Abstract
Wnt/β-catenin is involved in every aspect of embryonic development and in the pathogenesis of many human diseases, and is also implicated in organ fibrosis. However, the role of β-catenin-mediated signaling on liver fibrosis remains unclear. To explore this issue, the effects of PRI-724, a selective inhibitor of the cAMP-response element-binding protein-binding protein (CBP)/β-catenin interaction, on liver fibrosis were examined using carbon tetrachloride (CCl4)- or bile duct ligation (BDL)-induced mouse liver fibrosis models. Following repetitive CCl4 administrations, the nuclear translocation of β-catenin was observed only in the non-parenchymal cells in the liver. PRI-724 treatment reduced the fibrosis induced by CCl4 or BDL. C-82, an active form of PRI-724, inhibited the activation of isolated primary mouse quiescent hepatic stellate cells (HSCs) and promoted cell death in culture-activated HSCs. During the fibrosis resolution period, an increase in F4/80+ CD11b+ and Ly6Clow CD11b+ macrophages was induced by CCl4 and was sustained for two weeks thereafter, even after having stopped CCl4 treatment. PRI-724 accelerated the resolution of CCl4-induced liver fibrosis, and this was accompanied by increased matrix metalloproteinase (MMP)-9, MMP-2, and MMP-8 expression in intrahepatic leukocytes. In conclusion, targeting the CBP/β-catenin interaction may become a new therapeutic strategy in treating liver fibrosis.
Abbreviations: CREB, cAMP-response element-binding protein; CBP, CREB-binding protein; CCl4, carbon tetrachloride; BDL, bile duct ligation; PBDL, partial BDL; HSC, hepatic stellate cell; MMP, matrix metalloproteinase; TGF-β, transforming growth factor; HCV, hepatitis C virus; αSMA, α-smooth muscle actin,; H–E, hematoxylin and eosin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TIMP-1, tissue inhibitor of metalloproteinase; CXCL, c–x–c motif ligand; CCL, c–c motif ligand; SPARC, secreted protein acidic and rich in cysteine
Keywords: Liver, Fibrosis, Beta-catenin, Hepatic stellate cell, Macrophage
Highlights
-
•
PRI-724, a selective inhibitor of CBP/β-catenin, suppresses liver fibrogenesis.
-
•
PRI-724 promotes fibrosis resolution in the liver.
-
•
PRI-724 decreases collagen production from myofibroblasts.
-
•
PRI-724 increases the production of MMPs from macrophages.
Although liver fibrosis is becoming increasingly recognized as a major cause of morbidity and mortality in most chronic liver diseases, there are few—if any—treatment strategies available that specifically target the pathogenesis of fibrosis. In this study, we examined whether PRI-724, a selective inhibitor of the cAMP-response element-binding protein-binding protein (CBP)/β-catenin interaction, has therapeutic potential for liver fibrosis using mouse models. The results suggest a possibility that PRI-724 can be a candidate for new anti-fibrotic drugs for liver cirrhosis.
1. Introduction
Chronic tissue injury leads to fibrosis in many organs, including the liver, lungs, kidneys, and heart. In chronic liver disease, the development of fibrosis is the first step toward the progression to cirrhosis and its complications (such as organ failure, esophageal variceal bleeding, and hepatocellular carcinoma), irrespective of the underlying etiology (Bataller and Brenner, 2005), and there is currently no effective therapy. Although many pathways and cytokines such as transforming growth factor (TGF)-β, platelet-derived growth factor (PDGF), Toll-like receptor (TLR) 4, sphingosine-1-phosphate, AKT, and p38 mitogen-activated protein kinase (MAPK) have been characterized as mediators of liver fibrosis, the underlying molecular mechanism is still not well defined.
Wnt/β-catenin is involved in virtually every aspect of embryonic development and in the pathogenesis of many human diseases (Clevers, 2006), and is also involved in homeostatic self-renewal in adult tissues, such as liver and lung repair following an injury (Monga, 2011, Beers and Morrisey, 2011). Recently, Wnt/β-catenin has been reported to be associated with organ fibrosis (Dees and Distler, 2013, Chilosi et al., 2003) suggesting that they may be new therapeutic targets in liver fibrosis (Cheng et al., 2008). Hepatic stellate cells (HSCs) represent a major fibrogenic cell type in the liver (Bataller and Brenner, 2005). Following a liver injury, HSCs undergo an activation process and change their phenotype from quiescent retinoid storing HSCs to collagen-producing and contractile myofibroblast-like cells (Friedman, 2000). Wnt signaling is stimulated in activated HSCs compared to quiescent cells, and the inhibition of Wnt signaling by the transduction of the adenoviral Wnt co-receptor antagonist Dickkopf-1 restores HSC quiescence and increases apoptosis in cultured HSCs (Cheng et al., 2008). Macrophages may perform both injury-inducing and repair-promoting tasks simultaneously in an injured organ. The depletion of liver macrophages aggravates hepatocellular damage, while suppressing liver fibrosis following bile duct ligation (BDL) (Osawa et al., 2010). Moreover, the depletion of macrophages decreases myofibroblasts in a liver tumor (Osawa et al., 2013b). By contrast, macrophage depletion during the fibrosis resolution period induces the failure of matrix degradation (Duffield et al., 2005), suggesting that hepatic macrophages are involved in the regression of hepatic fibrosis (Friedman, 2005). The roles of β-catenin in macrophages have been reported. Macrophage-specific knockdown of β-catenin causes insufficient skin wound healing due to defects in migration, in the adhesion to fibroblasts, and in TGF-β production (Amini-Nik et al., 2014).
Following activation by upstream signaling from Wnt, β-catenin translocates to the nucleus. Nuclear β-catenin recruits the Kat3 transcriptional co-activators, cAMP-response element-binding protein (CREB)-binding protein (CBP) (Takemaru and Moon, 2000) or EP300 (p300) (Hecht et al., 2000), to stimulate the transcription of its target genes, and distinct roles have been reported for CBP and p300. CBP/β-catenin-mediated transcription is critical for proliferation/non-differentiation, whereas p300/β-catenin-mediated transcription initiates differentiation (Teo and Kahn, 2010, Lenz and Kahn, 2014). ICG-001, a selective inhibitor of the CBP/β-catenin interaction, attenuates bleomycin-induced lung fibrosis and reverses the established fibrosis (Henderson et al., 2010). ICG-001 also ameliorates renal interstitial fibrosis induced by unilateral ureteral obstruction (Hao et al., 2011). C-82 is a second-generation specific CBP/β-catenin antagonist developed by Prism Pharma, which inhibits the binding between β-catenin and CBP and increases the binding between β-catenin and p300 similar to ICG-001. PRI-724 is phosphorylated-C-82 and is rapidly hydrolyzed to its active form C-82 in vivo, and pre-clinical studies have shown a very acceptable toxicity profile (Lenz and Kahn, 2014). A phase I safety study for hepatitis C virus (HCV)-related cirrhosis patients has been ongoing in our hospital since September 2014. To attempt to clarify the precise roles of CBP/β-catenin on liver fibrosis, in this study, we investigated the effects of PRI-724 and of its active form, C-82, on chronic liver injury mouse models and primary isolated mouse HSCs.
2. Material and Methods
2.1. Animals and Treatments
Male wild-type (C57BL/6 and Balb/c) mice aged 8 to 11 weeks or 6 to 9 months were obtained from Japan SLC (Shizuoka, Japan). CCl4 administration or BDL induced liver fibrosis model was used for this study. The animals were intraperitoneally injected with 1 ml/kg body weight of CCl4 (1:4 v/v in mineral oil) (Sigma-Aldrich, St. Louis, MO, USA) twice a week. As a BDL model, common BDL is the most established model. However, the mortality of mice operated with common BDL was high at 5 weeks after the surgery. Thus, our established model, partial bile duct ligation (PBDL) was performed as previously reported (Osawa et al., 2010, Osawa et al., 2006). The left hepatic duct was ligated with 6-0 silk after anesthesia. The animals were intraperitoneally injected with or without 0.4 mg/mouse PRI-724 (Prism Pharma, Tokyo, Japan) dissolved in PBS 4 times a week. The experiments were conducted in accordance with the institutional guidelines (Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences) and the protocol was approved by the Research Committee of Komagome Hospital. All surgery was performed under anesthesia, and all efforts were made to minimize suffering.
2.2. Isolation of Mouse Primary HSCs
Mouse primary HSCs were isolated as previously described with slight modifications (Osawa et al., 2013a). The liver of C57BL/6 male wild-type or GFP mouse expressing EGFP ubiquitously under the CAG promoter (chicken β-actin promoter, rabbit β-globin poly A, CMV-IE enhancer) (8–11 week-old or 6–9 month-old) was perfused via the inferior vena cava with collagenase (Wako, Osaka, Japan) and pronase E (EMD Chemicals, Gibbstown, NJ, USA). After digestion, the cell suspension was filtered through nylon mesh and purified via 8.2% Nycodenz (Axis-Shield, Oslo, Norway) gradient centrifugation. The quiescent HSCs were obtained from normal liver and the in vivo stimulated HSCs were from fibrotic liver induced by eight week treatment of CCl4. The isolated HSCs were cultured in uncoated plastic dishes or thin layer matrigel (Corning, corning, NY, USA) coated dishes with DMEM high glucose (Wako) supplemented with 10% fetal bovine serum, 10 mM sodium pyruvate (Invitrogen, Carlsbad, CA, USA), MEM Non-essential amino acids (Invitrogen), 15 mM HEPES, and antibiotic solution at 37 °C in 5% CO2. After plating for 12 h (day 0), the quiescent HSCs were treated with or without C-82 (10 μM, Prism Pharma) dissolved in dimethyl sulfoxide (DMSO), and replacement of medium (C-82 or DMSO) was performed in every 5 days. The purity of HSCs was always 95% as determined by their typical shape and abundant lipid droplets. As necessary, the cells were fixed with ice-cold methanol and cell number per low magnification field was determined by nuclear counting with 4′,6-diamidino-2-phenylindole (DAPI) staining. Activated HSCs were obtained by continuous culture of quiescent HSCs for more than 20 days with replacement of medium in every 5 days. The culture-activated HSCs were treated with or without C-82. Medium change was not performed after C-82 treatment. Cell viability was examined using XTT based Cell Proliferation Kit (Biological Industries, Kibbutz Beit Haemek, Israel). Cell death was detected by propidium iodide staining, and morphological changes in the nuclei of cells undergoing apoptosis were determined by DAPI staining.
2.3. Isolation of Mouse Intrahepatic Leukocytes (IHLs)
IHL isolation was performed as previously reported (Kimura et al., 2011). Briefly, single-cell suspensions were prepared from the median lobe of the liver by digestion in RPMI 1640 (Wako) containing 0.02% collagenase IV and 0.002% DNase I (Sigma-Aldrich) for 40 min at 37 °C. The cells were overlaid on Lympholyte M (Cedarlane, Westbury, NY, USA) in PBS. After density separation, the isolated IHLs were used for fluorescence activated cell sorting (FACS) analysis and RNA extraction.
2.4. Gelatin Zymography
Gelatin zymography was performed with extracted proteins from the liver as described previously (Wielockx et al., 2001).
2.5. Statistical Analysis
Data are expressed as the mean ± SD of data collected from at least 3 independent experiments. Data between groups were analyzed by the 2-tailed Student's t-test. A P value of less than 0.05 was an indication of statistical significance.
2.6. Other Experimental Procedures
Other experimental procedures are described in the Supplementary experimental procedures. These include histological analysis, Western blot, hydroxyproline measurement, quantitative real time RT-PCR, FACS analysis, microarray analysis, measurement of serum MMP-9 and TIMP-1, and structure information of PRI-724 and C-82.
3. Results
3.1. Role of CBP/β-Catenin in Liver Fibrosis
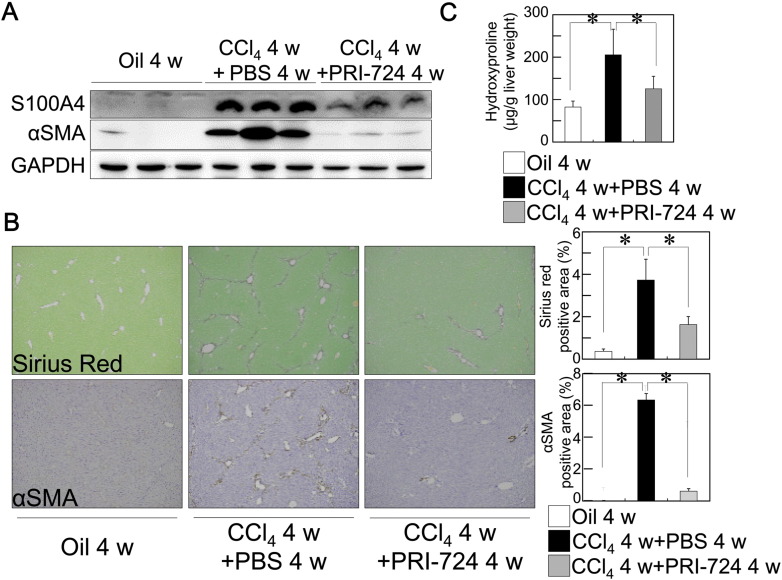
To examine whether CBP/β-catenin is involved in liver fibrosis, CCl4 was administrated to mice and the effect of PRI-724 was investigated. Following the administration of CCl4 to C57BL/6 (Fig. 1, Supplementary Fig. 1A, B) or Balb/c male mice (Supplementary Fig. 1C, D, E) (8–11 week-old), the nuclear translocation of β-catenin was observed in the non-parenchymal cells of the liver (Supplementary Fig. 1A; upper panel, C; left upper panel). Similarly, S100A4 expression, which is controlled by CBP/β-catenin and promotes liver fibrosis (Chen et al., 2015), was increased in non-parenchymal cells following the administration of CCl4, and this induction was reduced by the co-administration of PRI-724 (Fig. 1A, Supplementary Fig. 1A; lower panels, C; right upper and lower panels). Liver fibrosis was induced in CCl4-treated mice, as demonstrated by Sirius Red staining, the hydroxyproline content, and expression of αSMA, and PRI-724-treated mice showed a reduction in fibrosis (Fig. 1A, B, C, Supplementary Fig. 1D, E). Liver fibrosis is induced by hepatocyte damage followed by inflammatory cell infiltration with impaired hepatocyte regeneration. To investigate whether the reduction of liver fibrosis by PRI-724 is due to inhibition of hepatocellular damage, serum ALT levels were measured, which is contained mainly in the hepatocytes and is released into the bloodstream during hepatocellular damage. As shown in Supplementary Fig. 1B, ALT elevation by CCl4 treatment was not affected by PRI-724 suggesting that hepatocellular damage was not affected by PRI-724 treatment. PRI-724 alone had no effect on Sirius Red staining and αSMA expression (data not shown). These results suggest that CBP/β-catenin is activated in the non-parenchymal cells during fibrosis, and that this activation promotes liver fibrosis.
Fig. 1.
Inhibition of CBP/β-catenin reduced liver fibrosis induced by CCl4. C57BL/6 male mice (8–11 week-old) were treated with CCl4 (1 ml/kg (1:4 v/v in mineral oil), twice a week) plus PRI-724 (0.4 mg/mouse, four times a week) or PBS for four weeks. (A) The protein extracts from the livers were subjected to SDS-PAGE, and immunoblotting was performed with anti-S100A4, αSMA, and GAPDH antibodies. (B, C) Collagen deposition was assessed by Sirius Red staining (B, upper panel) and by the measurement of the hydroxyproline content (C). The expression of αSMA was examined by immunohistochemistry with an anti-αSMA antibody (B, lower panel) (original magnification × 42; graph on right panel). The results shown are representative of at least three independent experiments. Data are mean ± SD from at least 3 independent experiments. *, P < 0.05 using a 2-tailed Student's t-test.
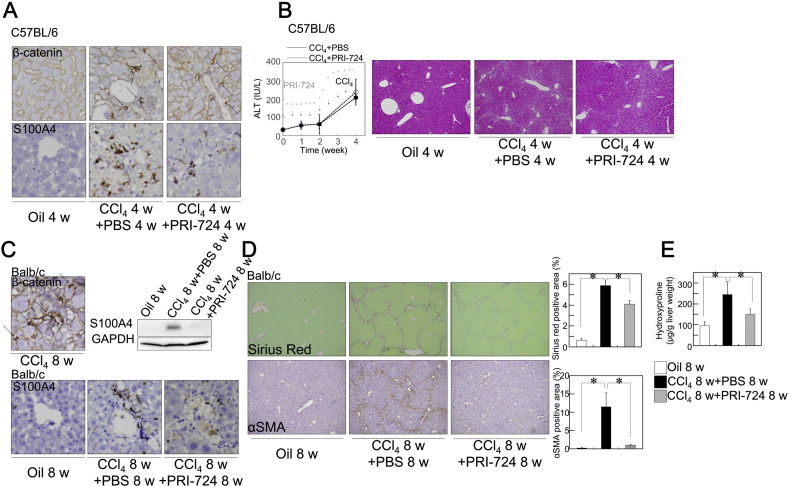
Supplementary Fig. 1.
Inhibition of CBP/β-catenin reduced liver fibrosis induced by CCl4. C57BL/6J male mice (A, B) or Balb/c male mice (C, D, E) (8–11 week-old) were treated with CCl4 (1 ml/kg (1:4 v/v in mineral oil), twice a week) plus PRI-724 or PBS (four times a week) for four (A, B) or eight (C, D, E) weeks. (A, C) Expression of β-catenin (A; upper panels, C; upper panel) and S100A4 (A; lower panels, C: lower panels) were examined by immunohistochemistry with anti-β-catenin (original magnification × 400) or S100A4 (original magnification × 200) antibodies. (B) Serum ALT levels were compared at the indicated time periods (left panel). Liver sections were stained with H-E (right panels) (original magnification × 42.). (D, E) Collagen deposition was assessed by Sirius Red staining (D, upper panel) and by the measurement of the hydroxyproline content (E). The expression of αSMA was examined by immunohistochemistry with an anti-αSMA antibody (D, lower panel) (original magnification × 42; graph on right panel). The results shown are representative of at least three independent experiments. Data are mean ± SD from at least 3 independent experiments. *, P < 0.05 using a 2-tailed Student's t-test.
3.2. Effect of CBP/β-Catenin Inhibition on HSC Activation
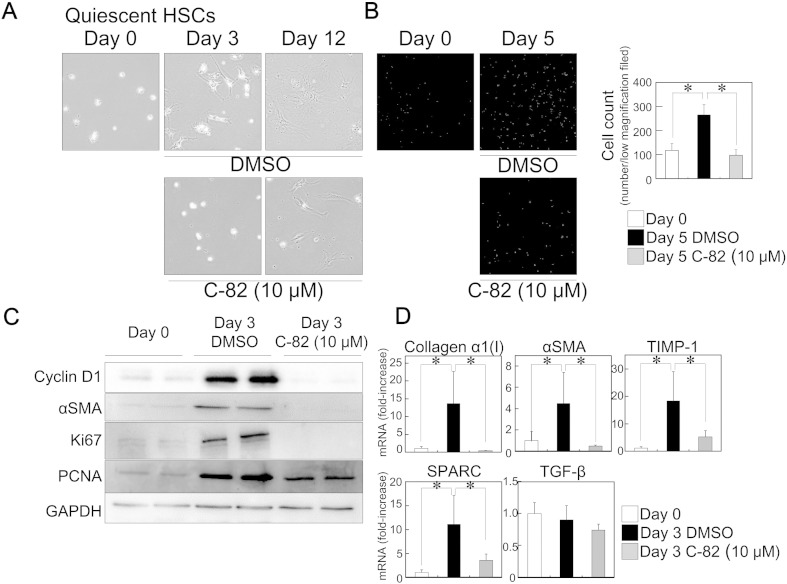
To explore the mechanism underlying the inhibitory effect of PRI-724 on liver fibrosis, we focused on HSCs, which represent a major fibrogenic cell type in the liver (Bataller and Brenner, 2001), and we examined the effects of C-82 (active form of PRI-724) on their activation. The isolated quiescent HSCs from C57BL/6 male mice (8–11 week-old) proliferated and displayed a stellate-shaped morphology (Fig. 2A, B, Supplementary Fig. 2A) during their activation on plastic dishes, accompanied by decreased lipid droplets containing vitamin A autofluorescence (data not shown). TCF/β-catenin-mediated molecule Cyclin D1, which is classical readout of β-catenin signaling, was increased during HSC activation, and C-82 inhibited the induction (Fig. 2C) suggesting that β-catenin signaling is activated in HSCs during their activation and C-82 inhibits the β-catenin signaling. In addition to the morphological changes, the protein expression of αSMA, and proliferation-related markers Ki67 and PCNA (Fig. 2C) and the mRNA expression of the fibrosis-related markers collagen α1(I), αSMA, TIMP-1, and SPARC were induced in HSCs during their activation (Fig. 2D). C-82 treatment suppressed these activation-related effects, and the removal of C-82 rapidly restored the features of HSC activation (Supplementary Fig. 2B). Microarray analysis revealed that C-82 treatment inhibited most of up-regulated gene expression by HSC activation (Supplementary Fig. 3). The HSCs grown on plastic dishes may not retain the phenotype as they as when situated in the extracellular matrix (ECM) of the liver tissue. To better mimic the in vitro setting, the isolated HSCs were plated on the thin layer matrigel. The cells displayed similar morphology (stellate-shaped) as that of cells on the plastic dish, and C-82 treatment suppressed this morphological change (data not shown). In addition, the in vivo stimulated HSCs were isolated from fibrotic liver by eight week treatment of CCl4. The cells were rapidly displayed the activated morphology compared with those from normal liver (Supplementary Fig. 4A). C-82 treatment also suppressed the activation-related effects (Supplementary Fig. 4A, B) in the in vivo stimulated HSCs, and the removal of C-82 rapidly restored the features of activation (Supplementary Fig. 4C). Because the young animals (8–11 week-old) may not represent age of the population that develop liver fibrosis, the effects of PRI-724 on grown C57BL/6 male mice (6–9 month-old) were examined. Similar to the young animals, the liver fibrosis induced by CCl4 was reduced by the co-administration of PRI-724 in the case of the aged animals as demonstrated by Sirius Red staining (Supplementary Fig. 4D). In addition, C-82 treatment also suppressed the activation-related changes of gene expression and morphology in the isolated HSCs from aged animals (Supplementary Fig. 3, Supplementary Fig. 4E). These results suggest that CBP/β-catenin promotes HSC activation. As the levels of TGF-β mRNA were comparable in all groups (Fig. 2D), the effect of C-82 does not appear to occur through regulation of TGF-β induction.
Fig. 2.
Inhibition of CBP/β-catenin suppressed HSC activation. Isolated mouse primary quiescent HSCs from C57BL/6 mice (8–11 week-old) were plated on plastic dishes and treated with or without C-82 (10 μM). The morphology of the cells was observed at the indicated time periods by phase-contrast microscopy (original magnification × 200) (A). The cells were fixed with ice-cold methanol and stained by DAPI. Cell number per low magnification field (original magnification × 42) was determined by nuclear counting (B). The protein extracts from the cells were subjected to SDS-PAGE, and immunoblotting was performed with anti-Cyclin D1, αSMA, Ki67, PCNA, and GAPDH antibodies (C). The expression of the indicated mRNA variants in the cells at day 3 was determined by quantitative real-time RT-PCR (D). The results shown are representative of at least three independent experiments. Data are mean ± SD from at least 3 independent experiments. *, P < 0.05 using a 2-tailed Student's t-test.
Supplementary Fig. 2.
Inhibition of CBP/β-catenin suppressed HSC activation. (A) Isolated primary HSCs from wild-type C57BL/6J or GFP mice (8–11 week-old) were plated on plastic dishes and treated with or without C-82 (10 μM) for the indicated time periods. (B) After C-82 treatment for 12 days, the culture medium was changed to C-82 supplemented or deficient medium. The morphology of the cells was observed by phase-contrast or fluorescent microscopy (original magnification × 200, GFP × 400). The results shown are representative of at least three independent experiments.
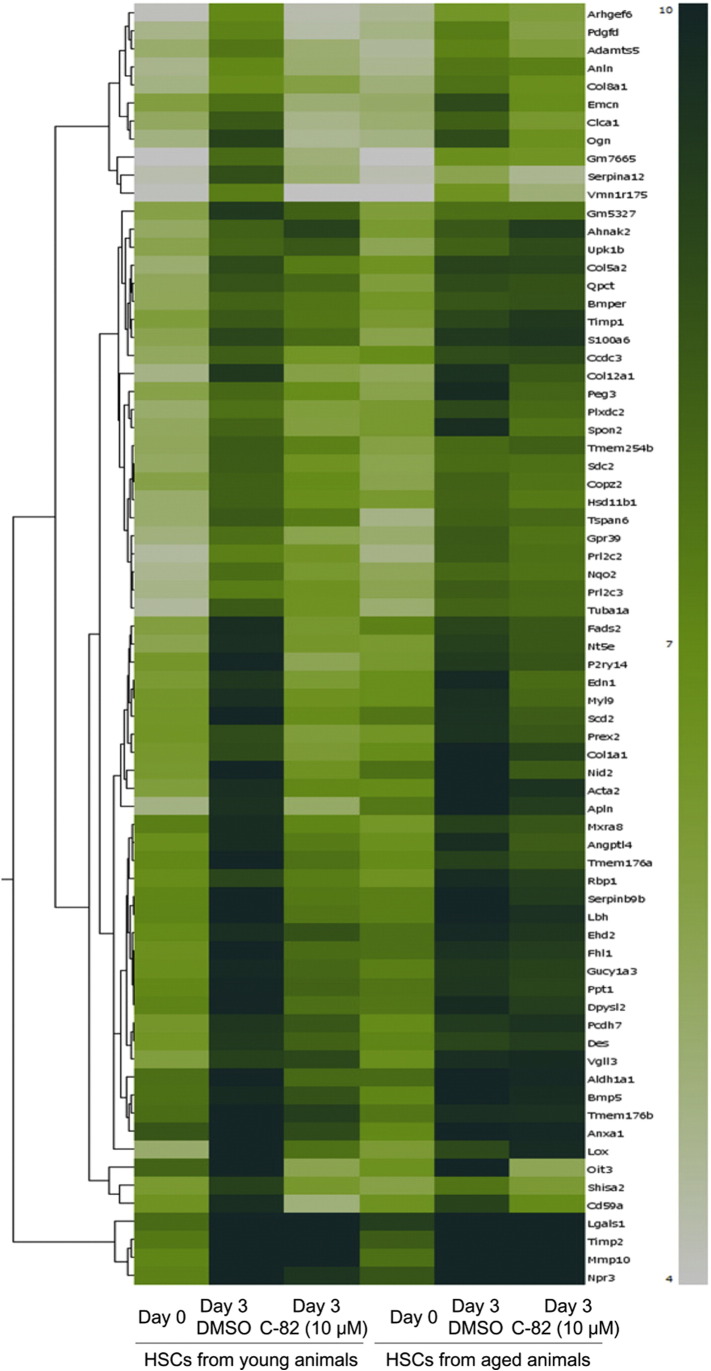
Supplementary Fig. 3.
Effects of CBP/β-catenin inhibition on mRNA expression in HSCs. Isolated primary HSCs from young (left 3 lanes; 8–11 week-old) or aged (right 3 lanes; 6–9 month-old) wild-type C57BL/6J mice were plated on plastic dishes and treated with DMSO or C-82 (10 μM) for 3 days. Hierarchical clustering of 5-fold upregulated probes after culture activation was shown. The columns represent samples of quiescent HSCs (day 0), vehicle (DMSO)-treated HSCs (day 3) and C-82-treated HSCs (day 3). The rows represent the increased 71 probesets at the culture activated phase compared with the quiescent phase, which are colored based on their normalized signal values as indicated by a heatmap. Probes are specified by their annotated Gene Symbols.
Supplementary Fig. 4.
Inhibition of CBP/β-catenin suppressed activation of HSC from fibrotic liver or aged animals. (A) Isolated mouse primary HSCs from fibrotic liver induced by CCl4 administration for 8 weeks (in vivo stimulated HSCs) in wild-type C57BL/6J mice (8–11 week-old) were plated on plastic dishes and treated with or without C-82 (10 μM) for the indicated time periods. (B) The expression of the indicated mRNA variants in the in vivo stimulated HSCs at day 3 was determined by quantitative real-time RT-PCR. (C) After C-82 treatment for 7 days, the culture medium was changed to C-82 supplemented or deficient medium. (D) C57BL/6J aged male mice (6–9 month-old) were treated with CCl4 (1 ml/kg (1:4 v/v in mineral oil) twice a week) plus PRI-724 (0.4 mg/mouse, four times a week) or PBS for four weeks. Collagen deposition was assessed by Sirius Red staining. (original magnification × 42; graph on lower panel). (E) Isolated primary HSCs from aged C57BL/6J male mice (6–9 month-old) were plated on plastic dishes and treated with or without C-82 (10 μM) for the indicated time periods. The morphology of the cells was observed by phase-contrast microscopy (original magnification × 200). The results shown are representative of at least three independent experiments. Data are mean ± SD from at least 3 independent experiments. *, P < 0.05 using a 2-tailed Student's t-test.
3.3. Cell Death Induction of Activated HSCs by Inhibition of CBP/β-Catenin
As described above, PRI-724 suppressed liver fibrogenesis, at least in part, though the inhibition of HSC activation. To address the considerations that arise in the clinical use of PRI-724 to treat liver cirrhosis patients, the effect of the reagent on an established fibrotic liver was examined. Liver fibrosis was induced by the administration of CCl4 for four weeks, or by PBDL surgery for 2 weeks, in C57BL/6 male mice (8–11 week-old), after which PRI-724 co-treatment begun. The PRI-724-treated mice showed reduced liver fibrosis in both the CCl4 model at eight weeks and in the PBDL model at five weeks accompanied by S100A4 reduction (Supplementary Fig. 5A, B), suggesting that PRI-724 is beneficial in the treatment of existing fibrosis. It has been previously reported that HSC apoptosis (Iredale et al., 1998, Wright et al., 2001) and the reversal of HSC activation (Troeger et al., 2012) contribute to fibrosis resolution. To investigate the effect of CBP/β-catenin inhibition on activated HSCs, C-82 was administered to activated HSCs obtained by culture on plastic dishes for more than 20 days. After treatment, the cells changed their shapes from stellate-like to shrunken (Fig. 3A), and cell viability was reduced (Fig. 3B). The mRNA expression of the fibrosis-related makers was decreased three days following C-82 treatment (Fig. 3C). Seven days following C-82 administration, the cells started to detach from the dishes and propidium iodide (Fig. 3A) staining revealed that cell death was induced in these cells. Apoptotic nuclear segmentation was found in the dead cells examined with DAPI staining (Supplementary Fig. 6A). The mRNA expression of survivin, which is regulated by CBP/β-catenin and protects fibroblasts from apoptosis (Sisson et al., 2012), was increased in the activated HSCs compared to quiescent cells, whereas C-82 treatment decreased the expression of this survival gene. In addition, the induction of TUNEL positive apoptotic non-parenchymal cells was higher following the administration of CCl4 plus PRI-724 than CCl4 plus PBS (Supplementary Fig. 6B). To investigate the effects of C-82 on cell death in the quiescent HSCs, serial cell toxicity assays using PI staining were performed from day 0 quiescent HSCs to day 20 activated HSCs. In contrast to the effects against activated HSCs, C-82 did not induce cell death in the quiescent HSCs (Fig. 3A,Supplementary Fig. 6C). These results suggest that CBP/β-catenin inhibition partially reduces an established liver fibrosis through the induction of cell death in activated HSCs.
Supplementary Fig. 5.
Inhibition of CBP/β-catenin reduced liver fibrosis induced by BDL. (A) C57BL/6J male mice (8–11 week-old) were treated with CCl4 (1 ml/kg (1:4 v/v in mineral oil), twice a week). Four weeks later, PRI-724 treatment (0.4 mg/mouse, four times a week) was started to the animals and CCl4 plus PRI-724 or PBS administration was continued for another four weeks. (B) C57BL/6J male mice (8–11 week-old) were subjected to PBDL. Two weeks later, PRI-724 treatment (0.4 mg/mouse, four times a week) was started. The animals were killed on 5 weeks after the surgery. Collagen deposition was assessed by Sirius Red staining (left panels, original magnification × 42; graph on middle panel) and by the measurement of the hydroxyproline content (right panel). The protein extracts from the livers were subjected to SDS-PAGE, and immunoblotting was performed with anti-S100A4 and GAPDH antibodies (lower panels). The results shown are representative of at least three independent experiments.
Fig. 3.
Inhibition of CBP/β-catenin induced cell death in activated HSCs. Activated HSCs were prepared by the continuous culture of quiescent HSCs from C57BL/6 male mice (8–11 week-old) on plastic dishes for more than 20 days. The activated HSCs were treated with or without C-82 (10 μM) for the indicated time periods. (A) The morphology of the cells was observed by phase-contrast microscopy (original magnification × 42). The necrotic cells were stained with propidium iodide and observed by fluorescent microscopy (original magnification × 42). (B) Cell viability at the indicated periods of time was assessed using XTT based cell viability assay. (C) The expression of the indicated mRNA variants in the cells at day 3 was determined by quantitative real-time RT-PCR. (D) The mRNA levels of survivin in the quiescent or activated HSCs, treated with or without C-82 (10 μM), were determined by quantitative real-time RT-PCR. The results shown are representative of at least three independent experiments. Data are mean ± SD from at least 3 independent experiments. *, P < 0.05 using a 2-tailed Student's t-test.
Supplementary Fig. 6.
Inhibition of CBP/β-catenin induced apoptosis in activated HSCs. (A) Activated HSCs were prepared by the continuous culture of quiescent HSCs from C57BL/6J male mice (8–11 week-old) on plastic dishes for more than 20 days. The activated HSCs were treated with or without C-82 (10 μM) for seven days and were stained by DAPI, which detects only dead cells. The apoptotic nuclei were shown (middle and right panels are phase contrast and fluorescent images of the same view) (original magnification, left panel × 200, middle and right panel × 600). (B) C57BL/6J male mice were treated with CCl4 (1 ml/kg (1:4 v/v in mineral oil), twice a week). Four weeks later, PRI-724 treatment (0.4 mg/mouse, four times a week) was started to the animals and CCl4 plus PRI-724 or PBS administration was continued for another four weeks. Apoptotic nuclei in mouse liver were identified using TUNEL staining. (C) Serial cell toxicity assays with PI staining from day 0 quiescent HSCs to day 20 activated HSCs were performed. The isolated HSCs cultured for the indicated periods of times were treated with or without C-82 (10 μM) for 7 days. The necrotic cells were stained with propidium iodide and observed by fluorescent microscopy (original magnification × 42). The results shown are representative of at least three independent experiments.
3.4. Acceleration of Fibrosis Resolution Through CBP/β-Catenin Inhibition
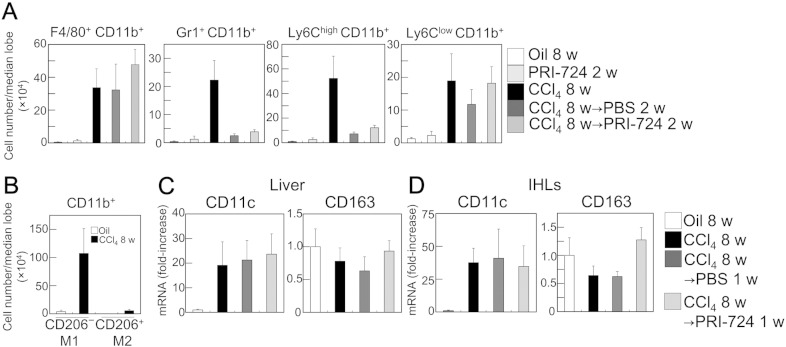
As described above, C-82 induced cell death in the activated HSCs, which contributed to liver fibrosis resolution. Next, the effect of PRI-724 during fibrosis resolution was investigated. Liver fibrosis was induced by the administration of CCl4 for eight weeks in C57BL/6 mice, and three days after the last CCl4 injection, PRI-724 treatment was started. The extent of liver fibrosis was decreased two weeks after the last round of CCl4 administration, and PRI-724 accelerated the resolution of fibrosis (Fig. 4A, B; left panel), although there was no significant difference at one week (data not shown). As both the expression levels of collagen α1(I) mRNA and of the αSMA protein induced by CCl4 were strongly decreased only two weeks after the last round of CCl4 administration (Fig. 4B: right panel, C), we hypothesized the existence of another mechanism that contributes to fibrosis resolution, besides the inhibition of quiescent HSC activation or cell death induction in activated HSCs. It has been previously reported that the CD11b+ F4/80+ Ly6Clow macrophage subset is responsible for fibrosis resolution (Ramachandran et al., 2012). In accordance with this report, a flow cytometric analysis of isolated IHLs and immunohistochemistry revealed that the amount of F4/80+ CD11b+ and Ly6Clow CD11b+ cells was increased following the administration of CCl4 for eight weeks, and was sustained during fibrosis resolution (Fig. 5A, Supplementary Fig. 7). By contrast, the induction of Gr-1+ CD11b+ and Ly6Chigh CD11b+ cells rapidly decreased during fibrosis resolution. PRI-724 treatment increased the mRNA expression of CXCL-1 and CXCL-2, which are macrophage-producing chemokines, in the liver during fibrosis resolution one week following the last round of CCl4 administration (Supplementary Fig. 7D), suggesting that this reagent affects liver macrophages. The classically activated M1 macrophages that suppress the fibrotic activities of fibroblasts (Huang et al., 2012, Song et al., 2000) as well as the regulatory M2 macrophages both have fibrotic (Song et al., 2000) and anti-fibrotic (Pesce et al., 2009, Wynn and Ramalingam, 2012) features. In this study, most of the CD11b+ cells in the IHLs from the CCl4-treated liver were CD206− cells (Fig. 5B), suggesting that the macrophages induced upon CCl4 administration are M1 macrophages. Similarly, the mRNA expression level of the M1 marker CD11c was increased in both liver tissues (Fig. 5C) and in IHLs isolated from the liver (Fig. 5D) following the administration of CCl4 for eight weeks, and were sustained during fibrosis resolution. PRI-724 treatment did not affect the mRNA expression of CD11c, suggesting that the reagent has no effect on the induction of M1 macrophages. By contrast, the number of CD11b+ CD206+ M2 macrophages was very low compared to M1 macrophages (Fig. 5B), and mRNA expression of the M2 marker CD163 did not increase during liver fibrosis and the resolution of fibrosis (Fig. 5C, D), suggesting the minor role of M2 macrophages. To investigate whether these two classifications (Ly6Chigh/low or M1/2) are completely overlapping, IHLs from the CCl4-treated liver were analyzed. Both of Ly6Chigh and Ly6Clow cells contained CD206− cells (data not shown), suggesting that these two are non-overlap classifications.
Fig. 4.
Inhibition of CBP/β-catenin accelerated fibrosis resolution. C57BL/6 male mice (8–11 week-old) were treated with CCl4 (1 ml/kg (1:4 v/v in mineral oil), twice a week) for eight weeks. The animals were then treated with or without PRI-724 (0.4 mg/mouse, four times a week) for two weeks. (A, B) Collagen deposition was assessed by Sirius Red staining (A, original magnification × 42; graph on the middle panel) and by the measurement of the hydroxyproline content (B, left panel). The mRNA levels of collagen α1(I) in the liver were determined by quantitative real-time RT-PCR (B, right panel). (C) The expression of αSMA was examined by Western blotting with an anti-αSMA antibody. The results shown are representative of at least three independent experiments. Data are mean ± SD from at least 3 independent experiments. *, P < 0.05 using a 2-tailed Student's t-test.
Fig. 5.
Macrophages increased in fibrotic liver and sustained during resolution. C57BL/6 male mice (8–11 week-old) were treated with CCl4 (1 ml/kg (1:4 v/v in mineral oil), twice a week) for eight weeks. The animals were then treated with or without PRI-724 (0.4 mg/mouse, four times a week) for the indicated periods of time. (A, B, D) IHLs were isolated from the median lobe of the animals' livers. The expression of the indicated cell-surface makers on the cells was determined by a FACS analysis (A, B). The expression of the indicated mRNA variants in the liver (C) and in the isolated IHLs (D) was determined by quantitative real-time RT-PCR. The results shown are representative of at least three independent experiments. Data are mean ± SD from at least 3 independent experiments. *, P < 0.05 using a 2-tailed Student's t-test.
Supplementary Fig. 7.
F4/80+ CD11b+ cells and Ly6Clow CD11b+ cells were sustained during fibrosis resolution. C57BL/6J male mice (8–11 week-old) were treated with CCl4 (1 ml/kg (1:4 v/v in mineral oil), twice a week) for eight weeks. The animals were then treated with or without PRI-724 (0.4 mg/mouse, four times a week) for the indicated time periods. (A) IHLs were isolated from the median lobe of the animals' livers. The expression of the indicated cell-surface makers on the cells was determined by a FACS analysis and representative FACS plots were shown (n = 4). (B, C) The expression of F4/80, Ly6C, and Gr-1 was examined by immunohistochemistry with anti-F4/80, Ly6C, and Gr-1 antibodies respectively (original magnification × 200). (D) The expression of the indicated mRNA variants in the liver was determined by quantitative real-time RT-PCR. The results shown are representative of at least three independent experiments. Data are mean ± SD from at least 3 independent experiments. *, P < 0.05 using a 2-tailed Student's t-test.
Macrophages have been reported to produce MMPs (Huang et al., 2012, Song et al., 2000, Shapiro et al., 1993), which are involved in fibrosis resolution (Wick et al., 2013, Pellicoro et al., 2014). Because MMP induction should be prior to resolution, the MMPs were analyzed at one week after CCl4 treatment for eight weeks. Gelatin zymography showed that the activation of MMP-9 in the liver was accelerated by PRI-724 during liver resolution (Fig. 6A). Similarly, the induced levels of protein expression of MMP-8 and mRNA expression of MMP-9, -2 and -8 in the liver were higher in PRI-724-treated animals (Fig. 6B, C). By contrast, MMP-13 and TIMP-1, an endogenous inhibitor of MMPs, were not affected by PRI-724. Moreover, the ratio of serum MMP-9/TIMP-1 was increased in PRI-724-treated animals (Fig. 6D). These results suggest that the acceleration of fibrosis resolution by PRI-724 may occur through changing in balance between MMPs and TIMP-1, and the effects may result in the fibrosis resolution at two weeks after CCl4 treatment. In isolated IHLs, S100A4 mRNA expression was increased by liver fibrosis, and this induction was suppressed by PRI-724 (Fig. 6E), suggesting that CBP/β-catenin was activated in IHLs and that the reagent affected this activation. Similar to the liver, the mRNA expression of MMP-9, -2, and -8 was increased in IHLs during fibrosis resolution, and PRI-724 accelerated the induction of MMPs. These results suggest that the enhanced production of MMPs from the M1 macrophages may be involved in the acceleration of fibrosis resolution by PRI-724.
Fig. 6.
Inhibition of CBP/β-catenin increased the expression of MMPs in IHLs during fibrosis resolution. C57BL/6 male mice (8–11 week-old) were treated with CCl4 (1 ml/kg, twice a week) for eight weeks. The animals were then treated with or without PRI-724 (0.4 mg/mouse, four times a week) for one week. The collagenase activity in the protein extracts from the livers was measured by gelatin zymography (A). The expression of MMP-8 in the liver was examined by Western blotting with an anti-MMP8 antibody (B). The expression of the indicated mRNA variants in the liver (C) and in the isolated IHLs (E) was determined by quantitative real-time RT-PCR. The ratio of serum MMP-9/TIMP-1 was determined by ELISA (D). The results shown are representative of at least three independent experiments. Data are mean ± SD from at least 3 independent experiments. *, P < 0.05 using a 2-tailed Student's t-test.
4. Discussion
The present study investigated the contribution of CBP/β-catenin to the progression of liver fibrosis in mice. The results, which suggest that the inhibition of CBP/β-catenin suppresses liver fibrosis through the inhibition of HSC activation, the activated HSC death, and the production of MMPs from macrophages, provide novel therapeutic possibilities for the treatment of liver fibrosis.
In the injured liver, the initial hepatocyte cell death stimulates subsequent inflammatory responses, leading to further liver injury and fibrosis. HSC activation and fibrogenesis are mediated by a complex cross-talk between damaged parenchymal and non-parenchymal cells, such as Kupffer cells, which activate HSCs through TGF-β production (Bataller and Brenner, 2005). In the CCl4- or BDL-induced liver injury model, the translocation of β-catenin and S100A4 expression were observed only in the non-parenchymal cells. In addition, PRI-724 did not affect the liver injuries examined histologically and biochemically. These results suggest that β-catenin is not involved in hepatocyte cell death. Moreover, the co-administration of PRI-724 had no effect on the number of F4/80-positive cells in the liver of CCl4-treated mice (data not shown), and C-82 treatment suppressed the activation of quiescent HSCs on the plastic dishes, suggesting that the inhibitory effect of PRI-724 on fibrogenesis is due to its direct effect on HSCs.
It has been reported that the levels of the β-catenin protein are elevated in fibroblasts during proliferation, that stabilized β-catenin-expressing mice show aggressive fibromatoses and hyperplastic cutaneous wounds (Cheon et al., 2002), and that Wnt signaling is required for TGF-β-mediated fibrosis to occur (Akhmetshina et al., 2012). In progenitor cells, the selective blocking of the CBP/β-catenin interaction by ICG-001 initiates a differentiation program (Lenz and Kahn, 2014). Thus, it is likely that C-82 shifts the balance from CBP/β-catenin to p300/β-catenin-mediated transcription, resulting in the induction of differentiation rather than proliferation, through which activation was inhibited in the quiescent HSCs. The death of activated HSCs has been reported to contribute to fibrosis resolution (Iredale et al., 1998, Wright et al., 2001, Issa et al., 2001), and the inhibition of Wnt signaling increases apoptosis in cultured HSCs (Cheng et al., 2008). In the activated HSCs, C-82-induced cell death is observed seven days after the beginning of the treatment. On the other hand, C-82 did not induce cell death in the quiescent HSCs for at least 15 days (data not shown), and the removal of C-82 started its activation. These results suggest that CBP/β-catenin inhibition induces cell death only in activated HSCs, but not in quiescent cells. During the activation, CBP/β-catenin-mediated transcription, such as that of survivin is increased and these transcripts may be required for the survival of activated HSCs.
The contribution of macrophages to fibrosis resolution has been reported (Duffield et al., 2005, Friedman, 2005). MMPs resolve the ECM resulting in fibrosis resolution (Iimuro and Brenner, 2008), and macrophages have been reported to produce MMPs (Huang et al., 2012, Song et al., 2000, Shapiro et al., 1993). The cell count of macrophages in the liver during fibrosis resolution was comparable in PRI-724-treated and untreated mice, suggesting that the reagent affects the property, but not the number, of cells. In prostate cancer PC3 cells, p300 knockdown by siRNA leads to decreased MMP-9 activity, accompanied by the decreased mRNA expression of MMP-9 and MMP-2 (Santer et al., 2011), suggesting that p300/β-catenin is involved in MMP expression. Thus, PRI-724 may shift the balance to p300/β-catenin transcription resulting in MMP expression. The expression of MMP-8, generally known as neutrophil collagenase, was also up-regulated by PRI-724. The neutrophil-dependent resolution of the repair of the cholestatic rat liver has been reported (Harty et al., 2008). However, Gr-1-positive cells, which indicate the presence of neutrophils, rapidly disappeared during fibrosis resolution. Besides neutrophils, MMP-8 expression is found in other inflammatory cells such as monocytes and macrophages (Prikk et al., 2002), plasma cells (Wahlgren et al., 2001), and T-cells (Kim et al., 2001), suggesting that neutrophils may play a minor role in the acceleration of fibrosis resolution by PRI-724.
These data do not provide evidence that the MMP production from macrophages is directly regulated by CBP/β-catenin and that the produced MMPs are involved in fibrosis resolution in our model. The gating strategy for macrophages in analysis of IHL does not exclude monocytes. Moreover, the mechanism by which CBP/β-catenin stimulates the activation of quiescent HSCs and induces the survival of activated HSCs remains unclear. The effects of CBP/β-catenin inhibitors against portal fibroblasts, which represent another type of collagen-producing cells besides HSCs in biliary fibrosis (Wells, 2014), have not yet been investigated. In addition, it is still unclear if the effects of the CBP/β-catenin inhibitor are primarily on HSCs, macrophages, other immune cells, and/or sinusoidal endothelium in vivo. Thus, further studies are needed to resolve these uncertainties.
5. Conclusions
The inhibition of CBP/β-catenin suppressed liver fibrogenesis and promoted fibrosis resolution. Thus, targeting CBP/β-catenin may represent a new therapeutic strategy in the treatment of liver fibrosis, and the phase I clinical study of PRI-724 on HCV-related liver cirrhosis patients is ongoing in our hospital.
Conflicts of Interest
No conflict of interest exists.
Author's Contributions
Yosuke Osawa performed animal experiments, cell isolations, cell-culture studies, fibrosis evaluation, PCR, Western blots, zymography, hydroxyproline measurement and created figures and contributed to the design of the study and the writing of the manuscript. Keisuke Oboki performed microarray analysis. Jun Imamura contributed to the experimental design. Ekumi Kojika performed animal treatments. Yukiko Hayashi and Tsunekazu Hishima performed IHC and histological analysis. Toshiji Saibara, Futoshi Shibasaki, and Michinori contributed to the study design. Kiminori Kimura performed FACS analysis and contributed to the study design.
The following are the Supplementary data related to this article.
Supplementary experimental procedures
Primer sequences used for quantitative real-time RT-PCR.
Supplementary Fig. 8.
Structure information of PRI-724 and C-82.
Acknowledgments
This work was supported by Grants from the Takeda Science Foundation, the Sagawa Foundation for Promotion of Cancer Research, the JSPS KAKENHI Grant Number 26460965, the Research Program on Hepatitis of Ministry of Health, Labor, and Welfare of Japan, and Clinical Research Fund of Tokyo Metropolitan Government.
Contributor Information
Yosuke Osawa, Email: osawa-gif@umin.ac.jp.
Keisuke Oboki, Email: ooboki-ks@igakuken.or.jp.
Jun Imamura, Email: jun.imamu@cick.jp.
Ekumi Kojika, Email: kojika@cick.jp.
Yukiko Hayashi, Email: yukhayashi@gmail.com.
Tsunekazu Hishima, Email: hishima@cick.jp.
Toshiji Saibara, Email: saibarat@kochi-u.ac.jp.
Futoshi Shibasaki, Email: shibasaki-ft@igakuken.or.jp.
Michinori Kohara, Email: kohara-mc@igakuken.or.jp.
Kiminori Kimura, Email: kkimura@cick.jp.
References
- Akhmetshina A., Palumbo K., Dees C., Bergmann C., Venalis P., Zerr P., Horn A., Kireva T., Beyer C., Zwerina J., Schneider H., Sadowski A., Riener M.O., Macdougald O.A., Distler O., Schett G., Distler J.H. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat. Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini-Nik S., Cambridge E., Yu W., Guo A., Whetstone H., Nadesan P., Poon R., Hinz B., Alman B.A. Beta-catenin-regulated myeloid cell adhesion and migration determine wound healing. J. Clin. Invest. 2014;124:2599–2610. doi: 10.1172/JCI62059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R., Brenner D.A. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin. Liver Dis. 2001;21:437–451. doi: 10.1055/s-2001-17558. [DOI] [PubMed] [Google Scholar]
- Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers M.F., Morrisey E.E. The three R's of lung health and disease: repair, remodeling, and regeneration. J. Clin. Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li J., Zhang J., Dai C., Liu X., Wang J., Gao Z., Guo H., Wang R., Lu S., Wang F., Zhang H., Chen H., Fan X., Wang S., Qin Z. S100A4 promotes liver fibrosis via activation of hepatic stellate cells. J. Hepatol. 2015;62:156–164. doi: 10.1016/j.jhep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- Cheng J.H., She H., Han Y.P., Wang J., Xiong S., Asahina K., Tsukamoto H. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G39–G49. doi: 10.1152/ajpgi.00263.2007. [DOI] [PubMed] [Google Scholar]
- Cheon S.S., Cheah A.Y., Turley S., Nadesan P., Poon R., Clevers H., Alman B.A. Beta-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M., Poletti V., Zamo A., Lestani M., Montagna L., Piccoli P., Pedron S., Bertaso M., Scarpa A., Murer B., Cancellieri A., Maestro R., Semenzato G., Doglioni C. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Dees C., Distler J.H. Canonical Wnt signalling as a key regulator of fibrogenesis — implications for targeted therapies? Exp. Dermatol. 2013;22:710–713. doi: 10.1111/exd.12255. [DOI] [PubMed] [Google Scholar]
- Duffield J.S., Forbes S.J., Constandinou C.M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- Friedman S.L. Mac the knife? Macrophages — the double-edged sword of hepatic fibrosis. J. Clin. Invest. 2005;115:29–32. doi: 10.1172/JCI23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., He W., Li Y., Ding H., Hou Y., Nie J., Hou F.F., Kahn M., Liu Y. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 2011;22:1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty M.W., Papa E.F., Huddleston H.M., Young E., Nazareth S., Riley C.A., Ramm G.A., Gregory S.H., Tracy T.F., Jr. Hepatic macrophages promote the neutrophil-dependent resolution of fibrosis in repairing cholestatic rat livers. Surgery. 2008;143:667–678. doi: 10.1016/j.surg.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Hecht A., Vleminckx K., Stemmler M.P., Van Roy F., Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W.R., Jr., Chi E.Y., Ye X., Nguyen C., Tien Y.T., Zhou B., Borok Z., Knight D.A., Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.C., Sala-Newby G.B., Susana A., Johnson J.L., Newby A.C. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-kappaB. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iimuro Y., Brenner D.A. Matrix metalloproteinase gene delivery for liver fibrosis. Pharm. Res. 2008;25:249–258. doi: 10.1007/s11095-007-9311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale J.P., Benyon R.C., Pickering J., Mccullen M., Northrop M., Pawley S., Hovell C., Arthur M.J. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa R., Williams E., Trim N., Kendall T., Arthur M.J., Reichen J., Benyon R.C., Iredale J.P. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548–557. doi: 10.1136/gut.48.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.H., Albertsson P., Xue Y., Nannmark U., Kitson R.P., Goldfarb R.H. Expression of neutrophil collagenase (MMP-8) in Jurkat T leukemia cells and its role in invasion. Anticancer Res. 2001;21:45–50. [PubMed] [Google Scholar]
- Kimura K., Sekiguchi S., Hayashi S., Hayashi Y., Hishima T., Nagaki M., Kohara M. Role of interleukin-18 in intrahepatic inflammatory cell recruitment in acute liver injury. J. Leukoc. Biol. 2011;89:433–442. doi: 10.1189/jlb.0710412. [DOI] [PubMed] [Google Scholar]
- Lenz H.J., Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105:1087–1092. doi: 10.1111/cas.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga S.P. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int. J. Biochem. Cell Biol. 2011;43:1021–1029. doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa Y., Hoshi M., Yasuda I., Saibara T., Moriwaki H., Kozawa O. Tumor necrosis factor-alpha promotes cholestasis-induced liver fibrosis in the mouse through tissue inhibitor of metalloproteinase-1 production in hepatic stellate cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa Y., Seki E., Adachi M., Suetsugu A., Ito H., Moriwaki H., Seishima M., Nagaki M. Role of acid sphingomyelinase of Kupffer cells in cholestatic liver injury in mice. Hepatology. 2010;51:237–245. doi: 10.1002/hep.23262. [DOI] [PubMed] [Google Scholar]
- Osawa Y., Seki E., Adachi M., Taura K., Kodama Y., Siegmund S.V., Schwabe R.F., Brenner D.A. Systemic mediators induce fibrogenic effects in normal liver after partial bile duct ligation. Liver Int. 2006;26:1138–1147. doi: 10.1111/j.1478-3231.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- Osawa Y., Suetsugu A., Matsushima-Nishiwaki R., Yasuda I., Saibara T., Moriwaki H., Seishima M., Kozawa O. Liver acid sphingomyelinase inhibits growth of metastatic colon cancer. J. Clin. Invest. 2013;123:834–843. doi: 10.1172/JCI65188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicoro A., Ramachandran P., Iredale J.P., Fallowfield J.A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- Pesce J.T., Ramalingam T.R., Mentink-Kane M.M., Wilson M.S., El Kasmi K.C., Smith A.M., Thompson R.W., Cheever A.W., Murray P.J., Wynn T.A. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prikk K., Maisi P., Pirila E., Reintam M.A., Salo T., Sorsa T., Sepper R. Airway obstruction correlates with collagenase-2 (MMP-8) expression and activation in bronchial asthma. Lab. Investig. 2002;82:1535–1545. doi: 10.1097/01.lab.0000035023.53893.b6. [DOI] [PubMed] [Google Scholar]
- Ramachandran P., Pellicoro A., Vernon M.A., Boulter L., Aucott R.L., Ali A., Hartland S.N., Snowdon V.K., Cappon A., Gordon-Walker T.T., Williams M.J., Dunbar D.R., Manning J.R., Van Rooijen N., Fallowfield J.A., Forbes S.J., Iredale J.P. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer F.R., Hoschele P.P., Oh S.J., Erb H.H., Bouchal J., Cavarretta I.T., Parson W., Meyers D.J., Cole P.A., Culig Z. Inhibition of the acetyltransferases p300 and CBP reveals a targetable function for p300 in the survival and invasion pathways of prostate cancer cell lines. Mol. Cancer Ther. 2011;10:1644–1655. doi: 10.1158/1535-7163.MCT-11-0182. [DOI] [PubMed] [Google Scholar]
- Shapiro S.D., Kobayashi D.K., Pentland A.P., Welgus H.G. Induction of macrophage metalloproteinases by extracellular matrix. Evidence for enzyme- and substrate-specific responses involving prostaglandin-dependent mechanisms. J. Biol. Chem. 1993;268:8170–8175. [PubMed] [Google Scholar]
- Sisson T.H., Maher T.M., Ajayi I.O., King J.E., Higgins P.D., Booth A.J., Sagana R.L., Huang S.K., White E.S., Moore B.B., Horowitz J.C. Increased survivin expression contributes to apoptosis-resistance in IPF fibroblasts. Adv. Biosci. Biotechnol. 2012;3:657–664. doi: 10.4236/abb.2012.326085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Ouyang N., Horbelt M., Antus B., Wang M., Exton M.S. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- Takemaru K.I., Moon R.T. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J. Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo J.L., Kahn M. The Wnt signaling pathway in cellular proliferation and differentiation: A tale of two coactivators. Adv. Drug Deliv. Rev. 2010;62:1149–1155. doi: 10.1016/j.addr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Troeger J.S., Mederacke I., Gwak G.Y., Dapito D.H., Mu X., Hsu C.C., Pradere J.P., Friedman R.A., Schwabe R.F. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren J., Maisi P., Sorsa T., Sutinen M., Tervahartiala T., Pirila E., Teronen O., Hietanen J., Tjaderhane L., Salo T. Expression and induction of collagenases (MMP-8 and -13) in plasma cells associated with bone-destructive lesions. J. Pathol. 2001;194:217–224. doi: 10.1002/path.854. [DOI] [PubMed] [Google Scholar]
- Wells R.G. The portal fibroblast: not just a poor man's stellate cell. Gastroenterology. 2014;147:41–47. doi: 10.1053/j.gastro.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick G., Grundtman C., Mayerl C., Wimpissinger T.F., Feichtinger J., Zelger B., Sgonc R., Wolfram D. The immunology of fibrosis. Annu. Rev. Immunol. 2013;31:107–135. doi: 10.1146/annurev-immunol-032712-095937. [DOI] [PubMed] [Google Scholar]
- Wielockx B., Lannoy K., Shapiro S.D., Itoh T., Itohara S., Vandekerckhove J., Libert C. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nat. Med. 2001;7:1202–1208. doi: 10.1038/nm1101-1202. [DOI] [PubMed] [Google Scholar]
- Wright M.C., Issa R., Smart D.E., Trim N., Murray G.I., Primrose J.N., Arthur M.J., Iredale J.P., Mann D.A. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685–698. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary experimental procedures
Primer sequences used for quantitative real-time RT-PCR.