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]