Abstract
Adipokines such as leptin play important roles in the regulation of energy metabolism, particularly in the control of appetite. Here, we describe a hormone, mimecan, which is abundantly expressed in adipose tissue. Mimecan was observed to inhibit food intake and reduce body weight in mice. Intraperitoneal injection of a mimecan-maltose binding protein (-MBP) complex inhibited food intake in C57BL/6J mice, which was attenuated by pretreatment with polyclonal antibody against mimecan. Notably, mimecan-MBP also induced anorexia in Ay/a and db/db mice. Furthermore, the expression of interleukin (IL)-1β and IL-6 was up-regulated in the hypothalamus by mimecan-MBP, as well as in N9 microglia cells by recombinant mouse mimecan. Taken together, the results suggest that mimecan is a satiety hormone in adipose tissue, and that mimecan inhibits food intake independently of leptin signaling by inducing IL-1β and IL-6 expression in the hypothalamus.
Keywords: Mimecan, Anorexia, Leptin, IL-1β, IL-6
1. Introduction
Mimecan, also known as an osteoinductive factor or osteoglycin, is a 12 kDa secreted protein corresponding to the 105 C-terminal amino acids (residues 176–280) of the preproprotein encoded by the osteoinductive factor gene (Oif), first identified in the organic matrix of bovine bone by chromatography (Bentz et al., 1989). Furthermore, a 25 kDa keratin sulfate proteoglycan in bovine cornea was shown to be encoded by the cDNA of this gene, representing the 223 C-terminal amino acids of the encoded protein (residues 58–280) (Funderburgh et al., 1997). The full-length mimecan cDNA was identified from the human pituitary, and was submitted to GenBank (accession no. AF100758) in our previous study (Hu et al., 2000). The genomic structure of mimecan is highly conserved among different species. The human mimecan precursor (298 amino acids) exhibits 92% homology with bovine mimecan (299 amino acids), suggesting a functional importance (Madisen et al., 1990). However, the physiological functions of mimecan remain elusive. Several studies suggest mimecan may be an essential component of normal vascular extracellular matrix, and it is involved in atherosclerosis (Kampmann et al., 2009). Mimecan is considered a major putative regulator of the left ventricular mass (Petretto et al., 2008), and the absence of mimecan has been observed in the development of colorectal cancer (Wang et al., 2007). In our previous study, mimecan was coexpressed with adrenocorticotropic hormone (ACTH) in corticotroph cells of the pituitary, and was up-regulated by glucocorticoids (Ma et al., 2010). Moreover, mimecan regulates ACTH secretion in corticotroph cells, and may play roles in the coordination of the hypothalamus–pituitary–adrenal axis (Ma et al., 2011). These previous results suggest that mimecan is an important factor involved in the physiological and biological functions. In the current study, we report that mimecan is highly expressed in adipose tissue, and it acts as a satiety factor inhibiting food intake by inducing interleukin (IL)-1β and IL-6 expression in the hypothalamus.
2. Materials and Methods
2.1. Fusion Protein Purification and Antibody Production
The cDNA encoding 12 kDa human mimecan (residues 175–279) was subcloned into pGEX-5X-2 (GE Healthcare) and overexpressed in Escherichia coli BL21 (DE3) cells. Purified mimecan-GST fusion protein was used for antibody production. Rabbits and mice were immunized with recombinant protein in Freund's adjuvant (Sigma-Aldrich, St. Louis, MO, USA) for polyclonal and monoclonal antibody production, respectively. Antibodies were purified using Protein G (GE Healthcare). The monoclonal subtype was identified as IgG1-κ. Human cDNA encoding 12 kDa mimecan (residues 175–279) was subcloned into pMAL-c2x (NEB) and overexpressed in BL21 (DE3) cells. Cells were grown at 37 °C to an optical density at 595 nm (A595) of 0.6–0.8, induced with 0.5 mM isopropyl-β-D-thiogalactoside (IPTG) for 5 h, and centrifuged. Cells were sonicated, centrifuged, and the fusion protein in the supernatant was purified by affinity chromatography (MBPTrap HP, GE Healthcare), gel filtration (Superdex 200, 10/300 GL, GE Healthcare), and ion exchange (HiTrap ANX FF, GE Healthcare) chromatography. Purity of the mimecan-MBP fusion protein was 96%, as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining. MBP was expressed and purified (98%) for use as a control. The cDNA encoding 25 kDa mimecan (residues 47–279) was subcloned into pET-28a (+) (Novagen) and overexpressed in BL21 (DE3) cells. The mimecan-His fusion protein (Mim-His) was purified (94%) by Ni-ion affinity chromatography (HisTrap HP, GE Healthcare). All proteins were buffer-exchanged with PBS, and endotoxin was removed (ActiClean Etox, Sterogene, Carlsbad, CA, USA).
2.2. In Vivo Experiments
All animal experiments were conducted in accordance with the institutional ethical guidelines on animal care, and approved by the Shanghai Jiaotong University Animal Care and Use Committee. Animals were housed individually for 1 week. C57BL/6J and Ay/a mice were fasted overnight (8:00 p.m.–8:00 a.m.). Mim-MBP or Mim-His was administered by i.p. injection. Nonfasted C57BL/6J mice were injected during the dark period (beginning at 8:00 p.m.). Food consumption was assessed hourly. The db/db mice were injected intraperitoneally with 0.05 μmol/kg Mim-MBP or MBP twice daily (9:00 a.m. and 9:00 p.m.) for 2 days, followed by 5 days when no protein was administered; this cycle was repeated four times (a total of 28 days). Food consumption and body weight were measured daily at 8:00 a.m. In the antibody neutralization test, C57BL/6J mice fasted for 24 h (08:00 a.m.–8:00 a.m.) were assigned to two groups at 11:00 a.m., and i.p. injected with anti-human mimecan polyclonal antibody or rabbit preimmune IgG (160 μg/g body weight). Nine hours later, all animals were injected i.p. with Mim-MBP (0.05 μmol/kg). Cannula placement was performed 1 week before i.c.v. injection. All rats were housed individually, and were fasted for 12 h (8:00 p.m.–8:00 a.m.). Then, 8 μL (2 nmol/kg) Mim-MBP (3 μg/uL) or MBP (2.6 μg/uL) was injected into the lateral ventricle. Knockout mice and WT littermates were fasted for 12 h (8:00 p.m.–8:00 a.m.), followed by i.p. injection of recombinant mouse leptin (R&D Systems, Minneapolis, MN, USA) at 5 mg/kg, or PBS control using the same volume.
2.3. Cell Culture
For primary neuronal cell culture, the hypothalamus of 18 day SD rat embryos were dissected in ice-cold PBS, washed three times in PBS, and digested with 0.2% trypsin at 37 °C for 20 min, followed by addition of a DMEM/F12 medium containing 10% fetal bovine serum (FBS). Cells were passed through a 74 μm filter, centrifuged for 7 min at 1000 rpm, and resuspended in the same medium, then seeded onto 24-well plates at 1.5 × 105 cells/well on wells coated with 100 μg/mL poly-d-lysine (PDL, Beyotime, Shanghai, China). After 4 h, medium was replaced with DMEM/F12 containing 2% B27 and 1 × glutamine. Forty-eight hours later, 2.5 μg/mL arabinoside (Ara-C; Sigma-Aldrich) was added. After 24 h, medium was replaced with DMEM/F12 containing 2% B27 and 1 × glutamine to remove the Ara-C. Medium was replaced with 50% fresh medium every 3 days. On day 7, cells were treated with 10, 50, or 100 nM recombinant mouse mimecan (R&D Systems) for 1, 2, or 4 h, and the expression of appetite-regulation neuropeptides and hypothalamic inflammatory factors was analyzed. Cells were infected for 48 h with Murine Stem Cell Virus (MSCV) encoding mouse mimecan. Murine microglia cell line N9 was grown in IMDM supplemented with 5% FBS and 2 mM glutamine. Before the experiment, cells were maintained in serum-free IMDM for 12 h.
2.4. Statistical Analysis
Analyses of data were performed with GraphPad Prism 5.0 software. Data were presented as means ± SEM and analyzed with the statistical tests indicated in the text and figure legends. P < 0.05 was considered statistically significant.
3. Results
3.1. Mimecan is a Hormone, Abundantly Expressed in Adipose Tissue
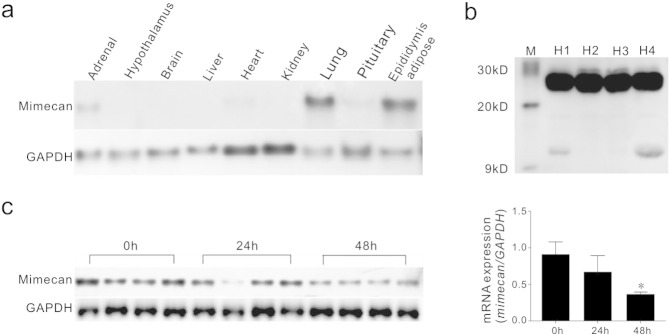
In our previous study, we showed that mimecan is highly expressed in the mouse lung, but expressed at lower levels in other tissues, such as adrenal, pituitary, and heart tissue (Hu et al., 2005). Using northern blot analysis in the present study, we found that mimecan is also highly expressed in mouse adipose tissue (Fig. 1a). Moreover, mimecan corresponding to two protein bands of approximately 25 kDa and 12 kDa was detected in the human circulation, particularly at high levels for the form corresponding to 25 kDa (Fig. 1b). Because adipose tissue secretes various hormones with important roles in the regulation of energy balance (Zhang et al., 1994, Scherer et al., 1995, Steppan et al., 2001, Pelleymounter et al., 1995, Halaas et al., 1995, Campfield et al., 1995), we characterized the role(s) of mimecan in energy regulation. Firstly, we investigated possible alterations of mimecan expression in adipose tissue during fasting. Notably, 24 h fasting decreased mimecan mRNA to 74% of control, and 48 h fasting reached a significant reduction to 40% (Fig. 1c), in a similar manner as the reduction by leptin mRNA during fasting for 48 h (Sivitz et al., 1996).
Fig. 1.
Mimecan expressed in various mouse tissues and secreted into the blood. (a) Mimecan mRNA expression in various tissues from C57BL/6J mice determined by northern blot analysis. (b) Mimecan corresponding to 25 kDa and 12 kDa was detected in human serum by western blot analysis using monoclonal antibody against mimecan. H1–H4 represents four healthy individuals. M: molecular weight markers. Body mass index (BMI) of the individuals: H1, 21.8; H2, 21.5; H3, 19.5; H4, 25.7. (c) Northern blot analysis of mimecan mRNA expression in adipose tissue from C57BL/6J mice fasting for 0, 24, and 48 h (n = 4 for each time point). The right histogram is the gray scale of mimecan mRNA expression. Data are expressed as means ± SEM. *P < 0.05 vs 0 h. Statistical analysis was performed by one-way ANOVA.
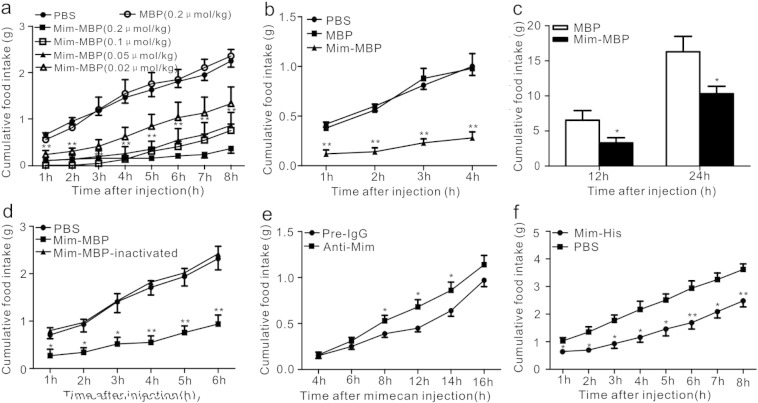
The sequence encoding the 12 kDa (residues 175–279) human mimecan protein was inserted into the expression vector pMAL-c2x, and the resulting fused mimecan-maltose binding protein (Mim-MBP) was expressed in E. coli (Kapust and Waugh, 1999) and purified to homogeneity as a 55 kDa monomer. After Mim-MBP was dissolved in phosphate-buffered saline (PBS, pH 7.4), it was administered to 12-week-old C57BL/6J mice by intraperitoneal (i.p.) injection at doses of 0.02, 0.05, 0.1, and 0.2 μmol/kg. Control groups were administered either the carrier protein MBP at a dose of 0.2 μmol/kg, or an equal volume of PBS. Mimecan administration resulted in a dose-dependent anorexic effect in mice when fasting for 12 h (Fig. 2a). There was no difference in food intake between the MBP and PBS groups, indicating that MBP was a proper control in this study. After receiving 0.02 μmol/kg Mim-MBP, cumulative food intake was significantly reduced by 65.8% (0.41 ± 0.14 g vs. 1.2 ± 0.27 g [mean ± SEM]) for the first 3 h and 43.6% (1.33 ± 0.36 g vs. 2.36 ± 0.14 g) for 8 h, compared to MBP. Furthermore, compared to the PBS group, cumulative food intake was reduced by 83.9% (0.19 ± 0.12 g vs. 1.18 ± 0.10 g) for 3 h and 61.7% (0.86 ± 0.27 g vs. 2.25 ± 0.13 g) for 8 h after injection with 0.05 μmol/kg Mim-MBP (Fig. 2a). With all comparisons, the anorexic effect of Mim-MBP plateaued with a dose of 0.05 μmol/kg, hence 0.05 μmol/kg was considered a dose maximal for the anorexic effect, and therefore used in subsequent experiments. With ad libitum access to food, C57BL/6J mice consumed significantly less food over 4 h after i.p. injection of mimecan (Fig. 2b) while still showing similar serum glucose concentrations (Fig. S1). Moreover, intracerebroventricular (i.c.v.) administration of 2 nmol/kg Mim-MBP induced a significant reduction in cumulative food intake over 12 and 24 h in Sprague–Dawley (SD) rats (Fig. 2c).
Fig. 2.
Intraperitoneal (i.p.) and intracerebroventricular (i.c.v.) injections of mimecan induced a reduction in food intake and the anorexic effect of mimecan was attenuated by pretreatment with polyclonal antibody or by heat-inactivated mimecan. (a) Cumulative food intake of C57BL/6J mice responding to i.p. injection of different doses of mimecan-MBP (Mim-MBP) at indicated time points after 12 h of fasting (8:00 p.m.–8:00 a.m., n = 8). (b) Suppression of food intake in C57BL/6J mice with ad libitum access to food when responding to i.p. injection of Mim-MBP (0.05 μmol/kg) (n = 5), compared to phosphate-buffered saline (PBS) or maltose binding protein (MBP). (c) Suppression of food intake in Spraque–Dawley (SD) rats responding to i.c.v. injection of mimecan-MBP (Mim-MBP) (2 nmol/kg) (n = 8). (d) Cumulative food intake of C57BL/6J mice after i.p. injection with active or inactive Mim-MBP (n = 5). (e) Antibody neutralization test in C57BL/6J mice. Mice were pretreated with polyclonal antibody against mimecan or rabbit preimmune IgG by i.p. injection (n = 12). (f) Suppression of food intake of C57BL/6J mice responding to i.p. injection of mimecan-His (Mim-His) (0.05 μmol/kg) (n = 6). *P < 0.05; **P < 0.01 vs. PBS at the indicated time points in (a) and (b). *P < 0.05; **P < 0.01 vs. Mim-MBP inactivated at time points in (d). Data are expressed as means ± SEM. *P < 0.05; **P < 0.01. Statistical analysis was performed by one-way ANOVA.
To determine whether the anorexic effect of mimecan was caused by endotoxin, which has been previously shown to suppress food intake (Gayle et al., 1998, Grossberg et al., 2011), Mim-MBP was heat-treated at 95 °C for 5 min to denature the protein. Endotoxin is unaffected under these conditions (Luheshi et al., 1999). Injection (i.p.) of this denatured Mim-MBP into mice failed to suppress food intake, and its effect on food intake was similar to PBS. However, after injection of the Mim-MBP without heat treatment, which came from the same container as the heat-inactivated protein, cumulative food intake was significantly reduced in C57BL/6J mice (Fig. 2d). Furthermore, i.p. injection with polyclonal antibody against mimecan significantly attenuated the anorexic effect of mimecan (Fig. 2e).
The 25 kDa form of mimecan could be detected in the serum from healthy individuals in greater amounts than the 12 kDa form. To ensure that the 25 kDa form mediated the anorexic effect, we then purified the mimecan-His (25 kDa, residues 47–279) fusion protein (Mim-His) that was expressed in E. coli. Notably i.p. injection of Mim-His also induced a dramatic reduction in food intake (Fig. 2f). Together, these results demonstrated that the proteins corresponding to both 25 kDa and 12 kDa could induce the reduction in food intake.
3.2. Mimecan Resulted in Reductions of Food Intake and Body Weight in db/db and Ay/a Mice
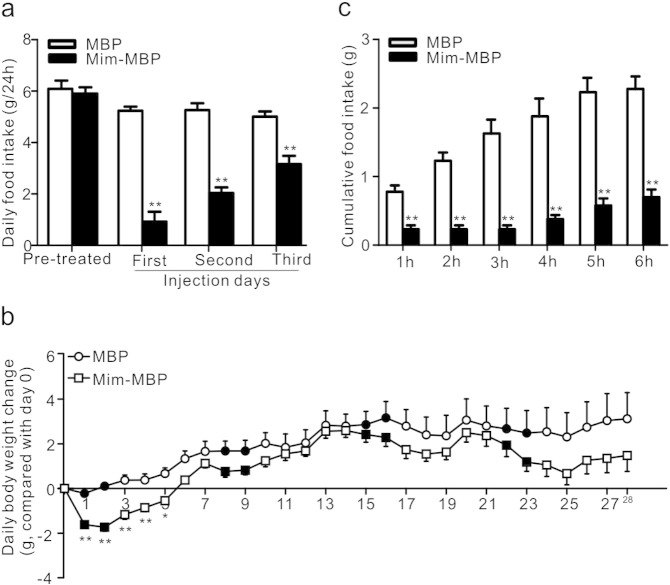
Leptin, a hormone derived from adipose tissue, is known to inhibit appetite (Zhang et al., 1994, Campfield et al., 1995, Halaas et al., 1995, Pelleymounter et al., 1995), but the interaction between leptin and mimecan remains unknown. The db/db mice carrying an inactivated leptin receptor (Chen et al., 1996) were used to investigate whether the anorexic effect of mimecan was mediated by leptin or its receptor. Daily food intake was significantly suppressed by Mim-MBP administration (0.05 μmol/kg, i.p.) twice daily for 3 successive days (Fig. 3a). Moreover, mice were administered Mim-MBP twice daily for 2 successive days, followed by 5 days off, with four repeated cycles (28 days total). As showed in Fig. 3b, the body weight of mice treated with MBP gradually increased during the observation. However, the body weight of mice treated with Mim-MBP was significantly reduced during the time when Mim-MBP was given for the first 2 days and remained until day 6 injection with Mim-MBP. The body weight of mice treated with Mim-MBP also gradually increased after day 6 injection with Mim-MBP, but the increment was less than that in the MBP-treated control mice (Fig. 3b). These results suggested that the anorexic effect of mimecan was independent of leptin signaling (Oh et al., 2006). Further evidence for the anorexic effect of mimecan was obtained from agouti mice (Ay/a), which have a locus mutation in the agouti gene inducing ectopic expression of the normal agouti protein (Fan et al., 1997). The ectopic production of agouti in the brain antagonizes melanocortin-4 receptors, causing genetic obesity by excess food intake. In Ay/a mice, Mim-MBP could still reduce food intake for at least 6 h after i.p. injection (Fig. 3c), showing that the mimecan-induced anorexia did not depend on melanocortin signaling, which is the most important pathway mediating appetite control.
Fig. 3.
The anorexic effect of mimecan was independent of leptin and melanocortin signaling. (a, b) Effect of mimecan on daily food intake (a) and body weight increment (b) in db/db mice with ad libitum access to food (0.05 μmol/kg; i.p. injection, n = 9–10). (b) Daily body weight change (g) = body weight on the indicated day — body weight on day 0. The solid circles and squares represent the changes of body weight during mimecan-MBP or MBP treatment, respectively. The empty circles and squares represent the changes of body weight after stopping injections of mimecan-MBP or MBP, respectively. (c) Cumulative food intake of Ay/a mice treated with Mim-MBP (0.05 μmol/kg) after fasting for 6 h (i.p. injection, n = 4–5). Data are expressed as means ± SEM. *P < 0.05; **P < 0.01. Statistical analysis was performed by one-way ANOVA.
3.3. Mimecan increased IL-1Β and IL-6 mRNA expressions in the hypothalamus and N9 microglia
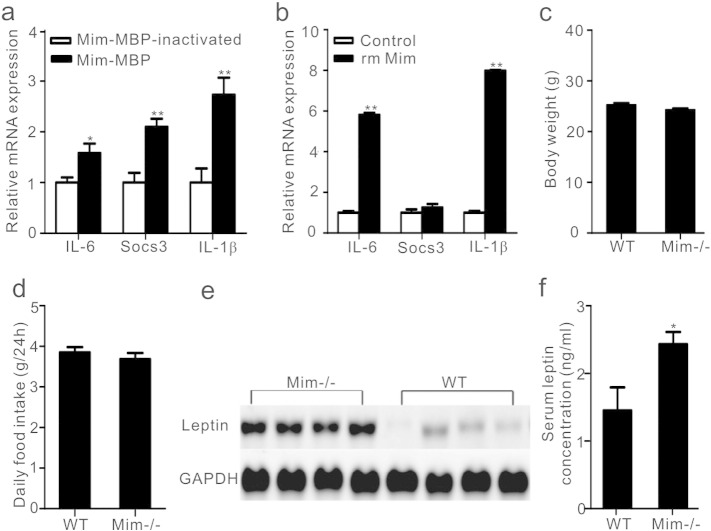
Previous studies have shown that IL-1β and IL-6 are the downstream effectors of leptin signaling in reducing food intake, and i.c.v. injection of IL-1β and IL-6 per se suppresses food intake (Luheshi et al., 1999, Scarlett et al., 2007, Schele et al., 2012). Therefore, we investigated the effect of mimecan on the hypothalamic cytokine profile. Notably, compared with mice treated with Mim-MBP inactivated by boiling, mRNA expression of IL-1β and IL-6 was significantly increased in the hypothalamus from mice injected i.p. with Mim-MBP (Fig. 4a). Consistent with increased IL-6 and IL-1β, the intracellular signal protein, SOCS3, a negative-feedback regulator of leptin signaling (Mori et al., 2004), was also elevated in hypothalamus. Unexpectedly, there was no significant increase in the mRNA expressions of IL-6, IL-1β, and SOCS3 in primary cultured hypothalamic neurons that were either transfected with MSCV-mimecan or treated with recombinant mouse mimecan (rm Mim) (Fig. S2), although the expression of IL-1β was slightly increased by the latter treatment. However, it has been reported that not only neurons, but also the microglia in the hypothalamus secrete cytokines regulating energy balance (Tang et al., 2007, Le Foll et al., 2014). In the present study, we found that in the mouse microglia cell line N9, incubation with recombinant mouse mimecan also resulted in an induction of IL-6 and IL-1β (Fig. 4b), suggesting that the cellular machinery required for interleukin induction was present in the brain, and mainly in the microglia.
Fig. 4.
Effect of mimecan on IL-1β, IL-6, and SOCS3 expression and features of mimecan knockout mice. (a) The mRNA expression of IL-1β, IL-6, and SOCS3 in the hypothalamus from C57BL/6J mice (n = 10) with ad libitum access to food, treated with active or inactive Mim-MBP (0.05 μmol/kg) by i.p. injection for 4 h. The reference gene was actin. (b) Effect of recombinant mouse mimecan (rm Mim) (100 nM for 4 h) on the expression of IL-1β, SOCS3, and IL-6 in N9 microglia cells. The reference gene was actin. (c, d) The body weight (c) and daily food intake (d) in Min−/− (knockout) mice and wild type (WT) littermates with ad libitum access to food (n = 25). (e) Leptin mRNA expression in adipose tissue from Min−/− mice and WT littermates (n = 4). (f) Levels of leptin in serum from Mim−/− mice and WT littermates (n = 12). Data are expressed as means ± SEM. *P < 0.05; **P < 0.01. Statistical analysis was performed by one-way ANOVA.
3.4. The deficiency of mimecan did not influence food intake but increase leptin expression
To further explore the physiological role of mimecan in energy homeostasis, we conducted subsequent experiments using mimecan knockout mice (Mim−/−) (Ma et al., 2011). Mimecan deficiency did not influence the growth or reproduction of these mice. Unexpectedly, there was no significant difference in body weight or food intake between Mim−/− mice and wild-type (WT) littermates, regardless of whether the animals were fed a standard diet or a high fat diet (Figs. 4c, d and S3). Notably, the levels of leptin in adipose tissue (Fig. 4e) and serum (Fig. 4f) were higher in Mim−/− mice fed standard diet than in WT littermates, which may explain the absence of orexigenic and obese phenotypes in Mim−/− mice. Moreover, the anorexic effect of leptin was partially attenuated in Mim−/− mice compared with WT mice (Fig. S4).
4. Discussion
Mimecan is extensively expressed in various tissues including bone, cornea, lung, and various other tissues. In the present study, we found mimecan was highly expressed in the adipose tissue as a hormone, was secreted into the circulation. Administration of mimecan by intraperitoneal or intracerebroventricular injection inhibited food intake in a dose-dependent manner in C57BL/6J mice and SD rats, and the anorexic effect was attenuated by pretreatment with polyclonal antibody against mimecan. These results suggested that mimecan is a hormone, playing important roles in the regulation of food intake.
Mimecan could induce anorexia either by i.p. injection or by i.c.v. injection. Moreover, much lower doses of mimecan were required to elicit anorexic effect when injected centrally than peripherally, suggesting mimecan may exert its anorexic effect in the central nervous system (CNS). It has been shown that the hypothalamus is the major center controlling energy metabolism. During the last decade, accumulated evidence supports the hypothesis that both IL-1β and IL-6 play integral roles in the regulation of energy metabolism at the level of the CNS. Several previous studies have shown that IL-1, IL-6, and their respective receptors are locally produced in the hypothalamus (Scarlett et al., 2007, Schele et al., 2012, Shirazi et al., 2013). Mice lacking either IL-1R or IL-6 develop late-onset obesity, and intracerebroventricular rather than intraperitoneal IL-6 treatment decreases obesity in obese IL6−/− mice (Garcia et al., 2006, Wallenius et al., 2002). Moreover, central injection of IL-1 or IL-6 induces a marked suppression of appetite (Deboer et al., 2009, Schobitz et al., 1995) and a recent study reported that IL-1β and IL-6 mediate GLP-1 induced anorexia in the hypothalamus (Shirazi et al., 2013). Notably, compared to mice treated with mimecan denatured by boiling, the expression of IL-1β and IL-6 in the hypothalamus was significantly increased after the injection of mimecan without heat treatment. It has been proposed that the effect of mimecan on feeding behavior maybe mediated via the IL-1β and IL-6 pathway in the CNS, which resembles GLP-1 induced anorexia (Shirazi et al., 2013). In the present study, we detected the induction of IL-1β and IL-6 by mimecan in microglia rather than primary hypothalamic neuronal cells. Furthermore, a previous study showed that IL-6 production occurs selectively in microglia rather than in neurons or astrocytes from the hypothalamus and cerebral cortex (Le Foll et al., 2014). Moreover, it was reported that leptin could induce IL-6 production in microglia mediated by the leptin receptor, insulin receptor substrate-1, and the phosphatidylinositol-3-kinase pathway (Tang et al., 2007). A recent study reported that amylin-induced IL-6 production in microglia from the ventromedial hypothalamus mediates anorexia via enhanced leptin signaling (Le Foll et al., 2014). Together, our results and previous reports strongly suggest that mimecan-induced anorexia is mediated by IL-1β and IL-6.
Although considerable data suggest that central IL-1β and IL-6 can regulate food intake during health conditions (Wallenius et al., 2002, Garcia et al., 2006, Shirazi et al., 2013), IL-1β and IL-6, as inflammatory factors, also play key roles in lipopolysaccharide ([LPS] endotoxin) -induced anorexia (Gayle et al., 1998, Grossberg et al., 2011).Thus, it is questionable whether the anorexic effect of mimecan is elicited by endotoxin, which may be a contaminant in the protein prepared from a prokaryotically expressed vectors (Kalra et al., 1991).
The following experiments confirmed the specific anorexic effect of mimecan. Heat-denatured mimecan with minimal denaturation of endotoxin failed to reduce food intake and induce IL-1β and IL-6 expression in the hypothalamus (Luheshi et al., 1999), but the injection of mimecan without heat treatment retained activity. Moreover, both IL-1β and IL-6 were induced in the microglia by recombinant mouse mimecan (R&D, Minneapolis MN, USA), which was purified from a eukaryotic expression system. In addition, mimecan-induced inhibition of food intake was significantly attenuated with polyclonal antibody pretreatment, again confirming that the anorexic effect resulted from mimecan rather than from the endotoxin.
Leptin was the first identified adipose-derived hormone that regulates food intake. Because mimecan is an anorexic hormone abundantly expressed in adipose tissue, similar to the characteristics of leptin, we further investigated the correlation between mimecan and leptin. In db/db mice, where the function of the leptin receptor is genetically deleted, mimecan-induced reduction of food intake was still observed, showing that the anorexic effect of mimecan at least partially involved an independent pathway from leptin signaling. Mim−/− mice showed a similar food intake and body weight compared to WT litters, regardless of whether the animals were fed a standard diet or a high fat diet. Importantly, leptin was significantly increased in either mRNA expression in adipose tissue or serum levels in the circulation from Mim−/− mice. Considering the notable anorexic effect of leptin, the absence of orexigenic and obese phenotypes in Mim−/− mice may be due to the higher levels of expression and secretion of leptin. Moreover, leptin-induced inhibition of food intake in Mim−/− mice involved a slight attenuation compared to WT litters, showing that mimecan is not essential for leptin-induced anorexia. Taken together, our results suggest that mimecan and leptin use two independent peripheral signal pathways that regulate food metabolism.
In conclusion, mimecan is a hormone highly expressed in adipose tissue and secreted into the circulation. Mimecan exhibits an anorexic effect, possibly by increasing IL-1β and IL-6 expression in the hypothalamus, and its anorexic effect differs from the mechanism of action of leptin signaling.
Conflicts of Interest
The authors declare that they have no competing interests.
Author Contributions
H.D.S. and C.X.Z. conceived the project. H.D.S., H.M.C., and S.X.L. designed the research. H.M.C., X.P.Y., H.J., J.H.M., S.X.L., X.S.L., R.Y.L., C.C.G., and Z.Q.W. performed the experiments. M.Z., X.P.Y., and C.M.P. analyzed the data. H.D.S., H.M.C., and X.P.Y. wrote the manuscript.
Funding
This work was supported in part by the National Natural Science Foundation of China (31171127, 81370888, 81430019, 81200568, and 81200643), Shanghai Science and Technology Committee (10JC1410400, 13ZR1460900), and the Foundation for Excellent Ph.D (YBPY2009007).
Acknowledgments
We are grateful to all volunteers who participated in this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.09.044.
Contributor Information
Cui-Xia Zheng, Email: zcx9566@163.com.
Huai-Dong Song, Email: huaidong_s1966@163.com.
Appendix A. Supplementary data
Supplementary material.
References
- Bentz H., Nathan R.M., Rosen D.M., Armstrong R.M., Thompson A.Y., Segarini P.R., Mathews M.C., Dasch J.R., Piez K.A., Seyedin S.M. Purification and characterization of a unique osteoinductive factor from bovine bone. J. Biol. Chem. 1989;264:20805–20810. [PubMed] [Google Scholar]
- Campfield L.A., Smith F.J., Guisez Y., Devos R., Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking Adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Chen H., Charlat O., Tartaglia L.A., Woolf E.A., Weng X., Ellis S.J., Lakey N.D., Culpepper J., Moore K.J., Breitbart R.E., Duyk G.M., Tepper R.I., Morgenstern J.P. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Deboer M.D., Scarlett J.M., Levasseur P.R., Grant W.F., Marks D.L. Administration of IL-1beta to the 4th ventricle causes anorexia that is blocked by agouti-related peptide and that coincides with activation of tyrosine-hydroxylase Neurons in the nucleus of the solitary tract. Peptides. 2009;30:210–218. doi: 10.1016/j.peptides.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Boston B.A., Kesterson R.A., Hruby V.J., Cone R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Funderburgh J.L., Corpuz L.M., Roth M.R., Funderburgh M.L., Tasheva E.S., Conrad G.W. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J. Biol. Chem. 1997;272:28089–28095. doi: 10.1074/jbc.272.44.28089. [DOI] [PubMed] [Google Scholar]
- Garcia M.C., Wernstedt I., Berndtsson A., Enge M., Bell M., Hultgren O., Horn M., Ahren B., Enerback S., Ohlsson C., Wallenius V., Jansson J.O. Mature-onset obesity in interleukin-1 receptor I Knockout Mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- Gayle D., Ilyin S.E., Flynn M.C., Plata-Salaman C.R. Lipopolysaccharide (LPS)- and muramyl dipeptide (MDP)-induced anorexia during refeeding following acute fasting: characterization of brain cytokine and neuropeptide systems mRNAs. Brain Res. 1998;795:77–86. doi: 10.1016/s0006-8993(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Grossberg A.J., Zhu X., Leinninger G.M., Levasseur P.R., Braun T.P., Myers M.G., Jr., Marks D.L. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J. Neurosci. 2011;31:11376–11386. doi: 10.1523/JNEUROSCI.2311-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D., Lallone R.L., Burley S.K., Friedman J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hu R.M., Han Z.G., Song H.D., Peng Y.D., Huang Q.H., Ren S.X., Gu Y.J., Huang C.H., Li Y.B., Jiang C.L., Fu G., Zhang Q.H., Gu B.W., Dai M., Mao Y.F., Gao G.F., Rong R., Ye M., Zhou J., Xu S.H., Gu J., Shi J.X., Jin W.R., Zhang C.K., Wu T.M., Huang G.Y., Chen Z., Chen M.D., Chen J.L. Gene expression profiling in the human hypothalamus–pituitary–adrenal axis and full-length cDNA cloning. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9543–9548. doi: 10.1073/pnas.160270997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S.M., Li F., Yu H.M., Li R.Y., Ma Q.Y., Ye T.J., Lu Z.Y., Chen J.L., Song H.D. The mimecan gene expressed in human pituitary and regulated by pituitary transcription factor-1 as a marker for diagnosing pituitary tumors. J. Clin. Endocrinol. Metab. 2005;90:6657–6664. doi: 10.1210/jc.2005-0322. [DOI] [PubMed] [Google Scholar]
- Kalra S.P., Dube M.G., Sahu A., Phelps C.P., Kalra P.S. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc. Natl. Acad. Sci. U. S. A. 1991;88:10931–10935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampmann A., Fernandez B., Deindl E., Kubin T., Pipp F., Eitenmuller I., Hoefer I.E., Schaper W., Zimmermann R. The proteoglycan osteoglycin/mimecan is correlated with arteriogenesis. Mol. Cell. Biochem. 2009;322:15–23. doi: 10.1007/s11010-008-9935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapust R.B., Waugh D.S. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 1999;8:1668–1674. doi: 10.1110/ps.8.8.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll C., Johnson M.D., Dunn-Meynell A., Boyle C.N., Lutz T.A., Levin B.E. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes. 2014 doi: 10.2337/db14-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi G.N., Gardner J.D., Rushforth D.A., Loudon A.S., Rothwell N.J. Leptin actions on food intake and body temperature are mediated by IL-1. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7047–7052. doi: 10.1073/pnas.96.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q.Y., Zhang X.N., Jiang H., Wang Z.Q., Zhang H.J., Xue L.Q., Chen M.D., Song H.D. Mimecan in pituitary corticotroph cells may regulate ACTH secretion and the HPAA. Mol. Cell. Endocrinol. 2011;341:71–77. doi: 10.1016/j.mce.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Ma Q.Y., Zuo C.L., Ma J.H., Zhang X.N., Ru Y., Li P., Pan C.M., Liu Z., Cao H.M., Chen M.D., Song H.D. Glucocorticoid up-regulates mimecan expression in corticotroph cells. Mol. Cell. Endocrinol. 2010;321:239–244. doi: 10.1016/j.mce.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Madisen L., Neubauer M., Plowman G., Rosen D., Segarini P., Dasch J., Thompson A., Ziman J., Bentz H., Purchio A.F. Molecular cloning of a novel bone-forming compound: osteoinductive factor. DNA Cell Biol. 1990;9:303–309. doi: 10.1089/dna.1990.9.303. [DOI] [PubMed] [Google Scholar]
- Mori H., Hanada R., Hanada T., Aki D., Mashima R., Nishinakamura H., Torisu T., Chien K.R., Yasukawa H., Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- Oh I.S., Shimizu H., Satoh T., Okada S., Adachi S., Inoue K., Eguchi H., Yamamoto M., Imaki T., Hashimoto K., Tsuchiya T., Monden T., Horiguchi K., Yamada M., Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- Pelleymounter M.A., Cullen M.J., Baker M.B., Hecht R., Winters D., Boone T., Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Petretto E., Sarwar R., Grieve I., Lu H., Kumaran M.K., Muckett P.J., Mangion J., Schroen B., Benson M., Punjabi P.P., Prasad S.K., Pennell D.J., Kiesewetter C., Tasheva E.S., Corpuz L.M., Webb M.D., Conrad G.W., Kurtz T.W., Kren V., Fischer J., Hubner N., Pinto Y.M., Pravenec M., Aitman T.J., Cook S.A. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat. Genet. 2008;40:546–552. doi: 10.1038/ng.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett J.M., Jobst E.E., Enriori P.J., Bowe D.D., Batra A.K., Grant W.F., Cowley M.A., Marks D.L. Regulation of central melanocortin signaling by interleukin-1 beta. Endocrinology. 2007;148:4217–4225. doi: 10.1210/en.2007-0017. [DOI] [PubMed] [Google Scholar]
- Schele E., Fekete C., Egri P., Fuzesi T., Palkovits M., Keller E., Liposits Z., Gereben B., Karlsson-Lindahl L., Shao R., Jansson J.O. Interleukin-6 receptor alpha is co-localised with melanin-concentrating hormone in human and mouse hypothalamus. J. Neuroendocrinol. 2012;24:930–943. doi: 10.1111/j.1365-2826.2012.02286.x. [DOI] [PubMed] [Google Scholar]
- Scherer P.E., Williams S., Fogliano M., Baldini G., Lodish H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Schobitz B., Pezeshki G., Pohl T., Hemmann U., Heinrich P.C., Holsboer F., Reul J.M. Soluble interleukin-6 (IL-6) receptor augments central effects of IL-6 in vivo. FASEB J. 1995;9:659–664. doi: 10.1096/fasebj.9.8.7768358. [DOI] [PubMed] [Google Scholar]
- Shirazi R., Palsdottir V., Collander J., Anesten F., Vogel H., Langlet F., Jaschke A., Schurmann A., Prevot V., Shao R., Jansson J.O., Skibicka K.P. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16199–16204. doi: 10.1073/pnas.1306799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz W.I., Bailey H.L., Donohoue P. Rat adipose ob mRNA levels in states of altered circulating glucose and insulin. Biochem. Biophys. Res. Commun. 1996;220:520–525. doi: 10.1006/bbrc.1996.0437. [DOI] [PubMed] [Google Scholar]
- Steppan C.M., Bailey S.T., Bhat S., Brown E.J., Banerjee R.R., Wright C.M., Patel H.R., Ahima R.S., Lazar M.A. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Tang C.H., Lu D.Y., Yang R.S., Tsai H.Y., Kao M.C., Fu W.M., Chen Y.F. Leptin-induced IL-6 production is Mediated by Leptin Receptor, Insulin Receptor Substrate-1, Phosphatidylinositol 3-Kinase, Akt, NF-kappaB, and p300 pathway in microglia. J. Immunol. 2007;179:1292–1302. doi: 10.4049/jimmunol.179.2.1292. [DOI] [PubMed] [Google Scholar]
- Wallenius V., Wallenius K., Ahren B., Rudling M., Carlsten H., Dickson S.L., Ohlsson C., Jansson J.O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ma Y., Lu B., Xu E., Huang Q., Lai M. Differential expression of mimecan and thioredoxin domain-containing protein 5 in colorectal adenoma and cancer: a proteomic study. Exp. Biol. Med. (Maywood) 2007;232:1152–1159. doi: 10.3181/0701-RM-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.