Abstract
Background
Recently, chronic hepatitis E has been increasingly reported in organ transplant recipients in European countries. In Japan, the prevalence of hepatitis E virus (HEV) infection after transplantation remains unclear, so we conducted a nationwide cross-sectional study to clarify the prevalence of chronic HEV infection in Japanese liver transplant recipients.
Methods
A total of 1893 liver transplant recipients in 17 university hospitals in Japan were examined for the presence of immunoglobulin G (IgG), IgM and IgA classes of anti-HEV antibodies, and HEV RNA in serum.
Findings
The prevalence of anti-HEV IgG, IgM and IgA class antibodies was 2.9% (54/1893), 0.05% (1/1893) and 0% (0/1893), respectively. Of 1651 patients tested for HEV RNA, two patients (0.12%) were found to be positive and developed chronic infection after liver transplantation. In both cases, HEV RNA was also detected in one of the blood products transfused at the perioperative period. Analysis of the HEV genomes revealed that the HEV isolates obtained from the recipients and the transfused blood products were identical in both cases, indicating transfusion-transmitted HEV infection.
Interpretation
The prevalence of HEV antibodies in liver transplant recipients was 2.9%, which is low compared with the healthy population in Japan and with organ transplant recipients in European countries; however, the present study found, for the first time, two Japanese patients with chronic HEV infection that was acquired via blood transfusion during or after liver transplantation.
Keywords: Hepatitis E virus, Chronic hepatitis E, Liver transplantation, Transfusion
Highlights
-
•
We conducted the multicenter survey for HEV infection in liver transplant recipients.
-
•
Though the chronic HEV infection is rare, transfusion-transmitted cases were detected.
-
•
Blood products can be a risk of chronic HEV infection in transplant recipients.
1. Introduction
Hepatitis E is caused by infection with the hepatitis E virus (HEV), and HEV isolates that infect humans are currently categorized into four genotypes (1–4) (Okamoto, 2007). Genotypes 1 and 2 are restricted to humans and mainly waterborne transmitted in developing countries. In contrast, genotypes 3 and 4 are known to undergo zoonotic transmission by consumption of uncooked or undercooked meat or viscera of reservoir mammals and autochthonous isolates cause sporadic infections in industrialized countries (Takahashi et al., 2003, Tei et al., 2003).
Acute hepatitis E varies in severity from inapparent to fulminant. Mortality has been reported to be between 1% and 4% in the general population but up to 25% in pregnant women (Datta et al., 1987). HEV infection has traditionally been considered to be a transient and self-limiting disease requiring no specific therapy in immunocompetent individuals (Wedemeyer et al., 2012). However, HEV infection may occasionally cause severe liver dysfunction, fulminant hepatitis, and liver failure in some patients with an underlying disease (Kamar et al., 2012, Suzuki et al., 2002). Furthermore, HEV genotype 3 can lead to chronic hepatitis and liver cirrhosis in immunocompromised patients such as solid-organ transplant (SOT) recipients (Kamar et al., 2008), patients with human immunodeficiency virus infection (Dalton et al., 2009), and patients with hematologic cancers receiving chemotherapy (Ollier et al., 2009). Various studies have investigated the presence of HEV infection in SOT recipients in European and American countries but not yet in Japan. We conducted the first nationwide survey to clarify the prevalence of HEV antibodies and the presence of liver transplant recipients with chronic HEV infection in Japan.
2. Methods
2.1. Study Subjects
From all the regions of Japan, 17 high-volume centers for liver transplantation participated in this study (from north to south): Hokkaido University Hospital in Hokkaido; Tohoku University Hospital in the Tohoku area; University of Tsukuba Hospital, University of Tokyo Hospital, Keio University Hospital and Juntendo University Hospital in the Kanto area; Shinshu University Hospital in the Chubu area; Kyoto University Hospital, Osaka University Hospital and Kobe University Hospital in the Kinki area; Okayama University Hospital and Hiroshima University Hospital in the Chugoku area; Ehime University Hospital and Tokushima University Hospital in the Shikoku area; and Kyushu University Hospital, Nagasaki University Hospital and Kumamoto University Hospital in the Kyushu area. The number of liver transplantations performed in these 17 centers accounts for approximately 75% of the total performed in Japan (The Japanese Liver Transplantation Society, 2014).
Between April 1, 2013 and December 31, 2014, blood samples were collected from 1893 recipients being followed up at the above-mentioned 17 centers after liver transplantation. Anti-HEV antibodies were tested for in all 1893 samples. Within all the 1893 participants, 1651 patients who agreed to have HEV RNA testing, including all patients with detectable anti-HEV Ig (immunoglobulin) G class antibody, were also tested for the presence of HEV RNA in serum. The samples were stored at − 80 °C until testing. The clinical data of the patients, including medical history, medication profiles and laboratory test results, were retrieved from medical records. This study was approved by the institutional review board at every participating hospital and performed in accordance with the Declaration of Helsinki and ethical guidelines for clinical research. All the patients gave written informed consent to participate in this study.
2.2. Detection of Anti-HEV Antibodies and HEV RNA
To detect anti-HEV IgA, IgM and IgG class antibodies, in-house enzyme-linked immunosorbent assay (ELISA) was performed using purified recombinant ORF2 protein as described previously (Mizuo et al., 2002). The optical density (OD) of each sample was read at 450 nm. The cut-off value used for anti-HEV IgA, IgM and IgG was 0.642, 0.440 and 0.175, respectively (Takahashi et al., 2005). The samples with OD values for anti-HEV IgA, IgM, or IgG equal to or greater than the respective cut-off value were considered to be positive for each antibody. The specificity of ELISA was validated by absorption with the same recombinant ORF2 protein that was used as the antigen probe, as described previously (Takahashi et al., 2005).
Total RNA was extracted from 100 μL of each serum or whole blood sample and subjected to nested reverse transcription-polymerase chain reaction (RT-PCR) for the detection of HEV RNA. A nested RT-PCR targeting the 137-nt ORF2/3 overlapping region was conducted for the screening of HEV RNA (Inoue et al., 2006). This method represents a high sensitivity and is able to detect 1–3 copies of HEV RNA in 100 μL serum. For the samples detected positive by the ORF2/3–137 PCR, we performed an additional nested RT-PCR targeting the 457-nt ORF2 region to confirm the presence of HEV RNA and to assess the subgenotype, as described previously (Mizuo et al., 2002). The HEV genotype/subgenotype was determined based on the phylogenetic analysis of the ORF2 sequence.
Quantitation of HEV RNA was performed by real-time detection RT-PCR as described previously (Takahashi et al., 2008).
3. Results
3.1. Characteristics of the Subjects
A total of 1893 liver transplant recipients participated in this study; their demographic characteristics and laboratory data are presented in Table 1. Median age was 57 years old and median time since liver transplantation to serum sampling was 81 months. At the time of blood sampling, the median values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil) and gamma-glutamyl transferase (γ-GT) were exclusively within normal limits. “Liver injury episode after transplantation” was defined as the presence of elevated liver enzymes acquiring the management with re-hospitalization, and 61.3% of the recipients were included.
Table 1.
Characteristics of liver transplant recipients.a
| Total number of recipients [n] | 1893 |
| Age [years; median (total range)] | 57 (0–83) |
| Sex [n (%); male/female] | 970 (51.2)/923 (48.8) |
| Time from transplantation [months; median (total range)] | 81 (0–297) |
| Laboratory data at sampling | |
| WBC [/μL; median (total range)] | 5565 (1070–97,000) |
| Lymphocyte [/μL; median (total range)] | 1576 (157–24,225) |
| AST [IU/L; median (total range)] | 24 (1–484) |
| ALT [IU/L; median (total range)] | 19 (3–619) |
| T-Bil [mg/dL; median (total range)] | 0.8 (0.1–40.0) |
| γ-GT [IU/L; median (total range)] | 36 (0–3118) |
| Immunosuppression [n (%)] | |
| Tacrolimus/cyclosporin | 1359 (71.8)/327 (17.3) |
| Mycophenolic acid | 685 (36.2) |
| Corticosteroids | 681 (36.0) |
| Re-hospitalization due to liver injury episode after transplantationa [n (%)] | 1161 (61.3) |
Note: WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T-Bil, total bilirubin; γ-GT, gamma-glutamyl transferase.
Liver injury episode after transplantation was defined as the presence of elevated liver enzymes acquiring the management with re-hospitalization.
3.2. Prevalence of Anti-HEV Antibodies and HEV RNA
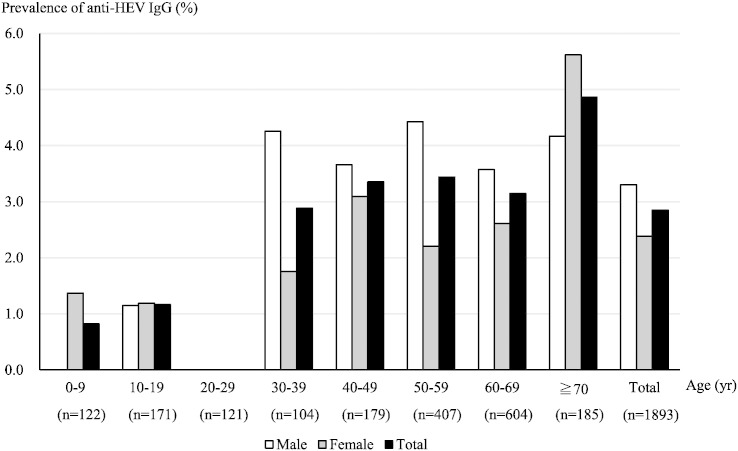
All of the 1893 serum samples were subjected to ELISA for the detection of anti-HEV antibodies (Table 2). Fifty-four patients (2.9%) were found to be positive for anti-HEV IgG class antibody. The age- and sex-specific prevalence of IgG class antibody in the recipients is shown in Fig. 1. The prevalence was slightly higher among the males (3.3%) than among the females (2.4%), although not statistically significant, and generally increased with age. Only one patient with detectable IgG class antibody was also positive for IgM class antibody (0.05%), while none had detectable IgA class antibody. In this patient, who was positive for IgG and IgM antibodies, HEV RNA was also detectable as noted below.
Table 2.
Prevalence of HEV antibodies and HEV RNA in liver transplant recipients.a
| Positive patient [n] | Percent [%] | |
|---|---|---|
| Anti-HEV IgG | 54/1893 | 2.9 |
| Anti-HEV IgM | 1/1893 | 0.05 |
| Anti-HEV IgA | 0/1893 | 0 |
| HEV RNA | 2/1651a | 0.12 |
Note: Ig, immunoglobulin.
The number of patients whose informed consent for testing HEV RNA was available.
Fig. 1.
The age- and sex-specific prevalence of anti-HEV IgG class antibody in liver transplant recipients in Japan.
The overall prevalence of anti-HEV IgG class antibody in liver transplant recipients was 2.9% (male, 3.3%; female, 2.4%). The prevalence generally increased with age..
n: the number of the recipients in each age group.
Two of the 54 patients with anti-HEV IgG tested positive for HEV RNA, while none of the remaining 1597 patients without detectable IgG class antibody who agreed to have the HEV RNA testing were positive for HEV RNA. Overall, HEV RNA was found to be positive at the prevalence rate of 0.12% (2/1651) (Table 2). The detailed clinical information and laboratory data at the time of diagnosis in these two HEV RNA-positive patients are shown in Table 3. Case 1 patient was a 60-year-old female living in the Kanto area who received liver transplantation for primary sclerosing cholangitis, while Case 2 patient was a 41-year-old male living in the Kyushu area who received liver transplantation for non-alcoholic steatohepatitis complicated with hepatocellular carcinoma. In these two patients, the duration from liver transplantation to blood sampling was lower than the 25 percentile (36 months), and the peripheral blood lymphocyte count was lower than the 25 percentile (1039 /μL). Whereas 71.8% and 35.9% of the patients negative for HEV RNA had been taking tacrolimus and corticosteroids respectively, both two positive patients had been taking tacrolimus and corticosteroids for immunosuppression. The HEV isolates were typed as genotype 3 in both patients (Table 3). The more detailed clinical courses of these two patients will be described elsewhere.
Table 3.
Characteristics of HEV RNA-positive liver transplant recipients.
| Variables | Case 1 | Case 2 |
|---|---|---|
| Age [years], sex | 60, female | 41, male |
| Living area | Kanto | Kyushu |
| Primary disease | Primary sclerosing cholangitis | Non-alcoholic steatohepatitis, hepatocellular carcinoma |
| Time from transplantation [months] | 8 | 3 |
| Laboratory data at sampling | ||
| WBC [/μL] | 1500 | 6170 |
| Lymphocyte [/μL] | 453 | 882 |
| AST [IU/L] | 23 | 36 |
| ALT [IU/L] | 27 | 48 |
| T-Bil [mg/dL] | 0.8 | 0.4 |
| γ-GT [IU/L] | 81 | 63 |
| Immunosuppression | Tacrolimus, corticosteroids | Tacrolimus, corticosteroids |
| Liver injury episode after transplantation |
+ | + |
| HEV genotype | 3 | 3 |
3.3. Elucidation of Possible Infectious Source and Route
To clarify whether these two HEV-viremic patients contracted HEV infection during or after liver transplantation and to investigate the possible infectious route of HEV in these patients, we retrospectively tested anti-HEV antibodies and HEV RNA in stored serum samples from each pair of patient and liver donor (Table 4).
Table 4.
The behavior of anti-HEV antibodies and HEV RNA in the chronic infection cases.
| Case 1 |
Case 2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-HEV IgG [OD value] |
Anti-HEV IgM [OD value] |
Anti-HEV IgA [OD value] |
HEV RNA [copies/mL] |
Anti-HEV IgG [OD value] |
Anti-HEV IgM [OD value] |
Anti-HEV IgA [OD value] |
HEV RNA [copies/mL] |
||
| Pre-transplantation | 0.410 (+) | 0.041 (−) | 0.062 (−) | – | Pre-transplantation | 0.016 (−) | 0.051 (−) | 0.197 (−) | – |
| At diagnosis (255 POD) |
> 3.000 (+) | 0.223 (−) | 0.089 (−) | 8.1 × 105 | At diagnosis (81 POD) |
0.204 (+) | 1.063 (+) | 0.082 (−) | 1.3 × 106 |
| After diagnosis (360 POD) |
> 3.000 (+) | 0.203 (−) | 0.105 (−) | 5.5 × 104 | After diagnosis (249 POD) |
> 3.000 (+) | 1.480 (+) | 0.143 (−) | 3.2 × 106 |
| Liver donor | 0.021 (−) | 0.029 (−) | 0.021 (−) | – | Liver donor | 0.006 (−) | 0.036 (−) | 0.010 (−) | – |
| Blood donor (FFP) |
NA | NA | NA | +a | Blood donor (Platelet) |
NA | NA | NA | 1.8 × 104 |
Note: OD, optical density; POD, postoperative day; FFP, fresh frozen plasma; NA, not available.
The titer of HEV RNA was not available.
In Case 1 patient, anti-HEV IgG class antibody was weakly positive; representing past HEV infection, while HEV RNA was not detected in the pre-transplant serum. Anti-HEV IgG became high-titer positive (OD value: > 3.000) on the 255th postoperative day (POD) accompanied by high-titer HEV RNA (8.1 × 105 copies/mL), and HEV RNA was persistently positive on the 360th POD. In Case 2 patient, anti-HEV IgG and IgM antibodies and HEV RNA were negative in the pre-transplant serum. On the 81st POD, the positive conversion of IgG and IgM antibodies was detected accompanied by high-titer HEV RNA (1.3 × 106 copies/mL). HEV RNA was persistently positive on the 249th POD. These results suggested that both patients acquired their HEV infection during or after liver transplantation and developed chronic infection of HEV. In addition, retrospective and prospective investigation of their laboratory data and virological analysis revealed that these recipients had presented mild liver injury and HEV viremia for at least more than 6 months. Therefore, these two patients were finally diagnosed as having chronic hepatitis E.
In both cases, HEV antibodies and HEV RNA were undetectable in the liver donors, excluding the possibility of de novo HEV infection from the transplanted liver. The two HEV-viremic patients had no history of eating raw or undercooked meat or shellfish after liver transplantation. During the perioperative period of liver transplantation, a total of 17 units of blood products (concentrated red blood cells, fresh frozen plasma or platelets) were transfused in Case 1 patient, and 26 units of blood products (concentrated red blood cells, fresh frozen plasma or platelets) transfused in Case 2 patient. Pilots of all the blood products transfused during the perioperative period had been stored in the Japanese Red Cross Society, and were also tested for the presence of HEV RNA. HEV RNA was detectable in one unit of the fresh frozen plasma transfused to Case 1 patient (the viral titer was not available) and one unit of the platelet preparation transfused to Case 2 patient (the viral titer was 1.8 × 104 copies/mL). Complete nucleotide sequence identity was obtained in the 412-nt sequence of ORF2 of each HEV isolate. As a result, in both cases, the HEV isolates detected from the recipient and the blood product were considered to be same, and the transfused blood products were determined to be the infectious source.
4. Discussion
HEV infection had not been considered responsible for chronic hepatitis until Kamar et al. reported the first case series of chronic hepatitis E in SOT recipients in 2008 (Kamar et al., 2008). Subsequently, chronic infections of HEV in transplant recipients have been described in several studies (Haagsma et al., 2009, Legrand-Abravanel et al., 2010). According to the latest review article in 2014, up to 50–60% of acute HEV infections can progress to chronic phase in SOT recipients and HEV infection can take rapid courses leading to liver cirrhosis in patients receiving immunosuppressants (Behrendt et al., 2014). Despite the high prevalence of HEV in the SOT recipients in European countries, chronic hepatitis E in Asian countries has been rarely reported (Behrendt et al., 2014).
By 2013, over 7000 liver transplants had been performed in Japan (The Japanese Liver Transplantation Society, 2014). The number of national notifications of acute hepatitis E has been increasing since anti-HEV IgA antibody measurement started to be covered by insurance in 2011 (The National Institute of Infectious Diseases. Infectious Diseases Weekly Report (IDWR), 2003–2013), and the isolation of autochthonous HEV as a causative agent of acute hepatitis has increased. However, no nationwide survey of HEV infection in liver transplant recipients had been performed. So the present study is the first nationwide survey to investigate the prevalence of HEV infection in liver transplant recipients in Japan. This large multicenter severance clarified that the presence of HEV infection in liver transplant recipients is not frequent in Japan compared with in Western countries as described below. However, two cases of transfusion-transmitted hepatitis E were detected; therefore, recipients have low but certain risk of HEV infection by blood products.
Though more than 60% of the recipients had some kind of episode of liver injury, the values of liver enzymes, T-Bil and γ-GT were exclusively within normal limits at the time of sampling. These episodes of liver injury were most commonly due to the biliary complication or the recurrence of hepatitis B and C, and seemed unrelated to HEV infection. In addition, even in the ongoing HEV infection cases, liver dysfunction was not severe or within normal limit sometimes. These laboratory data fluctuates easily in liver transplant recipients and it should be difficult to suspect active HEV infection from the clinical features alone.
In recent years, the prevalence and clinical courses of hepatitis E in transplant recipients were reported in European countries, North America and Iran (Legrand-Abravanel et al., 2010, Halac et al., 2012). In a review by Zhou et al. (2013), the overall prevalence of anti-HEV IgG class antibody in SOT recipients was 11.6% and 7.4% in liver transplant recipients. This review article included the results detected by several different ELISA kit, and different commercial serological measurements are known to indicate a variability of the prevalence rate (Kamar et al., 2012). Khan et al. (2011) analyzed the sensitivity and specificity of four ELISA systems for HEV antibodies (Abbott, Cosmic, TGH and Wantai) and revealed that Wantai test had the highest sensitivity and specificity (100% and 100%, respectively) among them. In this report, Cosmic test using our ELISA system also showed high sensitivity and specificity (98.1% and 100%, respectively) following Wantai test. Although it is difficult to compare, of course, the prevalence of IgG antibody in our study (2.9%) was lower than these reviewed prevalence rates. This is due to the intrinsic lower prevalence of HEV in the general population in Japan compared with in European countries (Kamar et al., 2012, Takahashi et al., 2010a). The prevalence in liver transplant recipients in Japan was also lower than 5.1% in Japanese blood donors whose age was 40–59 years old, and the male rate was 50% (Takeda et al., 2010). We have reported the prevalence of healthy people (Takahashi et al., 2010a), blood donors (Gotanda et al., 2007), dialysis patients (Mitsui et al., 2004) in Japan using the same ELISA method in this study, and the prevalence of Japanese recipients is also lower than these previous results. The low prevalence in the recipients is not thought to arise from the sensitivity of the measurement but from the special characteristics of the patients in this study.
The conceivable causes of the lower prevalence in the transplant recipients are as follows: First, it is possible that the reduction of the anti-HEV antibody titers was induced by immunosuppressive drugs (Sester et al., 2008). Second, avoiding consumption of raw or undercooked meat or shellfish after transplantation might reduce the chance of HEV infection, as normally seen in the general population. At 11 of the 17 institutions in this survey, the liver transplant recipients were prohibited from consuming raw or undercooked foods for at least 6 months after transplantation. Unfortunately, the serum samples before transplantation were not available from the majority of the studied patients, except for those from Case 1 and 2 patients with HEV viremia, and the prevalence of antibodies before and after transplantation could not be compared. However, on the basis of the assumption that half of acute HEV infections progress to chronic phase in transplant recipients (Behrendt et al., 2014), the liver transplant patients in Japan had less opportunity to be infected. In the two of HEV infection cases, IgA antibodies were not detected throughout the following course. Although anti-HEV IgA antibody measurement is covered by insurance in Japan because of its high sensitivity and specificity (Takahashi et al., 2005), HEV RNA detection seems to be the most suitable to detect the chronic infection in immunocompromised patients. The methods used in this study present a sufficient sensitivity and the low presence of ongoing HEV infection is likely to be true.
HEV infection is usually self-limiting in immunocompetent individuals and therefore specific therapies are not required in the majority of cases. However, it can lead to chronic hepatitis and liver cirrhosis in patients taking immunosuppressants after organ transplantation. Haagsma et al. (2009) reported two cases of liver transplantation in which chronic HEV infection developed gradually into graft cirrhosis and a second transplantation was needed. To prevent graft failure, persistent HEV infection in liver transplant recipients should not be overlooked and untreated. The first strategy for recipients with persistent HEV infection is considered to be reduction of the immunosuppressants, the second strategy is considered to be anti-viral therapies (Kamar et al., 2011). In our study, Case 1 patient had received anti-viral therapy and Case 2 had been followed up without any anti-viral therapy or reduction of the immunosuppressants after the diagnosis. More detailed information about the clinical courses of these cases will be published in a short time.
A previous study on the risk factors in SOT recipients for developing chronic hepatitis after acute HEV infection revealed that the recipients who developed chronic hepatitis had significantly lower counts of leukocytes, total lymphocytes, platelets, and CD2, CD3, and CD4 lymphocytes than transient patients (Kamar et al., 2008). In the present study, two cases with chronic infection were exposed to HEV in the early postoperative period and lower counts of lymphocytes in peripheral blood were observed. The reduced production of antibodies due to high dose immunosuppressants in the early postoperative period could contribute to the development of chronic infection even in a patient previously exposed to HEV. However, we cannot easily conclude from our results that these are the factors for risk of a chronic career after acute HEV infection, because we could neither detect any transient hepatitis patient nor compare the progress of acute and chronic infections.
HEV genotypes 3 and 4 usually cause sporadic infections, in industrialized countries most likely due to intake of contaminated food. HEV RNA has been detected in a variety of food products, especially in porcine livers (Colson et al., 2010). However, HEV can also be transmitted via the transplanted organ itself or through blood transfusion. Schlosser et al. reported that HEV transmission via the infected donor liver lead to chronic infection and graft cirrhosis development after liver transplantation (Schlosser et al., 2012). Several previous case reports suggested that HEV transmission by blood products is possible (Matsubayashi et al., 2004, Wedemeyer et al., 2012). According to the newest epidemiology of autochthones hepatitis E virus infection in Japan, 11 patients of 43 asymptomatic hepatitis E cases were detected from blood donors (Kanayama et al., 2015). In another report, 2% of HEV infections are ascribable to blood transfusion in Japan (Abe et al., 2006). Recently, Hewitt et al. reported that 42% of the patients in England transfused with HEV-positive blood products had evidence of infection with HEV and immunocompromised patients, including organ transplant recipients, showed prolonged viral infection (Hewitt et al., 2014). The two patients reported in our present study were the first cases with transfusion-transmitted chronic hepatitis E in liver transplant recipients in Japan, to the best of our knowledge. In addition, de novo HEV infection developed despite the presence of anti-HEV antibodies in Case 1. Takahashi et al. reported that the majority of HEV virions in serum possess lipids on their surfaces and inhibit neutralization by antibodies (Takahashi et al., 2010b). In Japan, screening of donated blood for HEV has only been conducted in the Hokkaido area, where HEV infection is most prevalent (Takahashi et al., 2010a). Patients receiving blood products include many immunocompromised individuals, so screening of blood donors for HEV in other areas in Japan is probably desirable: moreover, further investigations about HEV infection in other transplant recipients and immunosuppressed patients are required.
5. Conclusions
This study is the first multicenter survey for HEV infection in Asian liver transplant recipients and the presence of HEV infection is low in Japan; however, liver transplant recipients have a risk of transfusion-transmitted chronic hepatitis E. HEV infection should be considered as a differential diagnosis and molecular biological detection of HEV should be essential, when the recipient receives blood products and presents unknown liver injury.
Author Contributions
Yuki Inagaki: literature search, study design, patients' samples and data collection, data analysis, data interpretation, writing, tables and figures.
Yukio Oshiro: literature search, study design, participating institution recruitment, data interpretation, writing.
Tomohiro Tanaka, Tomoharu Yoshizumi, Hideaki Okajima, Kohei Ishiyama, Chikashi Nakanishi, Masaaki Hidaka, Hiroshi Wada, Taizo Hibi, Kosei Takagi, Masaki Honda, Kaori Kuramitsu, Hideaki Tanaka, Taiji Tohyama, Toshihiko Ikegami, Satoru Imura, Tsuyoshi Shimamura, Yoshimi Nakayama, Taizen Urahashi, Ken Shirabe, and Norihiro Kokudo: patients' samples and data collection.
Kazumasa Yamagishi: study design, data analysis.
Hiroshi Ohnishi, Shigeo Nagashima, and Masaharu Takahashi: samples measurement, data interpretation.
Hiroaki Okamoto: study design, samples measurement, data interpretation.
Nobuhiro Ohkohchi: study design, participating institution recruitment, data interpretation, corresponding author.
Yuki Inagaki and Yukio Oshiro contributed equally to the preparation of the text. All the authors reviewed and approved this manuscript.
Conflicts of interests
All authors report no conflict of interest.
Acknowledgments/Funding
This study was supported in part by a grant from the Ministry of Health, Labour and Welfare, Japan (H24-Hepatitis-General-002). The grant supported collecting the patients' samples and clinical data, and the measurement of the samples.
References
- Abe T., Aikawa T., Akahane Y., Arai M., Asahina Y., Atarashi Y. Demographic, epidemiological, and virological characteristics of hepatitis E virus infections in Japan based on 254 human cases collected nationwide. Kanzo. 2006;8:384–391. (in Japanese) [Google Scholar]
- Behrendt P., Steinmann E., Manns M.P., Wedemeyer H. The impact of hepatitis E in the liver transplant setting. J. Hepatol. 2014;61:1418–1429. doi: 10.1016/j.jhep.2014.08.047. [DOI] [PubMed] [Google Scholar]
- Colson P., Borentain P., Queyriaux B., Kaba M., Moal V., Gallian P. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010;202:825–834. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- Dalton H.R., Bendall R.P., Keane F.E., Tedder R.S., Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N. Engl. J. Med. 2009;361:1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- Datta R., Panda S.K., Tandon B.N., Madnagopalan N., Bose S.L., Acharya S.K. Acute sporadic non-A, non-B viral hepatitis of adults in India: epidemiological and immunological studies. J. Gastroenterol. Hepatol. 1987;2:333–345. [Google Scholar]
- Gotanda Y., Iwata A., Ohnuma H., Yoshikawa A., Mizoguchi H., Endo K. Ongoing subclinical infection of hepatitis E virus among blood donors with an elevated alanine aminotransferase level in Japan. J. Med. Virol. 2007;79:734–742. doi: 10.1002/jmv.20834. [DOI] [PubMed] [Google Scholar]
- Haagsma E.B., Niesters H.G., van den Berg A.P., Riezebos-Brilman A., Porte R.J., Vennema H. Prevalence of hepatitis E virus infection in liver transplant recipients. Liver Transpl. 2009;15:1225–1228. doi: 10.1002/lt.21819. [DOI] [PubMed] [Google Scholar]
- Halac U., Béland K., Lapierre P., Patey N., Ward P., Brassard J. Chronic hepatitis E infection in children with liver transplantation. Gut. 2012;61:597–603. doi: 10.1136/gutjnl-2011-300708. [DOI] [PubMed] [Google Scholar]
- Hewitt P.E., Ijaz S., Brailsford S.R., Brett R., Dicks S., Haywood B. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766–1773. doi: 10.1016/S0140-6736(14)61034-5. [DOI] [PubMed] [Google Scholar]
- Inoue J., Takahashi M., Yazaki Y., Tsuda F., Okamoto H. Development and validation of an improved RT-PCR assay with nested universal primers for detection of hepatitis E virus strains with significant sequence divergence. J. Virol. Methods. 2006;137:325–333. doi: 10.1016/j.jviromet.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Kamar N., Bendall R., Legrand-Abravanel F., Xia N.S., Ijaz S., Izopet J. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- Kamar N., Bendall R., Legrand-Abravanel F., Xia N.S., Ijaz S., Izopet J. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- Kamar N., Garrouste C., Haagsma E.B., Garrigue V., Pischke S., Chauvet C. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- Kanayama A., Arima Y., Yamagishi T., Kinoshita H., Sunagawa T., Yahata Y. Epidemiology of domestically acquired hepatitis E virus infection in Japan: assessment of the nationally reported surveillance data, 2007–2013. J. Med. Microbiol. 2015;64:752–758. doi: 10.1099/jmm.0.000084. [DOI] [PubMed] [Google Scholar]
- Khan A., Tanaka Y., Kurbanov F., Elkady A., Abbas Z., Azam Z. Investigating an outbreak of acute viral hepatitis caused by hepatitis E virus variants in Karachi, South Pakistan. J. Med. Virol. 2011;83:622–629. doi: 10.1002/jmv.22036. [DOI] [PubMed] [Google Scholar]
- Legrand-Abravanel F., Kamar N., Sandres-Saune K., Garrouste C., Dubois M., Mansuy J.M. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J. Infect. Dis. 2010;202:835–844. doi: 10.1086/655899. [DOI] [PubMed] [Google Scholar]
- Matsubayashi K., Nagaoka Y., Sakata H., Sato S., Fukai K., Kato T. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion. 2004;44:934–940. doi: 10.1111/j.1537-2995.2004.03300.x. [DOI] [PubMed] [Google Scholar]
- Mitsui T., Tsukamoto Y., Yamazaki C., Masuko K., Tsuda F., Takahashi M. Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: evidence for infection with a genotype 3 HEV by blood transfusion. J. Med. Virol. 2004;74:563–572. doi: 10.1002/jmv.20215. [DOI] [PubMed] [Google Scholar]
- Mizuo H., Suzuki K., Takikawa Y., Sugai Y., Tokita H., Akahane Y. Polyphyletic strains of hepatitis E virus are responsible for sporadic cases of acute hepatitis in Japan. J. Clin. Microbiol. 2002;40:3209–3218. doi: 10.1128/JCM.40.9.3209-3218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H. Genetic variability and evolution of hepatitis E virus. Virus Res. 2007;127:216–228. doi: 10.1016/j.virusres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Ollier L., Tieulie N., Sanderson F., Heudier P., Giordanengo V., Fuzibet J.G. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann. Intern. Med. 2009;150:430–431. doi: 10.7326/0003-4819-150-6-200903170-00026. [DOI] [PubMed] [Google Scholar]
- Schlosser B., Stein A., Neuhaus R., Pahl S., Ramez B., Krüger D.H. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J. Hepatol. 2012;56:500–502. doi: 10.1016/j.jhep.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Sester M., Gärtner B.C., Girndt M., Sester U. Vaccination of the solid organ transplant recipient. Transplant. Rev. (Orlando) 2008;22:274–284. doi: 10.1016/j.trre.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Aikawa T., Okamoto H. Fulminant hepatitis E in Japan. N. Engl. J. Med. 2002;347:1456. doi: 10.1056/NEJM200210313471819. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tamura K., Hoshino Y., Nagashima S., Yazaki Y., Mizuo H. A nationwide survey of hepatitis E virus infection in the general population of Japan. J. Med. Virol. 2010;82:271–281. doi: 10.1002/jmv.21678. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tanaka T., Takahashi H., Hoshino Y., Nagashima S., Jirintai Hepatitis E virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J. Clin. Microbiol. 2010;48:1112–1125. doi: 10.1128/JCM.02002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Hoshino Y., Tanaka T., Takahashi H., Nishizawa T., Okamoto H. Production of monoclonal antibodies against hepatitis E virus capsid protein and evaluation of their neutralizing activity in a cell culture System. Arch. Virol. 2008;153:657–666. doi: 10.1007/s00705-008-0045-6. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Kusakai S., Mizuo H., Suzuki K., Fujimura K., Masuko K. Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies against hepatitis E virus (HEV) is highly specific for diagnosis of acute HEV infection. J. Clin. Microbiol. 2005;43:49–56. doi: 10.1128/JCM.43.1.49-56.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Nishizawa T., Miyajima H., Gotanda Y., Iita T., Tsuda F. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. J. Gen. Virol. 2003;84:851–862. doi: 10.1099/vir.0.18918-0. [DOI] [PubMed] [Google Scholar]
- Takeda H., Matsubayashi K., Sakata H., Sato S., Kato T., Hino S. A nationwide survey for prevalence of hepatitis E virus antibody in qualified blood donors in Japan. Vox Sang. 2010;99:307–313. doi: 10.1111/j.1423-0410.2010.01362.x. [DOI] [PubMed] [Google Scholar]
- Tei S., Kitajima N., Takahashi K., Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- The Japanese Liver Transplantation Society Registry by the Japanese Liver Transplantation Society. Ishoku. 2014;49:261–274. (in Japanese) [Google Scholar]
- The National Institute of Infectious Diseases . 2003–2013. Infectious Diseases Weekly Report (IDWR) Available from http://www.nih.go.jp/niid/ja/idwr.html. [Google Scholar]
- Wedemeyer H., Pischke S., Manns M.P. Pathogenesis and treatment of hepatitis E virus infection. Gastroenterology. 2012;142:1388–1397. doi: 10.1053/j.gastro.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Zhou X., de Man R.A., de Knegt R.J., Metselaar H.J., Peppelenbosch M.P., Pan Q. Epidemiology and management of chronic hepatitis E infection in solid organ transplantation: a comprehensive literature review. Rev. Med. Virol. 2013;23:295–304. doi: 10.1002/rmv.1751. [DOI] [PubMed] [Google Scholar]