Abstract
Long-term linezolid use is limited by mitochondrial toxicity-associated adverse events (AEs). Within a prospective, randomized controlled trial of linezolid to treat chronic extensively drug-resistant tuberculosis, we serially monitored the translational competence of mitochondria isolated from peripheral blood of participants by determining the cytochrome c oxidase/citrate synthase activity ratio. We compared this ratio with AEs associated with mitochondrial dysfunction. Linezolid trough concentrations were determined for 38 participants at both 600 mg and 300 mg doses. Those on 600 mg had a significantly higher risk of AE than those on 300 mg (HR 3·10, 95% CI 1·23–7 · 86). Mean mitochondrial function levels were significantly higher in patients before starting linezolid compared to their concentrations on 300 mg (P = 0·004) or 600 mg (P < 0·0001). Increasing mean linezolid trough concentrations were associated with lower mitochondrial function levels (Spearman's ρ = − 0.48; P = 0.005). Mitochondrial toxicity risk increased with increasing linezolid trough concentrations, with all patients with mean linezolid trough > 2 μg/ml developing an AE related to mitochondrial toxicity, whether on 300 mg or 600 mg. Therapeutic drug monitoring may be useful to prevent the development of mitochondrial toxicity associated with long-term linezolid use.
Keywords: Mitochondrial toxicity, Adverse events, Linezolid, Tuberculosis, Drug resistant, Therapeutic drug monitoring
Highlights
-
•
Linezolid is increasingly being used to treat drug-resistant tuberculosis.
-
•
Long-term use of linezolid is limited by mitochondrial toxicity-associated adverse events.
-
•
We examined linezolid trough concentrations in chronic XDR-TB patients on linezolid.
-
•
Mitochondrial toxicity risk increased with increasing linezolid trough concentrations.
-
•
Therapeutic drug monitoring may help avoid mitochondrial toxicities in long-term linezolid treatment.
Linezolid is increasingly being used to treat drug resistant tuberculosis, with the beneficial effect of linezolid tempered by the mitochondrial toxicity-associated adverse events seen with long-term use. Within a prospective, randomized controlled trial of linezolid for the treatment of chronic, extensively drug-resistant tuberculosis, we found that the risk of mitochondrial toxicity increased with increasing linezolid trough concentrations, with all patients with a mean trough > 2 μg/ml developing an adverse event related to mitochondrial toxicity. If our results are confirmed by other studies, therapeutic drug monitoring may become an important component of long-term linezolid treatment to prevent mitochondrial toxicity-related adverse events.
1. Introduction
Linezolid is a protein synthesis inhibitor approved by the U.S. Food and Drug Administration for the treatment of vancomycin-resistant Enterococcus faecium infections, nosocomial pneumonias, and skin and skin structure infections. Linezolid binds to the 50S subunit of the bacterial ribosome, competing with recognition of the incoming aminoacyl-tRNA molecule that would normally bind to this site (Ippolito et al., 2008). Mammalian mitochondrial ribosomes, unlike cytosolic ribosomes, are thought to have been symbiotically acquired from free-living prokaryotes (Sagan, 1967). Although mammalian mitochondrial ribosomes have evolved substantially from their prokaryotic progenitors (Greber, BJ, et al., 2014, Greber, BJ, et al., 2015), in the region of the central loop of Domain V containing the cleft where linezolid (and the amino acid side chains of charged tRNAs) binds, the rRNA is very conserved. Further experimental evidence that linezolid binds to this region of the mammalian ribosome has been obtained (Leach et al., 2007). The use of linezolid for longer than 2–3 weeks is therefore limited by adverse events such as bone marrow suppression, lactic acidosis, and peripheral and optic neuropathy that have been associated with the inhibition of mitochondrial protein synthesis (De Vriese, AS, et al., 2006, McKee, EE, et al., 2006, Miro, O and Mensa, J, 2005).
Linezolid has been used off-label in the treatment of drug-resistant tuberculosis, with growing evidence for its efficacy but with use limited by these adverse events (Lee, M, et al., 2012, Lee, M, et al., 2015, Tang, S, et al., 2015, Sotgiu, G, et al., 2012, Koh, WJ, et al., 2012). In this analysis, we describe in more detail the adverse events associated with mitochondrial toxicity that occurred in the trial conducted by our group (Lee, M, et al., 2012, Lee, M, et al., 2015) and quantify the extent of mitochondrial toxicity in association with linezolid dose and trough concentrations.
2. Methods
2.1. Linezolid Clinical Trial
We conducted a prospective, randomized controlled trial of linezolid for the treatment of chronic, extensively drug-resistant tuberculosis (XDR-TB), as previously described (Lee et al., 2012). Patients failing their best background treatment regimen for the previous 6 months were randomized either to immediate or delayed (2 months) addition of linezolid 600 mg orally once daily to their failing regimen. The primary endpoint was time to sputum culture conversion on solid medium, with data censored at 4 months. After sputum smear conversion or receipt of linezolid for 4 months, there was a second randomization either to continue linezolid 600 mg once daily or to reduce the dose to 300 mg once daily. The optimized background regimen was also continued and additional drugs were permitted as needed. Patients were then followed to the end of therapy (about 2 years) plus one additional year for final treatment outcomes. This study was approved by the local institutional review boards and by the U.S. National Institute of Allergy and Infectious Diseases and is registered at clinicaltrials.gov (NCT00727844).
For this analysis, only adverse events associated with mitochondrial dysfunction – peripheral neuropathy, optic neuropathy, and bone marrow suppression – were included. Lactic acidosis did not occur in this trial. Peripheral neuropathies were graded by the Subjective Peripheral Neuropathy Score (SPNS) (McArthur, 1998) and SPNS grades ≥ 2 were included in this analysis, unless the grade 2 predated linezolid administration.
2.2. Pharmacokinetics
Blood for linezolid trough concentrations was collected in most patients at both 600 mg and 300 mg (unless the dose was never reduced to 300 mg). The ratio of the average trough value at 600 mg compared to 300 mg was computed as 2·27 and this factor was used to impute 300 mg trough values for those missing these data.
2.3. Mitochondrial Enzyme Assay
Blood for the mitochondrial enzyme assay was collected before starting linezolid, then every two weeks from weeks 3–23, monthly through month 7, and every 2 months thereafter. Additional blood was also collected during drug pauses due to adverse events and for two weeks after restarting linezolid. Mitochondria were isolated using the Mitochondria Isolation Kit (MITOISO2, Sigma) from peripheral blood mononuclear cells prepared by Ficoll density gradient centrifugation. Spectrometric measurement of the activities of cytochrome c oxidase, three subunits of which are translational products of mitochondrial ribosomes (Lemberg, MR, 1969, Attardi, G and Schatz, G, 1988), and citrate synthase, a mitochondrial protein synthesized by cytosolic ribosomes that serves as a reliable marker of mitochondrial content (Attardi, G and Schatz, G, 1988, Srere, PA, 1969, Garrabou, G, et al., 2007), was performed using the Cytochrome c Oxidase Assay Kit (CYTOCOX1, Sigma) and the Citrate Synthase Assay Kit (CS0720, Sigma), respectively. Enzyme activity was calculated as specific activity in μmole thionitrobenzoic acid formed (for citrate synthase) or μmole ferrocytochrome c oxidized (for cytochrome c oxidase) per minute per mg of protein. Mitochondrial function, as an indicator of the translational competence of mitochondrial ribosomes, was defined as the ratio of cytochrome c oxidase activity to citrate synthase activity. Due to the time needed to develop and optimize the mitochondrial function assay, however, mitochondrial function values were not collected during the first year of the study.
2.4. Polymorphisms in Mitochondrial 16S rRNA
A portion of mitochondrial DNA corresponding to nucleotides 515–3221 of the mitochondrial genome (GeneBank ID, NC_012920) (Anderson et al., 1981) containing both the 12S and 16S rRNA sequences was amplified by polymerase chain reaction and subjected to sequencing in both directions commercially (Solgent, Daejeon, S. Korea). Sequence reads were compared to the reference sequence (GeneBank ID, NC_012920) to identify single nucleotide polymorphisms (SNPs) using Sequencher software (Gene Codes Corp., Ann Arbor, MI, USA).
2.5. Statistical Analyses
Baseline characteristics between those who did or did not develop adverse events associated with mitochondrial toxicity were compared using a two-sample t-test for continuous variables and Fisher's exact test for binary variables. The main statistical analyses were conducted based on Cox models for time-to-event analyses. These models can evaluate covariates that vary over time, such as mitochondrial function and linezolid dose, and time-fixed covariates, such as randomization arm. The starting time for these analyses was based on two different points in the study:
-
1.
Time from initiation of linezolid 600 mg once daily;
-
2.
Time from second randomization to linezolid 600 mg or 300 mg dose, corresponding to patients who remained on linezolid (and in the study) long enough to undergo this second randomization.
The association of mitochondrial function and dose was evaluated using generalized estimating equations to account for correlation introduced by clustered data (i.e., multiple observations contributed by a single subject). Spearman's rank correlation coefficient and a corresponding p-value were estimated by randomly sampling a single observation per subject. Boxplots summarized the medians and interquartile ranges of the mean mitochondrial function values for each subject at a given dose.
2.6. Role of the Funding Source
The National Institutes of Health participated in study design, data collection, analysis, interpretation of data, writing of the report, and the decision to submit the paper for publication. The Republic of Korea Ministry of Health and Welfare was not involved in any of these aspects of this study. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
3. Results
The baseline characteristics of the 38 participants who received linezolid are listed in Table 1, according to the presence or absence of a mitochondrial toxicity-associated adverse event. Overall, 23/38 (60·5%) developed an adverse event and 15/38 (39·5%) did not. Patients who developed an adverse event had similar baseline characteristics to those who did not except that more were older (P = 0·01) and fewer had received BCG vaccination (P = 0·03). Patients failing their best background regimen for the previous six months were randomized to add linezolid 600 mg daily immediately or after a delay of two months while maintaining their existing (failing) treatment regimen. Median time from linezolid start to first adverse event was 237 days. At sputum smear conversion or receipt of 4 months of linezolid, patients were randomized a second time either to remain on linezolid 600 mg daily or to reduce to 300 mg daily. Median time to first adverse event after the second randomization was 92 days for patients on 600 mg and beyond the observation period (> 2 years) for those on 300 mg. Those continuing on 600 mg had a significantly higher risk of adverse events than those who reduced to 300 mg (hazard ratio 3·10, 95% confidence interval 1·23–7·86).
Table 1.
Baseline characteristics of 38 subjects who received linezolid.
| Characteristic⁎ | Mitochondrial toxicity-related adverse event |
|
|---|---|---|
| Yes (N = 23) | No (N = 15) | |
| Mean age, years (SD) | 43·9 (2·0) | 35·8 (2·2) |
| Male, no. (%) | 14 (60·9%) | 13 (86·7%) |
| Mean body mass index (SD) | 20·6 (0·9) | 19·3 (0·9) |
| Diabetes, no. (%) | 10 (43·5%) | 4 (26·7%) |
| BCG vaccination, no. (%) | 16 (69·6%) | 15 (100%) |
| Radiographic findings, no. (%) | ||
| Far advanced | 19 (82·6%) | 10 (66·7%) |
| Cavitation⁎⁎ | 10 (43·5%) | 7 (46·7%) |
| Bilateral | 23 (100·0%) | 14 (93·3%) |
| Median no. previous treatment episodes (IQR) | 5·0 (3·0–7·0) | 4·0 (3·0–7·0) |
| Adverse event, no. (%) | ||
| Peripheral neuropathy | 13 (56·5%) | |
| Optic neuropathy | 6 (26·1%) | |
| Myelosuppression | 4 (17·4%) | |
There were no significant differences at baseline between the two groups except for age (P = 0·01) and BCG (P = 0·03). Continuous variables summarized by means were tested using a two-sample t-test, while a continuous variable summarized using a median was tested using Wilcoxon's rank sum test. Categorical variables were tested using Fisher's exact test.
Excludes 7 patients with data missing for cavitation.
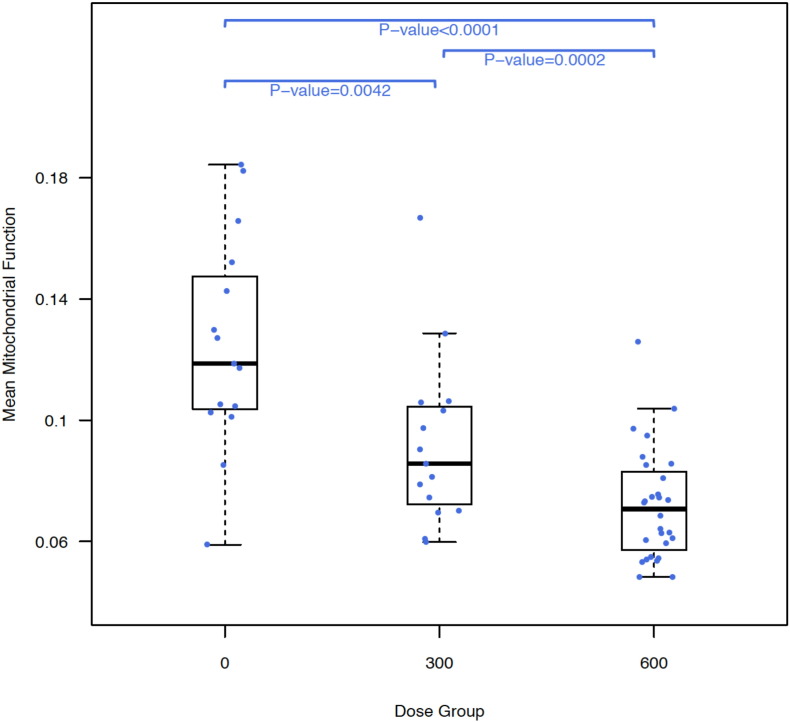
All subjects had blood drawn for mitochondrial function and linezolid trough concentrations, of whom 26 had troughs taken under both linezolid 600 mg and 300 mg doses, while 12 had only linezolid 600 mg trough concentrations. Mean mitochondrial function levels were significantly higher in patients before starting linezolid compared to their concentrations on 300 mg (P = 0·004) or 600 mg (P < 0·0001) and concentrations in patients on 300 mg were significantly higher than concentrations in patients on 600 mg (P = 0·0002; Fig. 1). Mean mitochondrial function level correlated with the risk of an adverse event. For example, a decrease in mitochondrial function of one standard deviation (i.e., a 0.04 drop) was associated with a doubling of the risk of an adverse event after the second randomization or after linezolid initiation (Table 2).
Fig. 1.
Boxplot of subject-level mean mitochondrial function by linezolid dose. Mean mitochondrial function values for patients receiving linezolid 300 mg daily and 600 mg daily were significantly lower than for the same patients at baseline (pre-linezolid). Mean mitochondrial function for patients on linezolid 600 mg daily was also significantly lower than for patients on 300 mg daily. Note that the bars of the boxplots summarize the median and interquartile values of the subject's mean values.
Table 2.
Cox regression results from different starting times to the first subsequent adverse event (AE).
| Baseline time for analysis: | Hazard ratio (95% CI) | Z-statistic | P-value |
|---|---|---|---|
| Mean linezolid trough and risk of AE | |||
| Time from second randomization | 2.00 (1.45, 2.77)⁎ | 4.19 | < 0.001 |
| Time from linezolid initiation | 2.05 (1.50, 2.79)⁎ | 4.52 | < 0.001 |
| Time varying mitochondrial function and risk of AE | |||
| Time from second randomization | 0.49 (0.30, 0.93)† | − 2.17 | 0.030 |
| Time from linezolid initiation | 0.53 (0.25, 0.93)† | − 2.21 | 0.027 |
Increased risk for each 1 μg/ml increase in linezolid trough concentration.
Decreased risk for each 1 standard deviation (0.04) increase in mitochondrial function level.
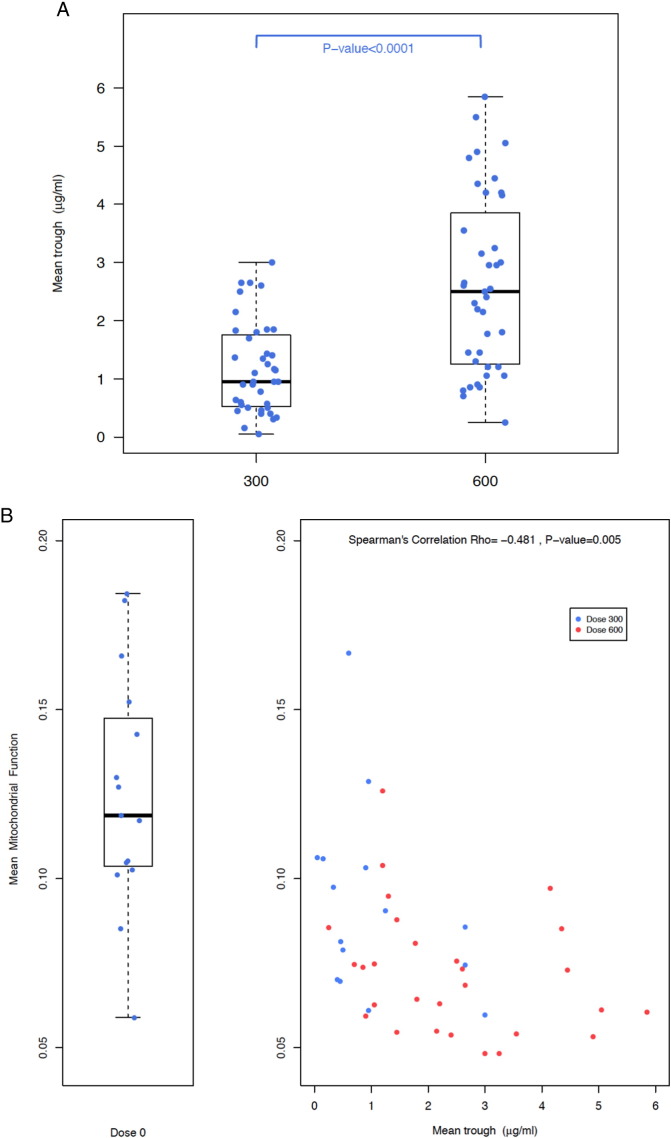
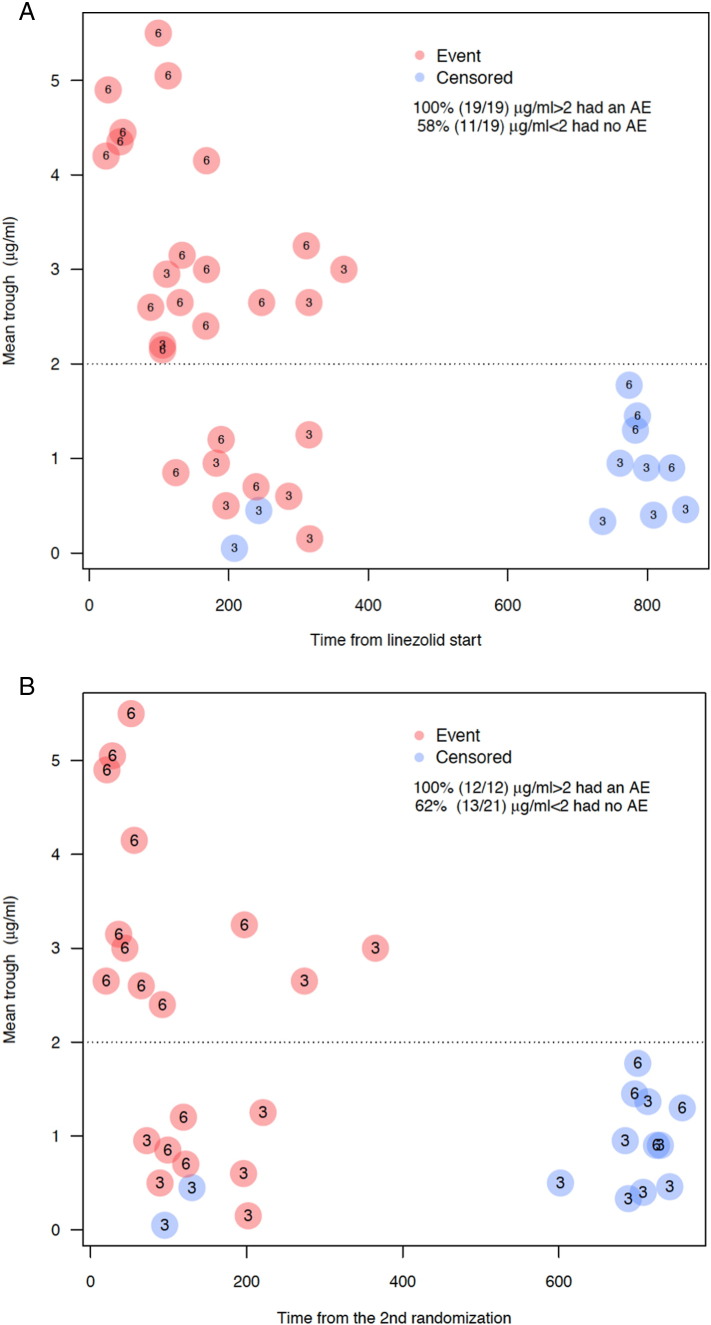
Mean linezolid trough concentrations were significantly lower for patients on the 300 mg dose compared to the 600 mg dose (P < 0·0001; Fig. 2A) and mean linezolid trough concentrations also inversely correlated with mean mitochondrial function levels, with increasing linezolid trough concentrations associated with decreasing mitochondrial function levels (Spearman's ρ = − 0·48; P = 0·005; Fig. 2B). Finally, mean linezolid trough concentrations directly correlated with the risk of an adverse event, such that for each 1 μg/ml increase in linezolid trough, there was a 2-fold increase in the risk of an adverse event (Table 2). This was true whether or not the risk was modeled from the time of linezolid initiation or the time of second randomization. Fig. 3 shows the time to adverse event from the time of linezolid start (Fig. 3A) or second randomization (Fig. 3B), stratified by mean linezolid trough concentration. All patients with a mean linezolid trough > 2 μg/ml developed an adverse event, whether on 300 mg or 600 mg, whereas at trough < 2 μg/ml, less than half developed an adverse event. There was no correlation between linezolid trough concentration and type of adverse event.
Fig. 2.
A: Mean linezolid trough concentration by linezolid dose. Mean linezolid trough concentration was significantly higher for patients on 600 mg daily compared to 300 mg daily. Note that the bars of the boxplots summarize the median and interquartile values of the subject's mean values. B: Mean mitochondrial function level by mean linezolid trough concentration. There is a significant inverse correlation such that higher mean linezolid trough concentrations correlate with lower mean mitochondrial function levels. Note that the bars of the boxplot summarize the median and interquartile values of the subject's mean values.
Fig. 3.
Time to adverse event from linezolid start (3A) or second randomization (3B) by mean linezolid trough concentration. All patients with a mean linezolid trough concentration > 2 μg/ml, regardless of dose, developed a mitochondrial toxicity-related adverse event. 6 = patient on 600 mg dose. 3 = patient on 300 mg dose.
Note: Five patients who reduced their linezolid dose from 600 mg to 300 mg due to adverse events before the second randomization are not included.
All patients were examined for polymorphisms in mitochondrial 16S rRNA but no associations were found between specific polymorphisms and mitochondrial toxicity-related adverse events (Table 3).
Table 3.
Mitochondrial ribosomal RNA single nucleotide polymorphisms (SNP) and their association with adverse events.
| SNP | Adverse event, N (%) |
Odds ratio (95% CI) | ||
|---|---|---|---|---|
| No | Yes | |||
| A2706G | No | 0 | 0 | – |
| Yes | 10 (100·0) | 27 (100·0) | ||
| A1736G | No | 9 (90·0) | 25 (92·6) | 0·72 (0·06–8·93) |
| Yes | 1 (10·0) | 2 (7·4) | ||
| A2833G | No | 10 (100·0) | 26 (96·3) | 1·19 (0·04–31·57) |
| Yes | 0 | 1 (3·7) | ||
| C1734T | No | 10 (100·0) | 26 (96·3) | 1·19 (0·04–31·57) |
| Yes | 0 | 1 (3·7) | ||
| C2772T | No | 10 (100·0) | 26 (96·3) | 1·19 (0·04–31·57) |
| Yes | 0 | 1 (3·7) | ||
| C2835T | No | 10 (100·0) | 26 (96·3) | 1·19 (0·04–31·57) |
| Yes | 0 | 1 (3·7) | ||
| C3206T | No | 9 (90·0) | 25 (92·6) | 0·72 (0·06–8·93) |
| Yes | 1 (10·0) | 2 (7·4) | ||
| G1719A | No | 10 (100·0) | 26 (96·3) | 1·19 (0·04–31·57) |
| Yes | 0 | 1 (3·7) | ||
| G3010A | No | 6 (60·0) | 20 (74·1) | 0·52 (0·11–2·42) |
| Yes | 4 (40·0) | 7 (25·9) | ||
| Ins2150 | No | 9 (90·0) | 26 (96·3) | 0·35 (0·02–6·13) |
| Yes | 1 (10·0) | 1 (3·7) | ||
| T2483C | No | 10 (100·0) | 26 (96·3) | 1·19 (0·04–31·57) |
| Yes | 0 | 1 (3·7) | ||
| T2626C | No | 10 (100·0) | 26 (96·3) | 1·19 (0·04–31·57) |
| Yes | 0 | 1 (3·7) | ||
4. Discussion
This analysis of 38 pulmonary XDR-TB patients treated with linezolid demonstrates the inverse association between linezolid trough concentrations and mitochondrial function, with higher trough concentrations associated with lower mitochondrial function. Furthermore, lower (impaired) mitochondrial function and higher linezolid trough concentrations also correlated with the development of mitochondrial toxicity-related adverse events. No specific genetic polymorphism in mitochondrial 16S rRNA was found to be associated with the development of adverse events in our analysis.
The oxazolidinone antibiotics inhibit bacterial growth by binding to 50S ribosomal RNA thereby inhibiting bacterial protein synthesis. Likewise, because of the structural resemblance of bacterial ribosomes with mitochondrial ribosomes, mitochondrial protein synthesis also appears to be inhibited by oxazolidinone antibiotics, causing mitochondrial toxicity (Leach, KL, et al., 2007, McKee, EE, et al., 2006, Miro, O and Mensa, J, 2005, Narita, M, et al., 2007). Linezolid, the prototype drug of this class, is approved by the U.S. FDA for the treatment of severe infections caused by resistant Gram-positive bacteria and is relatively well tolerated when used for the approved treatment duration of up to 4 weeks. When used for longer durations, however, adverse events associated with mitochondrial toxicity manifest, including peripheral and optic neuropathy, lactic acidosis, and bone marrow suppression (Narita, M, et al., 2007, Beekmann, SE, et al., 2008). The extent of this toxicity, resulting from the inhibition of mitochondrial protein synthesis, can be quantitated by measuring the mitochondrial enzymatic activity of cytochrome c oxidase, which has been shown to decrease with linezolid use and return to normal with linezolid discontinuation (De Vriese, AS, et al., 2006, Miro, O and Mensa, J, 2005, Garrabou, G, et al., 2007, Nagiec, EE, et al., 2005). Mitochondrial function was therefore defined as the activity of cytochrome c oxidase, representing the extent of mitochondrial protein synthesis, normalized to the activity of citrate synthase, a surrogate marker of mitochondrial mass considering the possible fluctuation of mitochondrial mass with linezolid use (Garrabou et al., 2007). Declining mitochondrial function then can be demonstrated by declining cytochrome c oxidase activity per unit citrate synthase activity. In our analysis, mitochondrial function correlated inversely with both linezolid dose and linezolid trough concentration. Patients with an average linezolid trough concentration above 2 μg/ml no matter the dose all developed adverse events related to mitochondrial toxicity.
One case–control study and several case reports, including a total of 7 patients, suggested an association between linezolid mitochondrial toxicity and the mitochondrial ribosomal RNA polymorphisms A2706G or G3010A (Palenzuela, L, et al., 2005, Carson, J, et al., 2007, Velez, JC and Janech, MG, 2010, Del Pozo, JL, et al., 2014). In our analysis, we did not find any association between mitochondrial toxicity adverse events and these or any other mitochondrial RNA polymorphism (Table 3). All of our patients, whether they developed an AE or not, had the A2706G polymorphism.
Newer oxazolidinones associated with a lower risk of mitochondrial toxicity are being developed. Tedizolid is the second oxazolidinone approved by the FDA for severe infections caused by resistant Gram-positive bacteria and, in short-term studies, appears to have a lower side effect profile than linezolid (Burdette and Trotman, 2015). The reason for this difference may be because tedizolid appears to have a shorter mitochondrial binding half-life than linezolid and thus allows for a period of mitochondrial recovery with every dosing interval (Flanagan et al., 2015). Long-term use of tedizolid in rats has not been associated with adverse events, in contrast to linezolid (Schlosser, MJ, et al., 2015, Wang, T, et al., 2014). Long-term studies of tedizolid in humans have not been published.
In our analysis, we focused more on the risk of an adverse event following the second randomization because this was the only time patients were randomized to differing doses of linezolid, allowing a more unbiased comparison of the risks of these differing doses. At the same time, these patients may be a select group relative to the more general set of patients enrolled at baseline because they demonstrated improved tolerability of linezolid. Thus we also analyzed the risk of linezolid from the time of linezolid start. Both analyses gave very similar results.
There are some limitations to our analysis. First, because mitochondrial function values were not collected during the first year of the study, five mitochondrial toxicity-related adverse events were without associated mitochondrial function values and therefore were not included in the analysis. Regardless, this limitation does not affect the association between linezolid trough concentration and adverse event. Second, our pharmacokinetics data only included linezolid trough concentrations and did not include measurements to determine the daily area under the plasma concentration-time curve (AUC24). However, linezolid trough concentrations rather than peaks correlate linearly with estimated AUC24 (Pea et al., 2010). Linezolid trough concentrations also correlate with mitochondrial toxicity-related AEs (Cattaneo, D, et al., 2013, Sousa, R, et al., 2011) and correlate just as well as AUC24 (Pea et al., 2012). Finally, although we did not find any correlation between mitochondrial RNA SNPs and linezolid mitochondrial toxicity, our analysis is limited by the small sample size.
Long-term use of linezolid is associated with development of the mitochondrial toxicities of peripheral neuropathy, optic neuropathy, lactic acidosis, and myelosuppression. Mitochondrial toxicity can be quantified by measuring mitochondrial function and impaired mitochondrial function is directly correlated with an increased risk of developing these adverse events. This risk increases until, at least in our analysis, all patients with an average linezolid trough concentration above 2 μg/ml developed an adverse event. If confirmed by other analyses, patients on long-term linezolid should have trough concentrations measured periodically and have their linezolid dose adjusted to maintain a trough concentration below 2 μg/ml. As tedizolid and other second-generation oxazolidinones that appear to have a lower risk of developing mitochondrial toxicity reach the market, therapeutic drug concentration monitoring may no longer be necessary.
Author Contributions
TS, KNO, LEV, SNC, and CEB designed the study. TS, ML, and YC collected the data. TS, H-SJ, YP, VD, and LEV conducted the laboratory analyses. LED, DF, JW, and YC analyzed the data. TS, LED, VD, DF, LCG, KNO, YX, CEB, and RYC interpreted the data. RYC drafted the manuscript. All authors reviewed and approved the final version.
Declarations of Interests
We declare no competing interests.
Acknowledgements
This project has been funded in whole or in part with federal funds from the Intramural Research Programs, National Institute of Allergy and Infectious Diseases and National Heart, Lung, and Blood Institute, National Institutes of Health; the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E; and by the Ministry of Health and Welfare, Republic of Korea. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- Anderson S., Bankier A.T., Barrell B.G. Sequence and organization of the human mitochondrial genome. Nature. 1981;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu. Rev. Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Beekmann S.E., Gilbert D.N., Polgreen P.M., Network I.E.I. Toxicity of extended courses of linezolid: results of an Infectious Diseases Society of America Emerging Infections network survey. Diagn. Microbiol. Infect. Dis. 2008;62(4):407–410. doi: 10.1016/j.diagmicrobio.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Burdette S.D., Trotman R. Tedizolid: the first once-daily oxazolidinone class antibiotic. Clin. Infect. Dis. 2015 doi: 10.1093/cid/civ501. [DOI] [PubMed] [Google Scholar]
- Carson J., Cerda J., Chae J.H., Hirano M., Maggiore P. Severe lactic acidosis associated with linezolid use in a patient with the mitochondrial DNA A2706G polymorphism. Pharmacotherapy. 2007;27(5):771–774. doi: 10.1592/phco.27.5.771. [DOI] [PubMed] [Google Scholar]
- Cattaneo D., Orlando G., Cozzi V. Linezolid plasma concentrations and occurrence of drug-related haematological toxicity in patients with Gram-positive infections. Int. J. Antimicrob. Agents. 2013;41(6):586–589. doi: 10.1016/j.ijantimicag.2013.02.020. [DOI] [PubMed] [Google Scholar]
- De Vriese A.S., Coster R.V., Smet J. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin. Infect. Dis. 2006;42(8):1111–1117. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- Del Pozo J.L., Fernandez-Ros N., Saez E., Herrero J.I., Yuste J.R., Banales J.M. Linezolid-induced lactic acidosis in two liver transplant patients with the mitochondrial DNA A2706G polymorphism. Antimicrob. Agents Chemother. 2014;58(7):4227–4229. doi: 10.1128/AAC.02856-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan S., McKee E.E., Das D. Nonclinical and pharmacokinetic assessments to evaluate the potential of tedizolid and linezolid to affect mitochondrial function. Antimicrob. Agents Chemother. 2015;59(1):178–185. doi: 10.1128/AAC.03684-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrabou G., Soriano A., Lopez S. Reversible inhibition of mitochondrial protein synthesis during linezolid-related hyperlactatemia. Antimicrob. Agents Chemother. 2007;51(3):962–967. doi: 10.1128/AAC.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber B.J., Bieri P., Leibundgut M. Ribosome. The complete structure of the 55S mammalian mitochondrial ribosome. Science. 2015;348(6232):303–308. doi: 10.1126/science.aaa3872. [DOI] [PubMed] [Google Scholar]
- Greber B.J., Boehringer D., Leitner A. Architecture of the large subunit of the mammalian mitochondrial ribosome. Nature. 2014;505(7484):515–519. doi: 10.1038/nature12890. [DOI] [PubMed] [Google Scholar]
- Ippolito J.A., Kanyo Z.F., Wang D. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 2008;51(12):3353–3356. doi: 10.1021/jm800379d. [DOI] [PubMed] [Google Scholar]
- Koh W.J., Kang Y.R., Jeon K. Daily 300 mg dose of linezolid for multidrug-resistant and extensively drug-resistant tuberculosis: updated analysis of 51 patients. J. Antimicrob. Chemother. 2012;67(6):1503–1507. doi: 10.1093/jac/dks078. [DOI] [PubMed] [Google Scholar]
- Leach K.L., Swaney S.M., Colca J.R. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol. Cell. 2007;26(3):393–402. doi: 10.1016/j.molcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Lee M., Cho S.N., Barry C.E., 3rd, Song T., Kim Y., Jeong I. Linezolid for XDR-TB–Final Study Outcomes. N. Engl. J. Med. 2015;373(3):290–291. doi: 10.1056/NEJMc1500286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Lee J., Carroll M.W. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N. Engl. J. Med. 2012;367(16):1508–1518. doi: 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg M.R. Cytochrome oxidase. Physiol. Rev. 1969;49(1):48–121. doi: 10.1152/physrev.1969.49.1.48. [DOI] [PubMed] [Google Scholar]
- McArthur J.H. The reliability and validity of the subjective peripheral neuropathy screen. J. Assoc. Nurses AIDS Care. 1998;9(4):84–94. doi: 10.1016/S1055-3290(98)80048-4. [DOI] [PubMed] [Google Scholar]
- McKee E.E., Ferguson M., Bentley A.T., Marks T.A. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob. Agents Chemother. 2006;50(6):2042–2049. doi: 10.1128/AAC.01411-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano A., Miro O., Mensa J. Mitochondrial toxicity associated with linezolid. N. Engl. J. Med. 2005;353(21):2305–2306. doi: 10.1056/NEJM200511243532123. [DOI] [PubMed] [Google Scholar]
- Nagiec E.E., Wu L., Swaney S.M. Oxazolidinones inhibit cellular proliferation via inhibition of mitochondrial protein synthesis. Antimicrob. Agents Chemother. 2005;49(9):3896–3902. doi: 10.1128/AAC.49.9.3896-3902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M., Tsuji B.T., Yu V.L. Linezolid-associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome. Pharmacotherapy. 2007;27(8):1189–1197. doi: 10.1592/phco.27.8.1189. [DOI] [PubMed] [Google Scholar]
- Palenzuela L., Hahn N.M., Nelson R.P., Jr. Does linezolid ause lactic acidosis by inhibiting mitochondrial protein synthesis? Clin. Infect. Dis. 2005;40(12):e113–e116. doi: 10.1086/430441. [DOI] [PubMed] [Google Scholar]
- Pea F., Furlanut M., Cojutti P. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob. Agents Chemother. 2010;54(11):4605–4610. doi: 10.1128/AAC.00177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pea F., Viale P., Cojutti P., Del Pin B., Zamparini E., Furlanut M. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J. Antimicrob. Chemother. 2012;67(8):2034–2042. doi: 10.1093/jac/dks153. [DOI] [PubMed] [Google Scholar]
- Sagan L. On the origin of mitosing cells. J. Theor. Biol. 1967;14(3):255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- Schlosser M.J., Hosako H., Radovsky A. Lack of neuropathological changes in rats administered tedizolid phosphate for nine months. Antimicrob. Agents Chemother. 2015;59(1):475–481. doi: 10.1128/AAC.03950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgiu G., Centis R., D'Ambrosio L. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur. Respir. J. 2012;40(6):1430–1442. doi: 10.1183/09031936.00022912. [DOI] [PubMed] [Google Scholar]
- Sousa R., Lopez R., Martinez-Pastor J.C. Usefulness of monitoring linezolid trough serum concentration in prolonged treatments. Rev. Esp. Quimioter. 2011;24(3):151–153. [PubMed] [Google Scholar]
- Srere P.A. Citrate synthase. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- Tang S., Yao L., Hao X. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur. Respir. J. 2015;45(1):161–170. doi: 10.1183/09031936.00035114. [DOI] [PubMed] [Google Scholar]
- Velez J.C., Janech M.G. A case of lactic acidosis induced by linezolid. Nat. Rev. Nephrol. 2010;6(4):236–242. doi: 10.1038/nrneph.2010.20. [DOI] [PubMed] [Google Scholar]
- Wang T., Guo D., Dong X., Mu L. Effect of linezolid on hematological and oxidative parameters in rats. J. Antibiot. (Tokyo) 2014;67(6):433–437. doi: 10.1038/ja.2014.21. [DOI] [PubMed] [Google Scholar]