Abstract
Background
CTCs provide prognostic information and their application is under investigation in multiple tumor types. Of the multiple variables inherent in any such process, none is more important to outcome than the appropriateness of the sample source. To address this question, we investigated CTCs in paired peripheral venous and arterial blood specimens obtained from stage IV uveal melanoma patients.
Methods
Blood specimens were obtained from both common femoral arteries and antecubital veins in 17 uveal melanoma patients with multiple hepatic metastases for CTC measurements.
Finding
CTCs were detectable with greater frequency (100%) and in larger numbers (median 5, range 1 to 168) in all arterial blood specimens than in venous samples (52.9%; median 1, range 0 to 8). Patients with hepatic as well as extra-hepatic metastasis showed higher number of arterial CTCs, compared to patients with liver-only metastasis (p = 0.003). There was no significant association between the number of arterial CTCs and the tumor burden within the liver in patients who had liver-only metastases.
Interpretation
Our data indicate that arterial blood specimens might be a better source of circulating uveal melanoma cells. Although less conveniently processed, perhaps arterial blood should be evaluated as sample source for measurement of CTCs.
Abbreviations: Ab, antibody; AKTi, AKT inhibitor; BCNU, bischlorethylnitrosourea; DEBDOX, drug-eluting beads with doxorubicin; EDTA, ethylenediaminetetraacetic acid; HMW-MAA, high molecular weight melanoma associated antigen; Ipi, ipilimumab; LN, lymph node; MEKi, MEK inhibitor; METi, MET inhibitor;; TACE, transarterial chemoembolization; VPA, valproic acid; XRT, radiation therapy
Keywords: Uveal melanoma, Circulating tumor cells, Hepatic metastasis, Arterial venous, Peripheral venous, CTC count
Highlights
-
•
CTCs were detectable in 100% of arterial blood obtained from metastatic uveal melanoma patients, while only 53% of venous blood was positive for CTCs.
CTCs have been investigated to provide prognostic information in multiple tumor types. Of the multiple variables, none is more important than the appropriateness of the sample source. Blood specimens were obtained from both femoral arteries and antecubital veins in 17 uveal melanoma patients with multiple hepatic metastases. CTCs were detectable with greater frequency (100%) and in larger numbers in all arterial blood specimens than in venous samples (52.9%). Our data indicate that arterial blood specimens might be a better source of circulating uveal melanoma cells. Although less convenient, arterial blood should be evaluated as sample source for measurement of CTCs.
1. Introduction
Uveal melanoma (UM) is the most common primary cancer of the eye in adults, with a reported incidence of 5.1 per million (Singh et al., 2011). The majority of UM cases (97.8%) occur in the Caucasian population (Singh et al., 2011). Despite the common embryologic origin of cutaneous and uveal melanocytes, the clinical, epidemiologic, and molecular characteristics of UM differ from those of cutaneous melanoma (Collaborative Ocular Melanoma Study G, 2001, Singh et al., 2001, Ewens et al., 2014). Local treatment of primary UM has improved; conservative non-surgical treatments such as brachytherapy with radioisotopes result in eye preservation and control the growth of primary UM. However, this improvement in local treatment did not significantly increase the overall survival for UM patients (Singh et al., 2011). Systemic metastases develop in up to 50% of the cases of UM patients. UM disseminates hematogeneously, as there is no major lymphatic drainage from the eye. Metastatic disease leads to death in the majority of patients because of the lack of effective systemic treatments (Kujala et al., 2003). The metastatic UM cells have significant tropism to the liver, and the liver is the first organ of metastasis in approximately 80% to 95% of patients who develop systemic recurrence. Several histologic, genetic, and demographic factors have been associated with metastases in UM, including large tumor size in primary cancer of the eye, monosomy 3, and BAP1 mutation (Collaborative Ocular Melanoma Study G, 2001, Ewens et al., 2014). It has been reported that 80% of metastatic uveal melanoma have mutation in BAP1 (Harbour et al., 2010). Published clinical observations suggested that UM cell metastases in the liver grow faster than metastases in other organs (All-Ericsson et al., 2002, Yoshida et al., 2014, Chattopadhyay et al., 2014). The lung is the second most common site of metastasis. A small percentage of patients first develop osseous and brain metastasis (Lorigan et al., 1991, Rietschel et al., 2005). It has been reported that distant micrometastasis resulting from the dissemination of tumor cells through the blood stream developed even before primary UM was clinically diagnosed and treated (Eskelin et al., 2000). It is also reported that the recurrence for patients undergoing enucleation displays a bimodal pattern, peaking three years with a second surge peaking at about nine years (Demicheli et al., 2014).
Due to their buoyancy, circulating tumor cells (CTCs) are found in the white blood cell fraction. CTCs have been investigated as a non-radiographic tool to monitor disease progression. The presence of CTCs suggests increased metastatic potential, and they have been investigated as a predictive marker for systemic recurrence. They could also serve as a source for diagnostic testing (liquid biopsy) in cases where the biopsy of metastases is difficult or risky. In such cases, evaluation of CTCs in blood would be more convenient and could be useful in obtaining critical information on the biological characteristics of cancer cells to facilitate a diagnostic or therapeutic decision. Furthermore, the genomic profile of CTCs may predict homing and colonization to specific distant organ sites (Li et al., 2008, Burger and Kipps, 2006).
CTCs have been detected in the majority of epithelial cancers, including those from the prostate (Danila et al., 2007), colon/rectum (Cohen et al., 2008), and breast (Cristofanilli et al., 2004). In patients with metastatic breast cancer, CTC counts above 5 per 7.5 ml of venous blood before the start of systemic therapy are associated with shorter median progression-free and overall survivals (Cristofanilli et al., 2008). The key mutation for therapeutic resistance has been found in CTCs in metastatic breast cancer patients and CTCs could potentially be used as a predictable marker for treatment response and resistance (Fernandez et al., 2014). In fact, the CellSearch® System was approved by the United States (US) Food and Drug Administration (FDA) for monitoring treatment effectiveness in metastatic prostate, colorectal, and breast cancer patients.
Venous blood collection is simple and minimally invasive, and this approach has made CTC testing readily available to many cancer patients. The major drawback is the fact that CTCs are not always detectable for patients with clinically evident metastatic disease. This observation raises the concern that a number of CTCs might have been sequestered or destroyed while circulating in the blood stream. Alternatively, it is possible that CTCs may have been lost during analysis due to technical reasons.
Recently, the field of CTC detection technologies has been significantly improved and various new approaches have been developed including filtration (Mazzini et al., 2014), dual immunomagnetic enrichment assay (Tura et al., 2014), fiber-optic array scanning (Krivacic et al., 2004), microfluidics (Dong et al., 2013) and photoacoustic-flow cytometry (Menyaev et al., 2013, Sarimollaoglu et al., 2014). However, none of these new technologies have been validated for approval by the US FDA.
Although accumulative evidence suggests that CTCs could provide prognostic information in breast cancer patients, the clinical benefits in measuring CTCs in UM patients remain controversial. It has been shown that the detection of CTCs in venous blood specimens of primary UM patients prior to their local treatment was 14% with an immunomagnetic enrichment method. However, there was no significant difference between the number of CTCs before and after their local therapies, and the number of CTCs was not correlated to the development of metastasis in a short median follow-up time of 16 months (Suesskind et al., 2011). Bidard et al. reported the result of CTC detection with the CellSearch® method in 40 stage IV UM patients with liver metastasis, in which eight out of 40 patients exhibited additional extra-hepatic metastasis. Surprisingly, no CTCs were detected in 70% of patients with hepatic metastasis. The median number of CTCs was 3 and the range of CTCs was 1 to 20 in 12 patients who showed positive CTCs in their venous blood specimens (Bidard et al., 2014). These results indicate that CTC measurement in venous blood may not be useful in stage IV UM patients since the detection rate of CTCs is very low. This also raises the critical question as to whether venous blood specimens are appropriate in evaluating CTCs in UM patients. UM CTCs might have been sequestered or destroyed in peripheral tissues or, alternatively, CTCs might have strong organ tropism and therefore they are repeatedly cleared from peripheral blood. The number of CTCs may also differ in different blood sources. To address these issues, we investigated the number of CTCs in paired blood specimens from both common femoral arteries and antecubital veins of the same patients with stage IV UM.
2. Patients and Methods
The protocol for blood specimen procurement was approved by the Institutional Review Board of Thomas Jefferson University. Seventeen UM patients, including ten patients who had liver only metastasis and seven patients who had hepatic and extra-hepatic metastases, were enrolled in this study between April 2014 and October 2014. All patients had treated their primary uveal melanoma between 2000 and 2013. Ten patients had received radioactive plaque as their treatment for primary uveal melanoma and seven patients had enucleation of their affected eye. None of these patients had a local recurrence of primary uveal melanoma at the time of CTC measurement. The patients signed the informed consent form prior to blood sample collection. All patients were scheduled to receive liver-directed therapy for metastatic UM. Prior to liver embolization treatment, 7.5 ml of blood samples was obtained from the common femoral arterial and the antecubital (forearm) veins, using CellSave tubes (Veridex, LLC, Raritan, NJ), and sent to the CTC measurement laboratory. The clinical information and the sources of blood were blinded to the CTC laboratory. Blood samples were maintained at room temperature and processed within 72 h of collection.
CTCs were analyzed using the standard CellSearch® protocol and CellTracks Circulating Melanoma Cell Kit® on the CellSearch® System (Janssen Diagnostics, LLC, Raritan, NJ). Briefly, cells expressing CD146 (Mel-CAM) were immunomagnetically enriched and stained with phycoerythrin (PE)-conjugated antibody specific to high molecular weight melanoma associated antigen (HMW-MAA), which is specific for melanoma cells. Allophycocyanin (APC)-conjugated anti-CD45 was used to identify leukocytes and anti-CD34 was used for detection of endothelial cells. 4,6-Diamidino-2-phenylindoledihydrochloride (DAPI) was used to detect cell nuclei. CTCs were defined as nucleated, CD146-positive cells, expressing HMW-MAA, but lacking expression of the common leukocyte antigen CD45 and CD34 endothelial markers. Samples were then scanned on the CellTracks® analyzer II fluorescent microscope (Veridex, LLC, Raritan, NJ) (Fig. 1). This technology is widespread and widely used in different counties, notably in the USA. The validity of this assay was confirmed by the Control Kit for Circulating Endothelial and Melanoma Cells provided by the manufacturer (Janssen Diagnostics, LLC). The control kit contains fixed cells from a SK-Mel-28 cell line in the bottles containing two populations of cell for high and low control. The control cells are fully compatible with CellSearch Endothelial Cell and CellTracks Circulating Melanoma Cell Kit reagents and are automatically identified by the CellTracks analyzer. The detected fixed SK-Mel-28 cells in two different lots for the studied melanoma CTC measurements were as follows: Lot #1: 1272 (mean) with the range of 1058–1486 in high control, and 62 (mean) with the range of 32 to 92 in low control; and Lot #2: mean of 1203 and the range of 719–1687 in high control, and mean of 53 and the range 11 to 95 in low control.
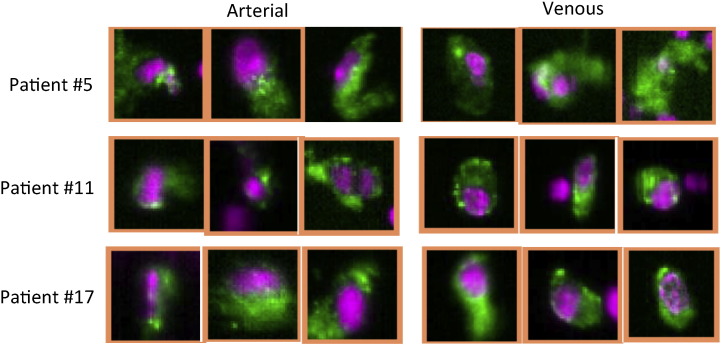
Fig. 1.
Gallery of circulating tumor cells (CTCs) from the CellSearch Analyzer. Representative cell images obtained from patients using the melanoma detection kit.
Figure shows representative images of CTCs from three patients on the CellTracks analyzer II. To be assigned as CTC, cells must have PE-stained nucleus (pseudocolored pink) and positive HMW-MAA staining (pseudocolored green).
We also conducted exploratory experiments by adding UM cells to the healthy donor peripheral blood. In the first spiking experiment, various numbers of melanoma cell (5, 15, 30, 100, 500, and 1500 cells) obtained from a long-term cultured metastatic UM cell line (TJU-UM001) were added to 6 tubes of whole blood specimen obtained from a healthy donor. Various numbers of fixed melanoma cells were diluted in CellSearch Dilution Buffer® and then added to 7.5 ml of whole blood. The information regarding numbers of melanoma cell in individual specimens was blinded to examiners and spiked UM cells were measured by the methods described above. UM cells were detected in all specimens spiked with at least 5 tumor cells in 7.5 ml blood (100% detection rate) (Supplement 1). Furthermore, in a separate experiment, we have prepared 3 tubes of the same healthy donor blood specimen. Each tube was first irradiated (25Gy) and then 20 UM cells were added. Melanoma cells were measured by the same method as described above. We have detected 4 tumor cells in each of these 3 tubes.
For patients with liver metastases only, the number of circulating CTCs in arterial blood specimens was compared to tumor burden within the liver. Using MRI of the abdomen patients were categorized into three groups: 1) less than 20% of liver involvement, 2) between 20% and 50% of liver involvement, and 3) more than 50% of liver involvement. The maximum diameter of tumor in liver metastasis was measured with MRI images.
2.1. Statistical Analysis
The CTC numbers per patient in subgroups of patients were summarized as medians and inter-quartile and full ranges. The difference between numbers of CTC in arterial and venous blood specimens was tested using the nonparametric Wilcoxon signed rank test for the paired data. The null hypothesis tested is that within-patient differences between CTC numbers in arterial and venous blood have a symmetric distribution around zero (the median difference is zero). The Spearman correlation coefficient was used to evaluate association between the size of the largest hepatic metastasis and the numbers of CTC. The nonparametric Kruskal–Wallis rank sum test was used to evaluate association between the numbers of CTC in arterial blood specimens of 10 patients who have liver-only metastases. The null hypothesis tested is that numbers of CTC in arterial blood specimens have the same distribution in patients who have hepatic and extra-hepatic metastases and in patients who have liver-only metastasis. The nonparametric Kruskal–Wallis rank sum test was used to compare numbers of CTC in arterial blood specimens of patients with three different levels of liver-only tumor involvement (< 20%, 20%–50%, > 50%). The null hypothesis tested is that the numbers of CTC have the same distribution in arterial blood specimens from patients with liver-only metastases but different level of tumor involvement. The data were analyzed in R (The R Foundation for Statistical Computing, http://www.R-project.org).
3. Results
Demographic information of patients and their treatment histories are shown in Table 1. The 17 UM patients with multiple hepatic metastases comprised seven males and ten females, of mean age 59.4 (range 35 to 79) years. Ten patients had liver-only metastasis by radiographic evaluation, and 7 patients had hepatic and extra-hepatic metastases. In 7 patients who have extra-hepatic metastatic, all patients have bone metastases and 3 patients have lung metastases (Table 1). Fifteen out of 17 patients had previous treatments including liver-directed treatments (n = 13) and systemic treatments (n = 9) before being enrolled into this study. Despite these treatments, their hepatic metastases were radiographically and clinically active and all patients subsequently received a liver-directed treatment after collection of blood specimens.
Table 1.
Demographic characteristic and treatment histories of 17 patients with stage IV uveal melanoma.
| ID | Gender | Age | Tumor volume in liver (%) | Largest tumor in liver (cm) | Extra-hepatic metastasis | Previous treatment liver-directed | Previous treatment others |
|---|---|---|---|---|---|---|---|
| 1 | F | 58 | 20–50 | 1.7 | None | Immunoembolization, radiosphere, TACE with BCNU | Ipi |
| 2 | M | 79 | < 20 | 2.2 | None | None | None |
| 3 | F | 77 | < 20 | 2.3 | Bone | Radiosphere, immunoembolization, TACE with BCNU | None |
| 4 | M | 68 | 20–50 | 3.2 | Bone, periportal LN, lung | Immunoembolization, TACE with BCNU | Carbo + Taxol, Xgeva, Ipi |
| 5 | F | 68 | < 20 | 3.4 | Lung, bone | Immunoembolization | MET Ab + MEKi, Ipi |
| 6 | M | 46 | < 20 | 3.5 | None | Immunoembolization | Adjuvant Sutent |
| 7 | M | 64 | < 20 | 3.9 | None | None | Adjuvant Sutent, METi |
| 8 | F | 35 | < 20 | 4.4 | Breast, pancreas, mediastinal LN, bone | Immunoembolization | Gemcitabine + Abraxane, VPA, MEKi, Cryoablation of pelvic metastasis |
| 9 | F | 78 | < 20 | 4.5 | Spine, skin | Immunoembolization, XRT to liver tumor, TACE with BCNU | None |
| 10 | F | 66 | < 20 | 4.6 | None | None | MEKi + AKTi, Ipi |
| 11 | F | 53 | < 20 | 4.7 | Bone, peritoneum, lung | Immunoembolization, TACE with BCNU | MET Ab, Ipi, VPA, PD-1 Ab |
| 12 | M | 63 | 20–50 | 6.9 | None | TACE with BCNU, DEBDOX | None |
| 13 | F | 50 | 20–50 | 7.4 | None | TACE with BCNU, DEBDOX | None |
| 14 | M | 36 | 20–50 | 8.2 | None | None | None |
| 15 | F | 40 | > 50 | 10.4 | None | TACE with BCNU, DEBDOX | Ipi |
| 16 | F | 58 | > 50 | 13.1 | None | TACE with BCNU | None |
| 17 | M | 71 | 20–50 | 14.3 | Muscle, brain, bone, peritoneum, skin | TACE with BCNU, DEBDOX | XRT to brain |
Note: Patients were sorted according to the size of the largest hepatic metastasis. Immunoembolization, embolization with granulocyte macrophage colony-stimulating factor plus Interleukin-2.
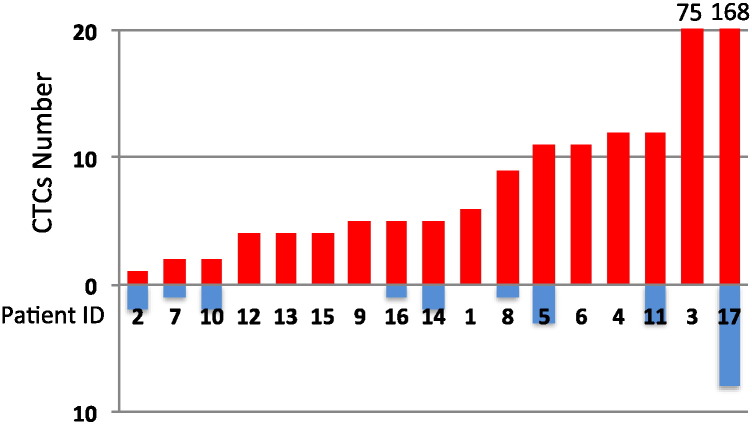
In the representative images, no obvious morphological difference of CTCs between arterial and venous blood was observed. CTCs clusters were not seen either (Fig. 1). CTCs were detected in all common femoral arterial blood specimens (100%) from these UM patients with multiple hepatic metastases (Fig. 2). The median number of CTCs in arterial blood was 5 (minimum 1, maximum 168), and inter-quartile range was from 4 to 11 CTCs. In contrast, CTCs were detectable in only 9/17 (52.9%) of peripheral venous blood specimens from the same patients. The median number of CTCs in the venous blood samples was 1 (minimum 0, maximum 8), and inter-quartile range from 0 to 2 CTCs with statistically significant difference between arterial blood specimens and peripheral venous blood specimens (p < 0.001).
Fig. 2.
Numbers of CTC in peripheral arterial and venous blood specimens.
Each column shows CTC numbers in arterial (red column) or venous (blue column) blood specimen in the same patient.
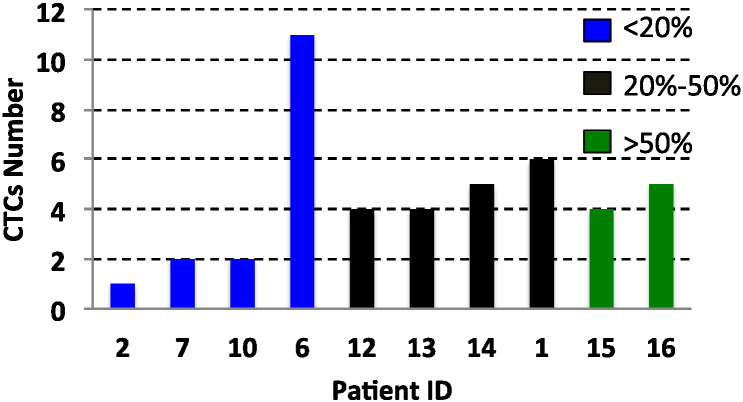
It is of note that there is no significant association between numbers of arterial or venous CTC and extent of tumor burden within the liver or the size of the largest tumor. The arterial CTC numbers of 10 patients who had liver-only metastases were not proportional to the volume of liver involvement by metastasis (p = 0.423) (Fig. 3). Furthermore, there was no significant correlation between the size of the largest hepatic metastasis and numbers of CTC in arterial blood of patients who have liver-only metastases (coefficient 0.074, p = 0.839).
Fig. 3.
Hepatic tumor volume and numbers of arterial CTC in patients with liver-only metastases.
Each column showed the CTC numbers in individual patients. Blue columns: liver involvement with tumor < 20%; purple columns: 20–50%; and green columns: > 50%.
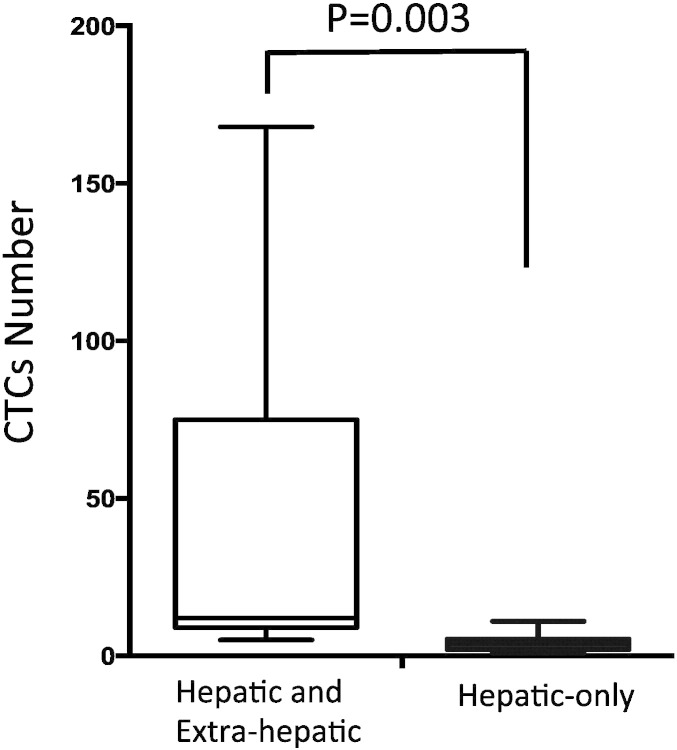
In terms of sites of metastasis, 7 patients who have hepatic as well as extra-hepatic metastases showed significantly higher numbers of CTC in arterial blood specimens (median 12, minimum 5, maximum 168, and inter-quartile range from 10 to 43.5), compared to the arterial numbers of CTC in 10 patients who have liver-only metastasis (median 4, minimum 1, maximum 11, and inter-quartile range from 2.5 to 5) (p = 0.003) (Fig. 4). There was no significant correlation between the presence of extra-hepatic metastasis and the total liver involvement by metastasis or the size of the largest hepatic metastasis.
Fig. 4.
Numbers of arterial CTC in uveal melanoma patients with and without extra-hepatic metastases.
Numbers of CTC detected in patients who had hepatic and extra-hepatic metastases liver-only metastases were higher than those obtained from patients with hepatic metastases only (p = 0.003).
4. Discussion
In this small pilot study, we detected CTCs in all arterial blood samples from UM patients who have multiple hepatic metastases. In contrast, the CTC detection rate and numbers of CTC were much lower in peripheral venous blood. Only half of venous blood specimens were positive for CTCs in stage IV UM patients. Since tumor cells may become apoptotic and fragmented while circulating in the peripheral venous system (Mehes et al., 2001), CTCs in the peripheral vein might not be the same as those in arterial blood and could be more fragile. In fact, our data have shown that numbers of CTC remarkably dropped in the peripheral venous circulation, compared to those in arterial blood, implying that venous blood specimens might not be an appropriate source for detection of CTCs in UM patients.
Due to the technical convenience and the assumption that CTCs obtained from the peripheral venous circulation represent the characteristics of metastasis, “venous blood specimens” have been used for detection of CTCs in cancer patients. This is based on the assumption that the sensitivity of the CTC detection methods is sufficient enough to detect CTCs in venous blood. Unfortunately, this assumption has not been proven to be true in various types of cancers including metastatic UM. As reported by Bidard's group, CTC detection with the CellSearch® method was only successful in 30% of UM patients with hepatic metastasis although numbers of CTC were associated with the presence of miliary hepatic metastasis (p < 0.004), metastasis volume (p = 0.005), progression-free survival (p = 0.003), and overall survival (p = 0.0009) (Bidard et al., 2014). Despite the correlation between venous CTCs and clinical outcome in a limited number of patients, it clearly shows that detection of venous CTCs is not sensitive enough to be used as a predictive marker for the presence of systemic metastasis. Similar results have been obtained from the investigation on stage IV breast cancer patients (Weissenstein et al., 2012). In 59 patients with metastatic breast cancer, CTCs were not detectable in 20 patients using cytokeratin and EpCAM antibodies. These results indicate that currently available technology using venous blood specimens is not suitable for early detection of metastatic disease. This also limits the clinical utilization of venous CTCs in stage IV cancer patients since radiographic imaging is much more sensitive in detecting and evaluating metastatic disease. A limited number of patients might have benefit by measuring CTCs after their treatments since better correlation has been seen between changes in numbers of CTC and their survival, compared to that of radiographic images (Budd et al., 2006); however, this observation would not be sufficient enough to change our standard practice in using radiographic images to evaluate the response to treatments in metastatic breast cancer patients. This would also raise the concern in using venous blood CTC as a surrogate marker to predict poor prognosis in patients with metastatic UM. In this regard, investigation of arterial blood specimens, rather than peripheral venous specimens, would be more sensitive in detection of CTCs in stage IV UM patients. Despite technical challenges, changing the source of blood specimens from peripheral vein to peripheral artery might open a new window of opportunity in utilizing CTCs as a surrogate prognostic marker for stage IV cancer patients, including UM.
It has been reported that a CTC-like cell could be detectable in the venous blood of healthy volunteers. By using the CellSearch melanoma kit, one CTC like cell was detected in the peripheral blood of 3 out of 55 healthy donors (Rao et al., 2011). This phenomenon could be explained by contamination of skin melanocytes during venipuncture and it might not necessarily be “false positive”. It is less likely that this is related to real “false positive” detection since the CellSearch System is a well established and validated technique with FDA approval for detection of various cancer cells. False positive results due to the contaminated skin melanocytes are not our concern for this study since arterial blood specimens were collected after flushing the catheter and discarding the first 5 ml of blood return before collecting blood specimens for CTC measurements.
It must also be emphasized that numbers of CTC did not correlate to the volume of hepatic metastasis in our patient population. It is also of note that CTC numbers tend to be higher in patients who had hepatic and extra-hepatic metastases. Our data indicate that the number of CTCs in the arterial blood is not reflective of the tumor volume in the liver, but instead, it may reflect different biological features of tumor cells such as less cohesiveness (scattering) and metastatic ability to other organs; therefore, they may have distinct prognostic contribution that cannot be obtained by standard radiographic evaluation.
Kinetics of CTCs has been investigated in colon cancer and hepatocellular carcinoma. Jiao et al. measured CTCs in different blood compartments before and after surgical intervention or radiofrequency ablation of hepatic metastasis in 29 colon cancer patients. CTCs were examined in both systemic and portal circulation by obtaining blood samples from the peripheral vein and the artery, the portal vein, and the hepatic vein. They reported that CTCs were much higher in the hepatic porto-systemic circulation, compared to peripheral systemic circulation, indicating that the majority of CTCs from hepatic metastases are trapped during the lung circulation (Jiao et al., 2009). Fang et al. detected and quantified CTCs in the peripheral veins and right atrium in patients with hepatocellular carcinoma using Ep-CAM antibody-conjugated magnetic beads. The detection rates of CTC were significantly higher in the right atrium, compared to peripheral venous blood (73.8% versus 52.4%). The number of CTCs were also higher in blood obtained from the right atrium (median 6, interquartile range 15.5), compared to those in peripheral venous blood (median 1, interquartile range 5.5) (Fang et al., 2014). These studies indicate that the lung circulation would be the major determining factor for CTCs in peripheral circulation and the development of systemic metastasis. In this regard, arterial CTCs passing through the lung circulation might have more potential in the development of systemic recurrence, which is consistent with our observation, in which CTC numbers were higher in patients with hepatic and extra-hepatic metastases. Future investigation on CTCs in pre- and post-lung circulation would provide important insight into this speculation.
The natural history of UM is poorly understood. Due to the lack of a lymphatic draining system, UM cells disseminate hematogeneously. In this regard, the detection of circulating melanoma cells may potentially be useful for the diagnosis, risk stratification, monitoring of disease progression, and analysis of treatment efficacy. Our data indicates that arterial blood, rather than venous blood, might be suitable for future investigation on CTCs in UM patients. This would also raise the concern as to whether venous blood specimens are a suitable source for investigation on CTCs in other types of cancer. Further investigation to address this fundamental question should be warranted.
The following is the supplementary data related to this article.
Spiked UM cells in healthy donor blood.
(A) 5, 15, 30, 100, 500 and 1500 UM cells were spiked into six individual tubes that contained 7.5 ml of blood from the same healthy donor. The number of UM cells was plotted versus the observed number of cells spiked. Detected UM cell numbers tended to be lower than added numbers probably due to the elimination of tumor cells by allogeneic blood lymphocytes. (B) Representative image of spiked UM cells in blood from the healthy donor. Tumor cells were surrounded by allogeneic lymphocytes.
Contributors
MT assembled and interpreted data on CTCs and wrote this manuscript. MJM, MC, and TS designed the study and interpreted data. DE, CG, and TS enrolled patients for this study and collected specimens. MT, ZM and KK analyzed CTCs. IC performed statistical analysis on the data. MO and RW coordinated this study.
All authors reviewed and approved the final draft of the paper.
Conflicts of interest
We declare that we have no conflicts of interest.
Acknowledgments
The authors thank Tracey Newhall, RN, Katherine Sohon, BS, Mitchell Berkowitz, BS, and Shingo Sato, BS for the coordination of this study and blood specimen collections. A part of the results in this study was presented at the American Association for Cancer Research (AACR) annual meeting in Philadelphia, PA (2015).
This study was supported by the Wills Vision Research Center at Jefferson Innovation Grant, Bonnie Kroll Research Fund, Mark Weinziel Research Fund, and Eye Melanoma Research Fund at Thomas Jefferson University.
References
- All-Ericsson C., Girnita L., Seregard S., Bartolazzi A., Jager M.J., Larsson O. Insulin-like growth factor-1 receptor in uveal melanoma: a predictor for metastatic disease and a potential therapeutic target. Invest. Ophthalmol. Vis. Sci. 2002;43(1):1–8. [PubMed] [Google Scholar]
- Bidard F.C., Madic J., Mariani P., Piperno-Neumann S., Rampanou A., Servois V., Cassoux N., Desjardins L., Milder M., Vaucher I. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastatic uveal melanoma. Int. J. Cancer. 2014;134(5):1207–1213. doi: 10.1002/ijc.28436. [DOI] [PubMed] [Google Scholar]
- Budd G.T., Cristofanilli M., Ellis M.J., Stopeck A., Borden E., Miller M.C., Matera J., Repollet M., Doyle G.V., Terstappen L.W. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin. Cancer Res. 2006;12(21):6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- Burger J.A., Kipps T.J. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay C., Grimm E.A., Woodman S.E. Simultaneous inhibition of the HGF/MET and Erk1/2 pathways affect uveal melanoma cell growth and migration. PLoS One. 2014;9(2):e83957. doi: 10.1371/journal.pone.0083957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S.J., Punt C.J., Iannotti N., Saidman B.H., Sabbath K.D., Gabrail N.Y., Picus J., Morse M., Mitchell E., Miller M.C. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26(19):3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- Collaborative Ocular Melanoma Study G Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch. Ophthalmol. 2001;119(5):670–676. doi: 10.1001/archopht.119.5.670. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Matera J., Miller M.C., Reuben J.M., Doyle G.V., Allard W.J., Terstappen L.W. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M., Reuben J., Uhr J. Circulating tumor cells in breast cancer: fiction or reality? J. Clin. Oncol. 2008;26(21):3656–3657. doi: 10.1200/JCO.2008.18.0356. (author reply 3657-3658) [DOI] [PubMed] [Google Scholar]
- Danila D.C., Heller G., Gignac G.A., Gonzalez-Espinoza R., Anand A., Tanaka E., Lilja H., Schwartz L., Larson S., Fleisher M. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin. Cancer Res. 2007;13(23):7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- Demicheli R., Fornili M., Biganzoli E. Bimodal mortality dynamics for uveal melanoma: a cue for metastasis development traits? BMC Cancer. 2014;14:392. doi: 10.1186/1471-2407-14-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Skelley A.M., Merdek K.D., Sprott K.M., Jiang C., Pierceall W.E., Lin J., Stocum M., Carney W.P., Smirnov D.A. Microfluidics and circulating tumor cells. J. Mol. Diagn. 2013;15(2):149–157. doi: 10.1016/j.jmoldx.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Eskelin S., Pyrhonen S., Summanen P., Hahka-Kemppinen M., Kivela T. Tumor doubling times in metastatic malignant melanoma of the uvea: tumor progression before and after treatment. Ophthalmology. 2000;107(8):1443–1449. doi: 10.1016/s0161-6420(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Ewens K.G., Kanetsky P.A., Richards-Yutz J., Purrazzella J., Shields C.L., Ganguly T., Ganguly A. Chromosome 3 status combined with BAP1 and EIF1AX mutation profiles are associated with metastasis in uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2014;55(8):5160–5167. doi: 10.1167/iovs.14-14550. [DOI] [PubMed] [Google Scholar]
- Fang Z.T., Zhang W., Wang G.Z., Zhou B., Yang G.W., Qu X.D., Liu R., Qian S., Zhu L., Liu L.X. Circulating tumor cells in the central and peripheral venous compartment — assessing hematogenous dissemination after transarterial chemoembolization of hepatocellular carcinoma. Onco Targets Ther. 2014;7:1311–1318. doi: 10.2147/OTT.S62605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez S.V., Bingham C., Fittipaldi P., Austin L., Palazzo J., Palmer G., Alpaugh K., Cristofanilli M. TP53 mutations detected in circulating tumor cells present in the blood of metastatic triple Negative breast cancer patients. Breast Cancer Res. 2014;16(5):445. doi: 10.1186/s13058-014-0445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J.W., Onken M.D., Roberson E.D., Duan S., Cao L., Worley L.A., Council M.L., Matatall K.A., Helms C., Bowcock A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L.R., Apostolopoulos C., Jacob J., Szydlo R., Johnson N., Tsim N., Habib N.A., Coombes R.C., Stebbing J. Unique localization of circulating tumor cells in patients with hepatic metastases. J. Clin. Oncol. 2009;27(36):6160–6165. doi: 10.1200/JCO.2009.24.5837. [DOI] [PubMed] [Google Scholar]
- Krivacic R.T., Ladanyi A., Curry D.N., Hsieh H.B., Kuhn P., Bergsrud D.E., Kepros J.F., Barbera T., Ho M.Y., Chen L.B. A rare-cell detector for cancer. Proc. Natl. Acad. Sci. U. S. A. 2004;101(29):10501–10504. doi: 10.1073/pnas.0404036101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala E., Makitie T., Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2003;44(11):4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- Li H., Alizadeh H., Niederkorn J.Y. Differential expression of chemokine receptors on uveal melanoma cells and their metastases. Invest. Ophthalmol. Vis. Sci. 2008;49(2):636–643. doi: 10.1167/iovs.07-1035. [DOI] [PubMed] [Google Scholar]
- Lorigan J.G., Wallace S., Mavligit G.M. The prevalence and location of metastases from ocular melanoma: imaging study in 110 patients. AJR Am. J. Roentgenol. 1991;157(6):1279–1281. doi: 10.2214/ajr.157.6.1950883. [DOI] [PubMed] [Google Scholar]
- Mazzini C., Pinzani P., Salvianti F., Scatena C., Paglierani M., Ucci F., Pazzagli M., Massi D. Circulating tumor cells detection and counting in uveal melanomas by a filtration-based method. Cancers. 2014;6(1):323–332. doi: 10.3390/cancers6010323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehes G., Witt A., Kubista E., Ambros P.F. Circulating breast cancer cells are frequently apoptotic. Am. J. Pathol. 2001;159(1):17–20. doi: 10.1016/S0002-9440(10)61667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menyaev Y.A., Nedosekin D.A., Sarimollaoglu M., Juratli M.A., Galanzha E.I., Tuchin V.V., Zharov V.P. Optical clearing in photoacoustic flow cytometry. Biomed. Opt. Express. 2013;4(12):3030–3041. doi: 10.1364/BOE.4.003030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C., Bui T., Connelly M., Doyle G., Karydis I., Middleton M.R., Clack G., Malone M., Coumans F.A., Terstappen L.W. Circulating melanoma cells and survival in metastatic melanoma. Int. J. Oncol. 2011;38(3):755–760. doi: 10.3892/ijo.2011.896. [DOI] [PubMed] [Google Scholar]
- Rietschel P., Panageas K.S., Hanlon C., Patel A., Abramson D.H., Chapman P.B. Variates of survival in metastatic uveal melanoma. J. Clin. Oncol. 2005;23(31):8076–8080. doi: 10.1200/JCO.2005.02.6534. [DOI] [PubMed] [Google Scholar]
- Sarimollaoglu M., Nedosekin D.A., Menyaev Y.A., Juratli M.A., Zharov V.P. Nonlinear photoacoustic signal amplification from single targets in absorption background. Photoacoustics. 2014;2(1):1–11. doi: 10.1016/j.pacs.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.D., Shields C.L., Shields J.A. Prognostic factors in uveal melanoma. Melanoma Res. 2001;11(3):255–263. doi: 10.1097/00008390-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Singh A.D., Turell M.E., Topham A.K. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118(9):1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Suesskind D., Ulmer A., Schiebel U., Fierlbeck G., Spitzer B., Spitzer M.S., Bartz-Schmidt K.U., Grisanti S. Circulating melanoma cells in peripheral blood of patients with uveal melanoma before and after different therapies and association with prognostic parameters: a pilot study. Acta Ophthalmol. (Copenh) 2011;89(1):17–24. doi: 10.1111/j.1755-3768.2009.01617.x. [DOI] [PubMed] [Google Scholar]
- Tura A., Luke J., Merz H., Reinsberg M., Luke M., Jager M.J., Grisanti S. Identification of circulating melanoma cells in uveal melanoma patients by dual-marker immunoenrichment. Invest. Ophthalmol. Vis. Sci. 2014;55(7):4395–4404. doi: 10.1167/iovs.14-14512. [DOI] [PubMed] [Google Scholar]
- Weissenstein U., Schumann A., Reif M., Link S., Toffol-Schmidt U.D., Heusser P. Detection of circulating tumor cells in blood of metastatic breast cancer patients using a combination of cytokeratin and EpCAM antibodies. BMC Cancer. 2012;12:206. doi: 10.1186/1471-2407-12-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Selvan S., McCue P.A., DeAngelis T., Baserga R., Fujii A., Rui H., Mastrangelo M.J., Sato T. Expression of insulin-like growth factor-1 receptor in metastatic uveal melanoma and implications for potential autocrine and paracrine tumor cell growth. Pigment Cell Melanoma Res. 2014;27(2):297–308. doi: 10.1111/pcmr.12206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spiked UM cells in healthy donor blood.
(A) 5, 15, 30, 100, 500 and 1500 UM cells were spiked into six individual tubes that contained 7.5 ml of blood from the same healthy donor. The number of UM cells was plotted versus the observed number of cells spiked. Detected UM cell numbers tended to be lower than added numbers probably due to the elimination of tumor cells by allogeneic blood lymphocytes. (B) Representative image of spiked UM cells in blood from the healthy donor. Tumor cells were surrounded by allogeneic lymphocytes.