Abstract
The in vivo neural activity of the pons during the perception of affective stimuli has not been studied despite the strong implications of its role in affective processing. To examine the activity of the pons during the viewing of affective stimuli, and to verify its functional and structural connectivity with other affective neural correlates, a multimodal magnetic resonance imaging methodology was employed in this study. We observed the in vivo activity of the pons when viewing affective stimuli. Furthermore, small-world connectivity indicated that the functional connectivity (FC) between the pons and the cortico-limbic affective regions was meaningful, with the coefficient λ being positively associated with self-reported emotional reactivity. The FC between the pons and the cortico-limbic-striatal areas was related to self-reported negative affect. Corroborating this finding was the observation that the tract passing through the pons and the left hippocampus was negatively related to self-reported positive affect and positively correlated with emotional reactivity. Our findings support the framework that the pons works conjunctively with the distributed cortico-limbic-striatal systems in shaping individuals' affective states and reactivity. Our work paves the path for future research on the contribution of the pons to the precipitation and maintenance of affective disorders.
Highlights
-
•
There is in vivo neural activity of the pons during the perception of affective stimuli.
-
•
The pons works conjunctively with other affective neural correlates in shaping the affective states and reactivity.
Identifying how the pons works conjunctively with the distributed cortico-limbic-striatal systems in shaping individuals' affective states and reactivity is important for understanding the role the pons plays in the precipitation and maintenance of affective disorders. This multimodal imaging study investigated the in vivo activity of the pons during affective processing. The activity in the pons associated with viewing affective stimuli and the functional and anatomical connectivity between the pons and other cortico-limbic affective regions suggest that the region plays an active role in affective processing.
1. Introduction
According to the literature, serotonin plays a crucial role in affective processing (Ren et al., 2013) as well as in regulating the biological rhythms of sleep, mood, and appetite (Monti and Jantos, 2008, Monti, 2011). Affective disorders induced by brain stem damage may be related to an altered neurotransmitter balance (Hurley et al., 2010). According to recent meta-analytic reviews, the serotonin transporter promoter variant has been found to be implicated in depression (Karg et al., 2011, Sharpley et al., 2014, Artigas, 2015). Additionally, serotonin transporter binding potential in the vicinity of the pons was reduced in people with bipolar disorders (Cannon et al., 2007). The basis pontis, however, is a crucial correlate in patients with pathological laughing and crying (PLC) episodes (Lee et al., 2003, Arif et al., 2005, Parvizi et al., 2009). The above pieces of clinical evidence indicate that the pons could be an important affective processing node. However, the current literature on the human affective processing network focuses largely on cortico-limbic correlates (Shah et al., 2012) and their inter-connectivity. These correlates include the limbic system, orbital, medial and lateral prefrontal cortex (PFC) (Price and Drevets, 2010). In vivo activity of the pons during affective perception has never been reported.
This study fills an important gap in the literature on the activity of the pons and its involvement in the perception of affective stimuli. To provide independent yet complementary information (Touroutoglou et al., 2014) on the role of the pons in affective processing, a multimodal approach was employed in this study to confirm the functional and structural connectivity between the pons and other correlates. We hypothesized that the viewing of affective stimuli would be associated with significant blood-oxygen-level-dependent (BOLD) signals at the pons because of the role of serotonergic neurons in affective processing. The observed significant activation of the pons was followed by a series of structural and functional connectivity studies. First, we verified the meaning of the connectivity between the seed region in the pons (the region of the pons showing significant activation while viewing affective stimuli) and the cortico-limbic affective processing network using the small-world (SW) connectivity method for data collected from the diffusion tensor imaging (DTI) scanning paradigm. The small-world network is characterized by regions highly connected to adjacent regions combined with fewer steps of information transfer from one region to another. This optimal wiring enables rapid and efficient information transfer with minimized connectivity cost. After confirming the significance of the SW connectivity, we investigated the functional and structural connectivity of the seed region and other cortical limbic regions using resting-state functional imaging data and the tractography built upon by DTI data. Given the lack of any previous literature on the connectivity of the pons and other affective processing correlates, we tested the null hypothesis of no significant functional and structural connectivity between the pons and the cortico-limbic affective correlates. Last but not least, to understand the meaningful role played by the activated pons region in affective processing, we examined whether the structural and functional connectivity between the pons and the other affective correlates was positively correlated with the following: 1. the affect states, measured by the Chinese Affect Scale (CAS) (Hamid and Cheng, 1996) and 2. emotional reactivity, measured by the Emotional Reactivity Scale (ERS) (Nock et al., 2008). To control for reward sensitivity to facial emotions, we used the international affective stimuli instead (Lang et al., 2008).
2. Materials and Methods
2.1. Participants
This study was approved by the Human Research Ethics Committee for Non-Clinical Faculties of The University of Hong Kong. We recruited 40 female Chinese participants for this study. Only women were invited to participate in this study to control for any gender-related confounding variance. All of them had finished at least a high school education (over 12 years of education). They all had normal or corrected-to-normal vision. They did not suffer from amblyopia (Freiburg Vision Test) (Bach, 2007) or color blindness/weakness (Yu et al., 2013). They had average intellectual abilities as estimated by the Test of Nonverbal Intelligence, 3rd edition (TONI-3) (Brown et al., 1997). They all presented with a normal mood as measured by the Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith, 1983) (See STable 1 in the Supplementary document for demographic information on these participants.) None of them had any history of traumatic brain injury/medical conditions/psychiatric disorders or was on any medications that could affect brain activity and functioning. All participants gave written informed consent for a protocol approved by the ethics committee of The University of Hong Kong. There was no significant mood change as measured by the affect grid (Russell et al., 1989) between pre- and post-scanning.
2.2. Task and Procedure
2.2.1. Stimuli
The experimental stimuli were 96 pictures involving human images selected from the International Affective Picture System (IAPS) (Lang et al., 2008). Among these 96 pictures, there were equal numbers of positive, neutral and negative pictures (32 pictures each). Their valence and arousal ratings (both original ratings and participants' ratings) are listed in STable 2 in the Supplementary materials. Analyses of the participant ratings showed that the valence of positive pictures was higher than the valence of neutral pictures (t(39) = 17.737, p < 0.001), and the valence of neutral pictures was higher than the valence of negative pictures (t(39) = 17.063, p < 0.001). Furthermore, the arousal of both positive (t(39) = 11.092, p < 0.001) and negative (t(39) = 6.923, p < 0.001) pictures was higher than for neutral pictures, while there was no significant difference in arousal between positive and negative pictures (t(39) = 1.736, p = 0.086).
The control stimuli were the mask images generated from the 96 IAPS pictures. A Matlab (The Mathworks, Inc.) program script was employed to scramble the pixels in these 96 pictures. These control stimuli matched the experimental stimuli in terms of illumination and hue.
To confirm if the participants were actually viewing the affective stimuli during scanning, a recognition procedure was performed after scanning. Each participant was required to identify the 96 target affective pictures among 96 foils (i.e., new IAPS pictures that also consist of 32 positive, 32 neutral and 32 negative pictures) (See STable 3 in the Supplementary document for percentage accuracy of classifying the IAPS pictures.).
2.2.2. Emotion-Processing Task (EPT)
This task was modified from the task paradigm developed by Lee et al. (2012). Each participant performed 2 runs of the task (for the task paradigm, see Fig. 1). Each run consisted of 6 meaningful picture blocks (i.e., positive, negative and neutral blocks) and 6 mask image blocks (i.e., masked positive, negative and neutral blocks). Each meaningful picture block consisted of eight trials of picture presentation (3.5 s) of the same type of emotion followed by a blank screen (0.25 s). Within each run, the mask image blocks were similar to the meaningful picture blocks except that the pictures presented were scrambled masks of the corresponding meaningful pictures. The mask image blocks were presented interleaved with the meaningful picture blocks. A meaningful picture block was separated from its mask image block in each run. This separation was designed to reduce the affective reactions in a block elicited by perceiving the similar brightness and colors presented in close proximity. The order of blocks was randomized for each participant and was balanced across participants. Participants were asked to passively view the pictures without any overt responses. A rest (approximately 30 s) was given between runs. Cortical activation while performing on the EPT was largely consistent with previous findings, confirming the validity of the paradigm (see STable 4 and STable 5 in the Supplementary materials).
Fig. 1.
EPT task paradigm. (A) Each participant was asked to passively view 2 runs of 6 blocks of meaningful pictures (i.e., positive [Pos], negative [Neg] and neutral [Neu]) and 6 blocks of masked images (i.e., positive [MPos], negative [MNeg] and neutral [MNeu]) during scanning. (B) Each block consisted of 8 trials of image presentation (3.5 s) and blank screen (0.25 s).Two of the trials is presented in this figure. The pictures for the 3.5 s image presentation shown here are for illustration and are different from those used in the formal study.
2.2.3. Procedure
Before entering the scanner, participants were required to complete questionnaires regarding their demographic information and affective status. They were then administered 10 trials of the EPT task as practice. The stimuli used in these practice trials were different from the stimuli used in the experimental task. During resting-state scanning, the participants were instructed to lie quietly and keep their eyes open without falling asleep. During task-based scanning, the visual stimuli presentations were delivered by functional magnetic resonance imaging visual stimulus system (SA-9900, Shenzhen Sinorad Medical Electronics, http://www.sinorad.com). The participant viewed the visual stimuli from a mirror mounted on the head coil. To confirm that the participants were paying attention to the affective stimuli during scanning, they were administered a recognition trial (i.e., the old-new classification task), where the 96 experimental stimuli were mixed with 96 foils immediately post-scanning. The participants had to differentiate the targets from the foils. Afterwards, they were asked to rate the valence and arousal of the experimental and control stimuli on a 75-point scale (for valence rating: 1 = very negative, 75 = very positive; for arousal rating: 1 = totally calm, 75 = extremely excited). The affect grid was administered again to confirm that the participants' pleasure and arousal states had not changed between pre- and post-scanning.
2.3. Data Acquisition and Analysis
2.3.1. Behavioral Data
All behavioral data and ratings in self-reported measurements were analyzed using the Statistical Package for the Social Sciences software (SPSS, v.20). The affect grid ratings before and after scanning were compared using paired t-tests. Accuracy rates during the post-scanning recognition trial were compared with a chance level of accuracy, i.e., 50%.
2.3.2. Imaging data
All images were collected using a 3.0 Tesla Siemens Prisma scanner at Peking University, which was equipped with a 20-channel head coil. A T1-weighted high-resolution anatomical image (MPRAGE, 192 continuous sagittal slices, TR = 2530 ms, TE = 2.98 ms, flip angle = 7°, matrix = 256 × 224, FOV [field of view] = 256 × 224 mm2, slice thickness = 1 mm, voxel size = 1 × 1 × 1 mm3) was obtained first. For the functional images of the EPT task, for achieving higher resolution and better signal to noise ratio, we focused only on the signals in the brain stem. Twenty slices were acquired using a T2*-weighted gradient echo planar imaging sequence (TR = 2000 ms, TE = 35 ms, flip angle = 90°, matrix = 96 × 28, FOV = 144 × 42 mm2, slice thickness = 1.5 mm, voxel size = 1.5 × 1.5 × 1.5 mm3), ZOOMit, named by Siemens, which can zoom into the anatomy and shape the image according to the region of interest. For the resting state functional images, whole-brain scanning was performed. Thirty-three functional slices were acquired using a T2*-weighted gradient echo planar imaging sequence (TR = 2000 ms, TE = 30 ms, flip angle = 90°, matrix = 64 × 64, FOV = 224 × 224 mm2, slice thickness = 4.2 mm, voxel size = 3.5 × 3.5 × 4.2 mm3). Similar parameters were used for the whole-brain scanning of the EPT task. For both the EPT task and the resting state scans, the axial slices were adjusted to be parallel to the straight gyrus, and the first 6 volumes were discarded to allow for T1 equilibration effects. Siemens RESOLVE sequence was employed for DTI images collection which acquired with 12 diffusion gradient directions (b = 1000) and two non-diffusion weighted (b = 0) references (TR = 8400 ms, TE = 63 ms, flip angle = 180°, FOV = 196 × 196 mm2, voxel size = 2 × 2 × 2 mm3, 65 slices covering the whole brain). Task-based BOLD signals were analyzed. Small-world connectivity was tested, and resting-state functional and DTI structural connectivity were examined (see the Supplementary materials for information about imaging data analysis). Small-word connectivity was captured based on the individual connectivity matrix constructed from cortical (e.g. dorsolateral prefrontal) and subcortical regions (e.g. limbic) together with corpus callosum and pons according to the SRI24/TZO atlas (Rohlfing et al., 2010), with average FA of tracts passing any pairs of nodal regions defined as connecting edges. Therefore, this measure would denote whether there is optimal wiring among brain regions as a whole network system, of which pontine and cortico-limbic affective regions are involved. Resting state functional connectivity enables the mapping of connectivity without relying on task-related activation patterns which could be variable, and can benefit by delineating to what extent natural variability of intrinsic functional connectivity might contribute to the affective processing (Touroutoglou et al., 2012).
3. Results
3.1. Behavioral Findings
The ratings of pre- and post-scan pleasure and arousal are shown in STable 1 in the Supplementary materials. No significant pre- and post-scanning difference was found for either the pleasure (t(39) = 1.834, p = 0.074) or arousal (t(39) = 1.425, p = 0.162) ratings of the affect grid, suggesting that participants' affect states did not change significantly during scanning. The accuracy rates of classifying both old and new pictures during the post-scanning recognition task are listed in STable 3 in the Supplementary materials. All the accuracy rates were higher than the chance level of accuracy (ts > 16, ps < 0.001), suggesting that the participants had viewed and remembered most of the photos during scanning.
3.2. Activation of the Pons
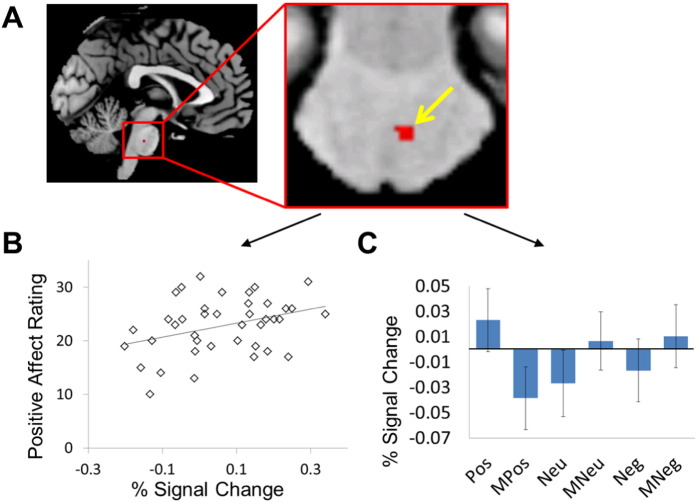
For the brainstem-only scanning data, positive pictures, relative to neutral pictures, elicited stronger activation in a pontine region (Z value = 3.674, cluster size = 6 voxels, peak coordinates: x = 2, y = − 25, z = − 35, see Fig. 2A). Although it is uncertain if this region is the pontine raphe nuclei (Cannon et al., 2006, Cannon et al., 2007), the region likely connects to the raphe nuclei and function.
Fig. 2.
EPT-related fMRI results. (A) Stronger activation was elicited by positive pictures than by neural pictures in the brain stem (MNI coordinates of the peak voxel was x = 2.5, y = − 25, z = − 35; SPM T map was height-thresholded at p < 0.005 for observation purposes only), as indicated by the arrow. Within a sphere (radius = 3 mm) centering at the peak voxel, stronger activation was also elicited by positive pictures than by positive masks. (B) The difference in the % signal change extracted from the sphere between positive pictures and positive masks was positively correlated with CAS positive affect ratings. (C) The % signal changes extracted from the sphere for each condition are shown. Pos = positive pictures, Neg = negative pictures, Neu = neutral pictures, MPos = positive masks, MNeg = negative masks, and MNeu = neutral masks.
Within the sphere with the 3 mm radius centering at the aforementioned peaking voxel (p < 0.05 for small volume correction), the region of interest (ROI), positive pictures also elicited stronger activations than positive masks (Z value = 3.340, cluster size = 5 voxels), and positive masks elicited weaker activations than neutral masks (Z value = 3.430, cluster size = 2 voxels). The % signal changes extracted from the sphere for the difference between positive pictures and positive masks were positively correlated with the CAS positive affect ratings (R = 0.352, p = 0.026, see Fig. 2B).
3.3. Small-World Connectivity
The structural brain network of each individual was constructed based on their cortical and subcortical regions, corpus callosum and the pons, and the small-world (SW) connectivity measures obtained greater than one is denoted as the network having SW property (Humphries and Gurney, 2008). Our sample has an average SW of 1.94 (SD = 0.15, Min = 1.65, Max = 2.39), which is greater than 1 (MD = 0.94, 95% bootstrap CI = 1.89 to 1.99). Pearson's r between SW and other behavioral data and ratings in self-reported measurements were then computed to identify associations. It was found that λ, the ratio of the shortest path length of an individual's matrix to the average shortest path length of random networks, was positively correlated with Emotional Intensity (R = 0.37, 95% bootstrap CI = 0.07 to 0.58; Figure S1), whereas γ, the ratio of the clustering coefficient of individual's matrix to the average clustering coefficient of random networks, and SW, the small worldness, did not correlate with any ratings in self-reported measurements.
3.4. Functional Connectivity of the Pons with Other Affective Processing Correlates
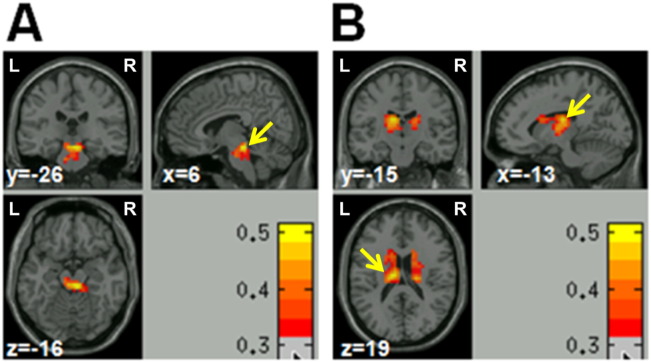
Significant positive FC was found between the seed region in the brain stem and almost all of the brain gray matter. A positive correlation between CAS positive affect ratings and the FC was found in a large cluster (containing 112 voxels, peak coordinates: x = 6, y = − 26, z = − 16, see Fig. 3A) covering the bilateral midbrain regions. However, a positive correlation between negative affect ratings and FC was found in another cluster covering the bilateral thalamus and caudate (containing 353 voxels, peak coordinates: x = − 13, y = − 15, z = 19, see Fig. 3B).
Fig. 3.
Resting-state functional connectivity (FC) analysis results. (A) Positive affect rating scores were positively correlated with the FC between the seed region and a cluster covering the bilateral midbrain regions, as indicated by the arrow. (B) Negative affect rating scores were positively correlated with the FC between the seed and a cluster covering the bilateral thalamus and caudate, as indicated by the arrows. Results were high-thresholded at p < 0.05 (cluster size > 100 voxels) in (A) and (B). L = left, R = right.
3.5. Tractography
Tracts passing through the ROI in the pons and the following regions (i.e., right amygdala, left and right posterior cingulate, left inferior frontal gyrus, left and right middle frontal gyrus (orbital part), left and right dorsolateral superior frontal gyrus, left superior medial frontal gyrus, left and right hippocampus, hypothalamus, left insula, left and right parahippocampal gyrus) were tracked successfully in at least half of our sample and were hence included in further analyses. Pearson's r was computed to identify any associations between the average FA of the tracts with behavioral data and ratings in self-reported measurements. It was observed that the FA of the ROI-to-left hippocampus tract (Fig. 4A) is negatively associated with the CAS positive affect ratings (R = − 0.38, p < 0.05, Fig. 4B) and positively associated with Emotional Sensitivity (R = 0.41, p < 0.05, Fig. 4C), Emotional Intensity (R = 0.36, p < 0.05, Fig. 4D), Emotional Persistence (R = 0.41, p < 0.05, Fig. 4E) and Total Emotionality (R = 0.43, p < 0.01, Fig. 4F). No other significant correlations were observed in other tracts with the ratings of self-reported measurements.
Fig. 4.
(A) The tract that passes through the region of interest (ROI) in the pons and left hippocampus of one subject overlaid on the sagittal slice of the corresponding skull-stripped T1-weighted anatomical image is presented for demonstration with the ROI mask shaded in yellow and the left hippocampus in purple. Fibers having dorsal-ventral as the principal diffusion direction are shaded in blue and anterior-posterior shaded in green. Significant correlations were observed between the average FA of this tract and other ratings of self-reported measurements across subjects, and the corresponding scatterplots are shown (B: with CAS positive affect, C: with Emotional Sensitivity, D: with Emotional Intensity, E: with Emotional Persistence, F: with Total Emotionality). FA = fractional anisotropy.
4. Discussion
Significant BOLD signals when viewing affectively positive stimuli were identified in a specific pontine region. It is the affective content but not other perceptual properties (verified by the masked images condition) that explains this significant finding. To the best of our knowledge, this study is the first to confirm the in vivo responses of the human pons during affective processing through non-invasive and high spatial resolution methodology. Our observation that the pons was specifically activated by affectively positive stimuli is consistent with the previous conceptualization of the serotonergic system in attenuating stress (Cools et al., 2008). It is also consistent with the finding that improved psychological well-being resulting from eight-week mindfulness meditation training was correlated with increased gray matter density in brain regions including the pontine raphe region (Singleton et al., 2014). The reduced serotonin transporter binding potential at the pons identified in patients with bipolar disorders corroborates our findings (Cannon et al., 2006, Cannon et al., 2007), which indicates that the pons and probably its connection to the raphe pontine region plays a unique role in processing affective information.
In the follow-up connectivity studies, SW connectivity measures are obtained based on the connectivity among cortical (e.g. dorsolateral prefrontal) and subcortical (e.g. limbic) regions together with corpus callosum and pons according to the SRI24/TZO atlas (Rohlfing et al., 2010), and we observed that the SW connectivity between the pons and the other cortico-limbic affective regions was meaningful. Furthermore, the coefficient λ is positively associated with emotional reactivity, suggesting that an optimal wiring of the brain has been established for such processing. The ROI is structurally and functionally connected with many cortico-limbic regions, including the ones involved in human affective processing (Wager et al., 2008). This finding is reasonable given the diffuse modulatory serotonergic effects on the brain. Indeed, previous data have suggested that the 5HT-mediated projections originating from the raphe nuclei to the prefrontal regions could be implicated in negative affective bias (Paulus et al., 2003, Evers et al., 2005). Striatal responses to emotional stimuli were previously found to be influenced by serotonergic manipulations, the extent of which correlated with the level of anxiety induced by the same manipulations (Roiser et al., 2008). The dorsolateral prefrontal cortex was previously postulated as playing a significant part in modulating subcortical affective processing (Phillips et al., 2003, Ochsner and Gross, 2005).
The meaning of the FC between the ROI in the pons and other affective processing correlates is revealed by its correlation with self-reported affect states and emotion reactivity. Positive correlations between CAS positive affect ratings and the FC of PRN-bilateral midbrain regions and between negative affect ratings and the PRN-thalamus/caudate FC were identified. Our resting-state results suggested that participants' affect states are associated with the neural connections between the pons and other brain areas, and furthermore, this association may be task-independent. The functional connection between the pons and a cluster containing both the dorsal and median raphe nuclei was positively correlated with participants' positive affect rating scores, while the connection between pons and a cluster involving both caudate and thalamus was positively correlated with participants' negative affect rating scores. Our results suggested that positive and negative affect states modulate the FCs between the pons and other brain areas differently.
Structurally, connections were identified between the ROI in the pons and the right amygdala, left and right posterior cingulate, left inferior frontal gyrus, left and right middle frontal gyrus, left and right dorsolateral superior frontal gyrus, left superior medial frontal gyrus, left and right hippocampus, hypothalamus, left insula, left and right parahippocampal gyrus. Previous animal studies have reported bidirectional connectivity between the PRN and the hypothalamus/amygdala (Sim and Joseph, 1992, Hermann et al., 1997).The tract that passes through the ROI in the pons and left hippocampus was negatively related to self-reported positive affect and positively correlated with emotion reactivity. It is possible that activity potentiation or suppression in the pons produces downstream effects on neuronal activities or 5HT transmissions in the cortico-limbic and basal ganglia structures (van de Kar and Lorens, 1979, Mokler et al., 1998, Hajos et al., 2003). Direct connectivity between PRN and cortical regions should be examined in future research to decode the mechanisms underpinning the pons' effect on cortical processing. A previous animal study has reported projection between the PRN and the anterior forebrain (Vertes, 1984). Additionally, regions in the pons may connect to other cortico-striatal-limbic regions via the mediation of dorsal and median raphe nuclei, both of which have strong reciprocal connections (Vertes, 1991).
Taken together, our findings support the framework that the pons works conjunctively with distributed prefronto-limbic-striatal systems in shaping individuals' affective states and reactivity, which is supported by both anatomical and functional connectivity patterns surrounding the pons. However, the current analyses do not allow us to make inferences regarding the causal influences of pons' activities on these cortico-limbic structures and networks, which could be resolved by future studies employing analytical methods for addressing effective connectivity, such as dynamic causal modeling (DCM) (Friston et al., 2003, Razi et al., 2015). Built upon existing anatomical and pharmacological evidence linking the pons with prefrontal, limbic and striatal networks, including the present observations, DCM could provide additional important information on how PRN activities might dynamically exert a modulatory causal influence on, or be modulated by, the functioning of the other networks, either during rest or when processing affective stimuli (Ethofer et al., 2006). The integrity of such causal influences is likely to underlie adaptive affective processing and states, the disruption of which could constitute the neurobiological basis for affective disorders at a more systematic level than regional abnormalities (de Almeida et al., 2009). Animal studies have identified direct retino-raphe projection modulating serotonergic activity and hence affective behavior (Ren et al., 2013, Pickard et al., 2015). Research on the existence of such a direct projection in humans may help to explain how the visual environment affects mood and affective states, as seen in seasonal affective disorders. We were not able to capture significant BOLD signals in the basis pontis, and hence, the perceptual basis of PLC is unclear. Future research is required to confirm the perceptual underpinnings in addition to the motor basis of the condition.
5. Limitations
To ensure and preserve the acquisition of high-resolution images of the raphe nuclei during scanning, we decided to scan only the brain stem and not the whole brain during task-based fMRI. This decision limited the understanding of the functional activation and roles of the pons together with other cortico-limbic regions during affective processing as well as task-related FC between brain regions (Davey et al., 2012). The recruitment of healthy volunteers limited the range of variance of affective responses and hence limited the types of analyses that could be applied to the data acquired. Future studies may consider recruiting a clinical sample of patients with affective disorders to understand how the pons participates in precipitating and maintaining the disorders (Elliott et al., 2011). This study recruited only female participants. Hence, the data acquired may not be fully generalizable to a male sample. Future studies should expand the gender (Bradley et al., 2001, Ameri et al., 2008) and age (Gruhn and Scheibe, 2008) representation to provide a comprehensive investigation of the role of the pons in affective processing across age, gender, and the range of affective responses. It is also important to study how pons-cortico-limbic networks are implicated in the etiology and treatment outcomes of major depressive disorder (Bach-Mizrachi et al., 2006, Lanzenberger et al., 2012, Hill et al., 2013).
6. Conclusions
Our work adds direct evidence regarding the functioning of the pons in association with other affective processing networks in humans, for which in vivo neural activity has never been previously reported. Our findings provide evidence on how a specific region in the pons is implicated during the processing of affective stimuli and during the resting-state, as well as its anatomical and functional connectivity with the rest of the brain, thus paving the path for future research on the contributions of the pons and cortico-limbic network to the precipitation and maintenance of affective disorders.
Authors' Contribution
TLee, JGao, and CChan conceptualized the research idea and planned the study. KSo reviewed the literature. DSun, WMen, and JGe collected the data. DSun, NWong, and RShao analyzed and interpreted the data. TLee wrote the manuscript. All authors read and approved the final manuscript.All authors had access to the data, and all authors agreed to submit the paper for publication.
Acknowledgments
The project was funded by The Research Grant Council General Research Fund (Ref # HKU-17613815). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.10.020.
Contributor Information
Tatia M.C. Lee, Email: tmclee@hku.hk.
Jia-Hong Gao, Email: jgao@pku.edu.cn.
Chetwyn C.H. Chan, Email: chetwyn.chan@polyu.edu.hk.
Appendix A. Supplementary data
A pontine region is a neural correlate of the human affective processing network.
References
- Ameri S.S., Vaidyanathan U., Bernat E.M., Patrick C.L. Sex differences in electroencephalography (EEG), electromography(EMG) and skin conductance reactivity to pleasant and unpleasant picture processing. Psychophysiology. 2008;45:S112. [Google Scholar]
- Arif H., Mohr J.P., Elkind M.S.V. Stimulus-induced pathologic laughter due to basilar artery dissection. Neurology. 2005;64:2154–2155. doi: 10.1212/01.WNL.0000166033.90390.2A. [DOI] [PubMed] [Google Scholar]
- Artigas F. Developments in the field of antidepressants, where do we go now? Eur. Neuropsychopharmacol. 2015;25:657–670. doi: 10.1016/j.euroneuro.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Bach M. The Freiburg visual acuity test-variability unchanged by post-hoc re-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:965–971. doi: 10.1007/s00417-006-0474-4. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H., Underwood M.D., Kassir S.A., Bakalian M.J., Sibille E., Tamir H. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Codispoti M., Sabatinelli D., Lang P.J. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- Brown L., Sherbenou R., Johnsen S.K. Third Edition. Pro-Ed; Austin, TX: 1997. Test of Nonverbal Intelligence. [Google Scholar]
- Cannon D.M., Ichise M., Fromm S.J., Nugent A.C., Rollis D., Gandhi S.K. Serotonin transporter binding in bipolar disorder assessed using [C-11]DASB and positron emission tomography. Biol. Psychiatry. 2006;60:207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Cannon D.M., Ichise M., Rollis D., Klaver J.M., Gandhi S.K., Charney D.S. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [C-11]DASB; Comparison with bipolar disorder. Biol. Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Cools R., Roberts A.C., Robbins T.W. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn. Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Yucel M., Allen N.B., Harrison B.J. Task-related deactivation and functional connectivity of the subgenual cingulate cortex in major depressive disorder. Front. Psychiatry. 2012;3:14. doi: 10.3389/fpsyt.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida J.R.C., Versace A., Mechelli A., Hassel S., Quevedo K., Kupfer D.J. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol. Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R., Zahn R., Deakin J.F.W., Anderson I.M. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2011;36:153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethofer T., Anders S., Erb M., Herbert C., Wiethoff S., Kissler J. Cerebral pathways in processing of affective prosody: a dynamic causal modeling study. NeuroImage. 2006;30:580–587. doi: 10.1016/j.neuroimage.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Evers E.A.T., Cools R., Clark L., van der Veen F.M., Jolles J., Sahakian B.J. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005;30:1138–1147. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gruhn D., Scheibe S. Age-related differences in valence and arousal ratings of pictures from the international affective picture system (IAPS): do ratings become more extreme with age? Behav. Res. Methods. 2008;40:512–521. doi: 10.3758/brm.40.2.512. [DOI] [PubMed] [Google Scholar]
- Hajos M., Gartside S.E., Viktor V., Sharp T. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology. 2003;45:72–81. doi: 10.1016/s0028-3908(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Hamid P.N., Cheng S.T. The development and validation of an index of emotional disposition and mood state: the Chinese affect scale. Educ. Psychol. Meas. 1996;56:995–1014. [Google Scholar]
- Hermann D.M., Luppi P.H., Peyron C., Hinckel P., Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J. Chem. Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hill X.L., Pascovich C., Urbanavicius J., Torterolo P., Scorza M.C. The median raphe nucleus participates in the depressive-like behavior induced by MCH: differences with the dorsal raphe nucleus. Peptides. 2013;50:96–99. doi: 10.1016/j.peptides.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Humphries M.D., Gurney K. Network “small-world-ness”: a quantitative method for determining canonical network equivalence. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley R.A., Flashman L.A., Chow T.W., Taber K.H. The brainstem: anatomy, assessment, and clinical syndromes. J. Neuropsychiatr. Clin. Neurosci. 2010;22:Iv–I7. doi: 10.1176/jnp.2010.22.1.iv. [DOI] [PubMed] [Google Scholar]
- Karg K., Burmeister M., Shedden K., Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited evidence of genetic moderation. Arch. Gen. Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- Lanzenberger R., Kranz G.S., Haeusler D., Akimova E., Savli M., Hahn A. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. NeuroImage. 2012;63:874–881. doi: 10.1016/j.neuroimage.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Lee T.M.C., Cheung C.C.Y., Lau E.Y.Y., Mak A., Li L.S.W. Cognitive and emotional dysfunction after central pontine myelinolysis. Behav. Neurol. 2003;14:103–107. doi: 10.1155/2003/872916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.M., Leung M.K., Hou W.K., Tang J.C., Yin J., So K.F. Distinct neural activity associated with focused-attention meditation and loving-kindness meditation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokler D.J., Lariviere D., Johnson D.W., Theriault N.L., Bronzino J.D., Dixon M. Serotonin neuronal release from dorsal hippocampus following electrical stimulation of the dorsal and median raphe nuclei in conscious rats. Hippocampus. 1998;8:262–273. doi: 10.1002/(SICI)1098-1063(1998)8:3<262::AID-HIPO8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Monti J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011;15:269–281. doi: 10.1016/j.smrv.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Monti J.M., Jantos H. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog. Brain Res. 2008;172:625–646. doi: 10.1016/S0079-6123(08)00929-1. [DOI] [PubMed] [Google Scholar]
- Nock M.K., Wedig M.M., Holmberg E.B., Hooley J.M. The emotion reactivity scale: development, evaluation, and relation to self-injurious thoughts and behaviors. Behav. Ther. 2008;39:107–116. doi: 10.1016/j.beth.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Parvizi J., Coburn K.L., Shillcutt S.D., Coffey C.E., Lauterbach E.C., Mendez M.F. Neuroanatomy of pathological laughing and crying: a report of the American Neuropsychiatric Association Committee on Research. J. Neuropsychiatr. Clin. Neurosci. 2009;21:75–87. doi: 10.1176/jnp.2009.21.1.75. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Rogalsky C., Simmons A., Feinstein J.S., Stein M.B. increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception i: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pickard G.E., So K.-F., Pu M. Dorsal raphe nucleus projecting retinal ganglion cells: why y cells? Neurosci. Biobehav. Rev. 2015;57:118–131. doi: 10.1016/j.neubiorev.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi A., Kahan J., Rees G., Friston K.J. Construct validation of a DCM for resting state fMRI. NeuroImage. 2015;106:1–14. doi: 10.1016/j.neuroimage.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C.R., Luan L.J., Lau B.W.M., Huang X., Yang J., Zhou Y. Direct retino-raphe projection alters serotonergic tone and affective behavior. Neuropsychopharmacology. 2013;38:1163–1175. doi: 10.1038/npp.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T., Zahr N.M., Sullivan E.V., Pfefferbaum A. The SRI24 multichannel atlas of normal adult human brain structure. Hum. Brain Mapp. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser J.P., Levy J., Fromm S.J., Wang H.Y., Hasler G., Sahakian B.J. The effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology. 2008;33:1992–2006. doi: 10.1038/sj.npp.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.A., Weiss A., Mendelsohn G.A. Affect grid — a single-item scale of pleasure and arousal. J. Pers. Soc. Psychol. 1989;57:493–502. [Google Scholar]
- Shah A., Jhawar S.S., Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J. Clin. Neurosci. 2012;19:289–298. doi: 10.1016/j.jocn.2011.04.039. [DOI] [PubMed] [Google Scholar]
- Sharpley C.F., Palanisamy S.K., Glyde N.S., Dillingham P.W., Agnew L.L. An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behav. Brain Res. 2014;273:89–105. doi: 10.1016/j.bbr.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Sim L.J., Joseph S.A. Efferent projections of the nucleus raphe magnus. Brain Res. Bull. 1992;28:679–682. doi: 10.1016/0361-9230(92)90246-t. [DOI] [PubMed] [Google Scholar]
- Singleton O., Hölzel B.K., Vangel M., Brach N., Carmody J., Lazar S.W. Change in brainstem gray matter concentration following a mindfulness-based intervention is correlated with improvement in psychological well-being. Front. Hum. Neurosci. 2014;8:33. doi: 10.3389/fnhum.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A., Bickart K.C., Barrett L.F., Dickerson B.C. Amygdala task-evoked activity and task-free connectivity independently contribute to feelings of arousal. Hum. Brain Mapp. 2014;35:5316–5327. doi: 10.1002/hbm.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A., Hollenbeck M., Dickerson B.C., Barrett L.F. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage. 2012;60:1947–1958. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kar L.D., Lorens S.A. differential serotonergic innervation of individual hypothalamic nuclei and other forebrain regions by the dorsal and median midbrain raphe nuclei. Brain Res. 1979;162:45–54. doi: 10.1016/0006-8993(79)90754-6. [DOI] [PubMed] [Google Scholar]
- Vertes R.P. a lectin horseradish peroxidase study of the origin of ascending fibers in the medial forebrain bundle of the rat. The upper brainstem. Neuroscience. 1984;11:669–690. doi: 10.1016/0306-4522(84)90052-6. [DOI] [PubMed] [Google Scholar]
- Vertes R.P. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Barrett L.F., Bliss-Moreau E., Lindquist K., Duncan S., Kober H. The neuroimaging of emotion. In: Lewis M., Haviland-Jones J.M., Barrett L.F., editors. Handbook of Emotions. Guilford Press; New York: 2008. pp. 456–470. [Google Scholar]
- Yu Z.-P., Cao K., Cao Y. People's Medical Publishing House; Beijing: 2013. Color Vision Examination Plates. [Google Scholar]
- Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A pontine region is a neural correlate of the human affective processing network.