Abstract
Post-transplant diabetes mellitus (PTDM) is a frequent consequence of solid organ transplantation. PTDM has been associated with greater mortality and increased infections in different transplant groups using different diagnostic criteria. An international consensus panel recommended a consistent set of guidelines in 2003 based on American Diabetes Association glucose criteria but did not exclude the immediate post-transplant hospitalization when many patients receive large doses of corticosteroids. Greater glucose monitoring during all hospitalizations has revealed significant glucose intolerance in the majority of recipients immediately after transplant. As a result, the international consensus panel reviewed its earlier guidelines and recommended delaying screening and diagnosis of PTDM until the recipient is on stable doses of immunosuppression after discharge from initial transplant hospitalization. The group cautioned that whereas hemoglobin A1C has been adopted as a diagnostic criterion by many, it is not reliable as the sole diabetes screening method during the first year after transplant. Risk factors for PTDM include many of the immunosuppressant medications themselves as well as those for type 2 diabetes. The provider managing diabetes and associated dyslipidemia and hypertension after transplant must be careful of the greater risk for drug-drug interactions and infections with immunosuppressant medications. Treatment goals and therapies must consider the greater risk for fluctuating and reduced kidney function, which can cause hypoglycemia. Research is actively focused on strategies to prevent PTDM, but until strategies are found, it is imperative that immunosuppression regimens are chosen based on their evidence to prolong graft survival, not to avoid PTDM.
Introduction
Diagnosis and Incidence
-
Known and Potential Risk Factors
Pre-existing diabetes risk
Associated candidate genes
Role of immunosuppression agents
Potential role of “stress,” inflammation, and infection
Potential role of vitamin D or other factors
-
The Impact of PTDM on Transplant Outcomes
Introduction
Kidney transplant outcomes
Outcomes after other organ transplant groups
-
Treating Diabetes After Transplant
Treatment of the hospitalized patient
Outpatient glucose management
Preventing cardiovascular disease
Eye care, foot care, preventing infections, and reproductive health
Importance of team-based care
Prevention of PTDM
Summary
I. Introduction
Solid organ transplantation is currently an important option for the treatment of many types of organ failure, including kidney, liver, heart, pancreas, lung, and small bowel. The introduction of cyclosporine heralded a new era in transplant outcomes, and outcomes have continued to improve with new regimens and improvements in care. Immunosuppression has so improved graft and patient survival after kidney transplant that the number one cause of graft failure is patient death of other causes unrelated to graft failure (1, 2). Kidney transplant improves patient survival and is also more cost-effective than dialysis. All of these improvements have, in turn, led to more individuals receiving solid organ transplants (Figure 1) who require long-term care.
Figure 1.
Total solid organ transplants performed in the United States. Numbers represent transplants performed from January 1988 to March 31, 2015, and reported to the United Network for Organ Sharing.
There was great hope that new steroid-free immunosuppression regimens would significantly reduce many side effects, including diabetes risk, but, in fact, even without corticosteroids, risk of diabetes remains a concern. In fact, the increasing frequency of obesity, particularly in kidney transplant candidates, has also increased the risk of post-transplant diabetes mellitus (PTDM).
This review will discuss the diagnosis of PTDM, based on the latest consensus guidelines, and how different screening practices and guidelines in the past affect our knowledge of the epidemiology of PTDM and perhaps the reported consequences of PTDM. We will discuss the factors that contribute to PTDM, including genetics, family traits, the prescribed immunosuppressants themselves, the potential role of inflammation, and factors yet to be fully proven.
Treatment of any type of diabetes can be challenging after transplant, whether pre-existing diabetes or PTDM. We will describe best practices for glucose management in the hospital and review the small studies of type 2 diabetes treatment agents that have assessed their safety in transplant recipients and other considerations for management of diabetes in an outpatient setting. Finally, we will discuss what approaches are being taken to prevent or reduce PTDM or its impact on outcomes of transplant recipients.
II. Diagnosis and Incidence
In 2003, the first International Consensus Guidelines for new-onset diabetes after transplantation (NODAT) were published (3). Although these criteria were focused on the diagnosis of diabetes after kidney transplant, they have been largely adopted by other transplant groups. Based on American Diabetes Association and World Health Organization (WHO) criteria for nontransplant patients at that time, diagnosis of NODAT could result from a fasting glucose ≥ 126 mg/dL (7 mmol/L) on more than one occasion, random glucose ≥ 200 mg/dL (11.1 mmol/L) with symptoms, or a 2-hour glucose level after a 75-g oral glucose tolerance test (OGTT) of ≥ 200 mg/dL (11.1 mmol/L) (4, 5).
In October 2013, a second international consensus panel met to update criteria and other data regarding NODAT and to evaluate the addition of hemoglobin A1C as a criterion, as it had been defined by the American Diabetes Association in 2010 in nontransplant adults (4, 5). The summary of their discussion and major recommendations were published in 2014 (see Table 1; Ref. 6).
Table 1.
Diagnosis of PTDM
| Based on recent International Consensus Guidelines (6), the diagnosis of PTDM can be made using any of the following American Diabetes Association/World Health Organization criteria for the diagnosis of diabetes (4, 5) once the transplant recipient has been discharged from the hospital and tapered to their maintenance immunosuppression. However, HbA1c should not be used alone to screen for diagnosis of PTDM within the first year after transplant. |
| 1) Fasting glucose >126 mg/dL (7 mmol/L) on more than one occasion. |
| 2) Random glucose >200 mg/dL (11.1 mmol/L) with symptoms. |
| 3) Two-hour glucose after a 75-g OGTT of >200 mg/dL (11.1 mmol/L). |
| 4) HbA1c >6.5%. |
The first recommendation of the group was to change the name from NODAT to PTDM because many cases of diabetes first identified after transplant are likely not new. There is considerable variation among transplant centers as to how or if candidates are screened for diabetes before transplant, but most centers use only fasting glucose and hemoglobin A1C for screening candidates. These methods are less sensitive than the OGTT for identifying diabetes in patients with chronic renal failure (7). Thus, it is unknown how many of those first recognized as having diabetes after transplant are truly new.
Second, the timing of the PTDM diagnosis was re-evaluated. The original criteria did not exclude diagnosis of NODAT from the immediate postoperative hospitalization when many recipients receive large doses of corticosteroids. With greater inpatient glucose screening, there is increasing awareness of the number of transplant recipients who have glucose intolerance or could be diagnosed with PTDM during this immediate post-transplant period (8, 9). Because the hyperglycemia does not always persist after discharge and the diagnosis of diabetes in most nontransplant populations is generally reserved for the outpatient setting, the consensus panel recommended delaying the evaluation for and diagnosis of PTDM until the recipient had been discharged from the hospital, was stable, and had tapered to likely chronic immunosuppression doses.
The third major recommendation of the consensus panel concerned the use of hemoglobin A1C for diagnosis of PTDM. For many reasons, including reduced red blood cell survival after transplant, hemoglobin A1C is a less reliable measure for identifying significant glucose intolerance in the first 12 months after transplant (10, 11). The panel concluded that whereas hemoglobin A1C can be used to diagnose diabetes if elevated (≥6.5%), it should not be used alone as a screen for PTDM, particularly in the first year after transplant.
It should be noted that, currently, the diagnosis of PTDM has no “end date.” If an adult or a pediatric solid organ transplant recipient is diagnosed with diabetes 1, 10, or 40 years later, it is still eligible to be called PTDM. Thus, even with the best of strategies to prevent the immediate onset of significant hyperglycemia, glucose intolerance related to age cannot be avoided, and the significance or impact of the diagnosis of PTDM made in the first year vs five years after transplant has yet to be determined.
Incidence varies with the type of transplant because of differences in age, body mass index (BMI), and underlying risk of the candidate group. Incidence also varies among transplant centers because of variations in the patients they serve with respect to race/ethnicity or prevalence of obesity, which affect risk, as well as variations in their usual immunosuppression regimen. However, the data published before the first consensus conference were quite variable, many centers did not fully embrace those first criteria, and no data have been published since the second criteria published in 2014. Thus, whereas we have summarized the incidence of PTDM published in Table 2, these should be considered relative, not absolute, risk rates because those rates are likely to be significantly changed from what we and others have published in the past once the new criteria for diagnosis of PTDM are implemented (9, 12–16).
Table 2.
Incidence of PTDM as Described After 2003 International Consensus Guidelines (3)
| Type of Transplant | Incidence, % | Refs. |
|---|---|---|
| Kidney | 10–74 | 9, 13, 15, 43, 126, 211 |
| Heart | 11–38 | 212 |
| Liver | 7–30 | 213 |
| Lung | 32 | 165 |
Studies of incidence published after the first International Consensus Guidelines are provided. However, even these have not always described their methods of screening for diabetes before or after transplant. Because there are no studies yet published after the more recent international guidelines, which excludes hyperglycemia that occurs in the immediate post-transplant hospitalization and follow-up, the overall incidence could change for kidney transplant, in particular. There are too few studies reported after lung transplant to report incidence.
III. Known and Potential Risk Factors
A. Pre-existing diabetes risk
Just as with type 2 diabetes, PTDM is more likely to occur in individuals with pre-existing type 2 diabetes risk factors, including age and a family history of type 2 diabetes, and in those with race or ethnicity considered at higher risk than Caucasians for type 2 diabetes—particularly African Americans and Hispanics (16) (Figure 2). Although Native Americans are among those with the highest risk for type 2 diabetes, there are less data identifying them at high risk for PTDM because most Native Americans presenting for transplant have already been diagnosed with diabetes. Importantly, obesity, whether defined as elevated BMI or increased waist circumference, has also been associated with increased risk of type 2 diabetes as well as PTDM. Obesity can predate the transplant, but weight gain after transplant can also contribute.
Figure 2.
Potential contributors to PTDM. Risk factors for PTDM including pre-existing diabetes risk, immunosuppressant agents used for treatment, inflammation, and other factors. FH, family history; DM, diabetes; HDL, high-density lipoprotein; TX, transplant; Vit D, vitamin D; Mg, magnesium; HepC, hepatitis C.
B. Associated candidate genes
Multiple studies have found an association between type 1 and type 2 diabetes candidate gene single nucleotide polymorphisms (SNPs) with risk for PTDM, including several genes implicated in maturity onset diabetes of the young syndromes. For example, in Hispanic kidney transplant recipients, SNPs in insulin receptor substrate (IRS)-1 and hepatocyte nuclear factor 4 were associated with diabetes after kidney transplant (17). Additional type 2 candidate gene SNPs associated with PTDM include TCF7L2, KCNJ11-Kir6.2, and some but not all variants of KCNQ1 (18–22). Those carrying multiple SNPs in diabetic genes were at even greater risk. PTDM has also been associated with SNPs in multiple IL genes, particularly IL-2, IL-7R, IL-17R, IL-1B, IL-4, IL-17-RE, IL-17R, and IL-17RB (23). Finally, SNPs in the transcription factor NFATc4 and adiponectin are also associated with PTDM (24, 25). One of the commonly used classes of immunosuppressant agents, the calcineurin inhibitors (CNIs; cyclosporine and tacrolimus), bind to nuclear factor of activated T-cells (NFAT). NFAT, in turn, can down-regulate adiponectin transcription, so individuals with SNP in this candidate gene may be at greater risk for glucose intolerance related to CNI-containing regimens.
Some genes associated with risk of end-organ failure leading to solid organ transplant are also associated with risk of PTDM. Specifically, several retrospective studies have identified autosomal dominant polycystic kidney disease (PCKD) as increasing the risk of PTDM after kidney transplant (13, 26–28). However, at least one retrospective cohort study, where the diabetes screening protocol was not well described, showed no significant difference in new-onset diabetes from matched controls (29). Yet several studies have suggested a potential mechanism for risk. In one study, carriers of the C variant gene polymorphism, among those with autosomal dominant PCKD, were found to have higher body fat content and glucose response to oral glucose challenge, but no insulin secretory defect (30). One of the candidate genes for type 2 diabetes and PTDM, hepatocyte nuclear factor 4, has also been identified as a disease modifier in an established PCKD animal model, resulting in greater cystic changes in the kidney (31). Thus, individuals with this SNP in addition to autosomal dominant PCKD may need transplant earlier and may be at greater risk for diabetes as well.
Cystic fibrosis (CF) is a genetic disease that can lead to the need for lung transplant. CF is also associated with a distinct type of diabetes, CF-related diabetes (CFRD). Thus, it is not surprising that those individuals with CF who have not developed CFRD before transplant are at higher risk for developing diabetes after lung transplant than other lung transplant candidates (32). Although some transplant groups refer to this as post-transplant diabetes, it might also be considered CFRD, for which this group is also at risk. Which designation should be used has not been discussed by any consensus panel or transplant organization.
C. Role of immunosuppression agents
Corticosteroids are well established to cause hyperglycemia through several mechanisms: by inducing or worsening pre-existing insulin resistance, increasing hepatic gluconeogenesis, and long-term, by stimulating appetite and weight gain (33–35). The impact is dose-dependent. High-dose corticosteroids, often used as part of induction protocols in the immediate post-transplant hospitalization, have much greater impact than chronic low-dose corticosteroids that are common to many maintenance immunosuppression protocols. A recent prospective randomized trial of early withdrawal of corticosteroids vs remaining on low-dose chronic prednisone (5 mg/d) from 6 months to 5 years after kidney transplant showed that incidence of PTDM was minimally impacted (36).
The remaining available immunosuppressive agents vary with respect to risk for PTDM. Mycophenolate mofetil and azathioprine have not been shown to have a large impact on insulin action or glucose metabolism and so do not appear to have a major role in PTDM. There is increasing evidence that the other commonly used immunosuppressants, particularly CNIs (eg, tacrolimus and cyclosporine) and inhibitors of the mammalian target of rapamycin (mTOR; eg, sirolimus or rapamycin and everolimus), may contribute to PTDM. Our work in animals suggests a dose-dependent effect of both tacrolimus and sirolimus on glucose metabolism (37), although there are no comparable human studies. However, there are reports that both glucose intolerance and the dyslipidemia that occurs predominantly with mTOR inhibitors improve as the dose is reduced.
Cyclosporine was the first CNI to be introduced, and it greatly improved graft survival with lower doses of corticosteroids. Because lower doses of corticosteroids were needed, both to prevent and treat rejection, less hyperglycemia was observed, initially leading many to believe these agents had no impact on insulin secretion or action. It wasn't until tacrolimus was introduced that the association between CNIs and glucose intolerance was first identified (38, 39). Tacrolimus therapy increased diabetes risk in transplant recipients already known to be at highest risk, such as African Americans and those with prior history of hepatitis C (40, 41). Although tacrolimus appeared to increase relative risk for developing PTDM more than cyclosporine treatment, it became apparent that cyclosporine was also associated with risk, 18% compared to 8%, respectively, based on data from the U.S. Renal Database for the 2-year incidence of PTDM after kidney transplant (42). A greater relative risk of tacrolimus compared to cyclosporine was also confirmed by a randomized controlled trial (43). Tacrolimus was also shown to increase the incidence of prediabetes after kidney transplant (33% at 12 mo) (44).
It should be noted that not all published studies have shown that tacrolimus and/or cyclosporine significantly affects glucose intolerance. Shihab et al (45) showed that switching from cyclosporine to tacrolimus resulted in improved renal function and no increase in new-onset diabetes or hyperglycemia. However, this study defined diabetes in a way that may have underestimated PTDM and was inconsistent with the published 2003 International Consensus Guidelines; specifically, they defined diabetes patients as those who had a fasting glucose > 140 mg/dL and required insulin treatment for more than 30 consecutive days, with anything less considered hyperglycemia. Robertson et al (46) studied the impact of cyclosporine on glucose homeostasis in 19 nondiabetic multiple sclerosis patients, noted to have “ideal BMI,” who were randomized to receive cyclosporine or placebo for up to 1 year; they were unable to show any effect of the prescribed cyclosporine on pancreatic β-cell function or iv glucose tolerance, although the doses used were less than normally prescribed early after transplant. The absence of an effect might suggest that pre-existing risk for diabetes may be additive because this population was not well described but was less likely to have a comparable BMI or other risk than a transplant population.
A number of investigator groups have evaluated the mechanism by which CNIs, tacrolimus or cyclosporine, impact glucose metabolism and diabetes risk in vitro, in animal models, and in patients (14, 37, 47, 48). Multiple mechanisms have been implicated for CNI-associated PTDM (Figure 3). CNIs have been shown to impair insulin secretion in clinical studies (49–53). Animal studies confirmed that treatment with tacrolimus worsened glucose tolerance both in vivo and in vitro and in normal as well as insulin-resistant models, predominantly through decreasing insulin secretion (54, 55). We have shown that short-term tacrolimus treatment of normal male and female Sprague-Dawley rats caused hyperglycemia with mild hyperinsulinemia in response to glucose challenge in a dose-dependent manner, suggesting mild insulin resistance; with longer duration of treatment, hyperinsulinemia was no longer observed, suggesting loss of insulin secretion (37, 54). Tacrolimus was also shown to reduce β-cell mass and increase islet apoptosis, but it had less impact on insulin signaling, suggesting that its predominant action was on insulin production (56). CNI treatment of animal models has also demonstrated decreased glucokinase activity and reduced insulin gene expression (57–59).
Figure 3.
Sirolimus and CNI impact on insulin signaling pathway. PDK1, phosphoinositide-dependent protein kinase 1; Rictor, rapamycin-insensitive companion of mTOR; Proctor, protein observed with Rictor; Raptor, regulatory-associated protein of mTOR; PRAS40, proline-rich Akt substrate 40; mLST8, mTOR-associated protein, LST8 homolog; YY1, Yin Yang 1; mSIN1, mammalian stress-activated protein kinase interactions protein; p, phosphorylation. Note that direct stimulation is denoted by  , inhibition is denoted by
, inhibition is denoted by  , and tentative inhibition by
, and tentative inhibition by  .
.
Tacrolimus has also been shown to induce β-cell apoptosis in both isolated rodent and human islets. Increased apoptosis was also observed after 24-hour treatment of isolated human islets with tacrolimus through the up-regulation of caspase-3 cleavage and activity (60). The apoptosis was associated with decreased Akt phosphorylation and IRS-2 mRNA and protein levels, suggesting that CNIs impact human β-cell survival in part through regulation of IRS-2 (61). Another study confirmed the effect of CNIs on abolishing glucose-induced IRS-2 mRNA and protein levels in rat islet β-cells (62). Pancreas allograft biopsies of pancreas transplant recipients have also demonstrated reversible cytoplasmic swelling and vacuolization of islets with tacrolimus and cyclosporine treatment (63).
Although most of the clinical data have suggested that tacrolimus, in particular, has its predominant impact through reduced insulin secretion, more recent data suggest that tacrolimus more than cyclosporine-based regimens can also increase insulin resistance after kidney transplant. This was first reported in animal studies but was also suggested in recent clinical studies as measured by homeostasis model assessment of insulin resistance and fasting insulin concentration (64–66). In human adipocytes, both cyclosporine and tacrolimus inhibited glucose uptake independent of insulin signaling by removing GLUT4 from the cell surface through an increased rate of endocytosis (67). In Wistar rats, cyclosporine reduced IRS-1 gene expression in liver and perirenal fat (68). The impact of CNI on insulin signal transduction may also be tissue-specific because cyclosporine treatment decreased IRS-1 protein in fat but not in liver (68).
However, some human studies have not confirmed these results. In 10 healthy human volunteers with normal BMI (24.7 ± 0.5 kg/m2), Øzbay et al (69) showed that an acute infusion of CNIs over 5 hours increased insulin sensitivity as determined by hyperinsulinemic-euglycemic clamp. A greater increase in sensitivity was observed with cyclosporine (25%) than tacrolimus (13%), but there was no change in first-phase insulin secretion or insulin pulsatility. Although the concentration achieved is comparable to goal doses after treatment, their BMI did not suggest that the impact of CNIs on glucose metabolism may require more than 5 hours or other additional susceptibility as with elevated BMI.
Rickels et al (70) compared type 1 diabetic islet transplant recipients with nondiabetic kidney and portally drained simultaneous pancreas-kidney (SPK) recipients using glucose-potentiated arginine testing. Although islet transplant recipients had impaired insulin secretion, the acute insulin response in tacrolimus-treated SPK and nondiabetic kidney transplant recipients was similar to kidney transplant donors at 3–48 months (SPK) and 6–60 months (nondiabetic kidney) after transplant on maintenance immunosuppression. It should be noted that the BMI values were matched between the groups and were lower than most transplant recipients that develop PTDM: 24 ± 1 kg/m2 for the normal controls; 21 ± 1 kg/m2 for islet transplant recipients; 23 ± 1 kg/m2 for SPK; 23 ± 1 kg/m2 in nondiabetic kidney recipients; and 24 ± 1 kg/m2 for kidney donors. These studies do not exclude the potential impact of CNIs on insulin action but again suggest that recipients may not all be equally at risk.
Cottrell (71) used OGTT and frequently sampled iv glucose tolerance testing with minimal model analysis to evaluate insulin sensitivity and β-cell function over 48 months in a cross-sectional study of 33 SPK recipients treated with a cyclosporine-based regimen. In this cohort that was selected because they were euglycemic, β-cell function was reduced at 3–6 months but improved by 12 months and normalized by 24–48 months, maintaining euglycemia with greater insulin secretion. Again, the candidates were type 1 diabetes patients and were treated with both cyclosporine and corticosteroids, so it is difficult to isolate the potential impact of the cyclosporine from the corticosteroids or to compare the results to a nonselected, kidney-transplant population with PTDM. Additionally, in this group the pancreas graft was placed into the systemic circulation, which results in significant systemic hyperinsulinemia that makes the insulin concentrations harder to interpret.
Finally, eight islet transplant recipients treated predominantly with tacrolimus and minimal corticosteroids were also compared to patients with type 1 diabetes and normal controls using a frequently sampled iv glucose tolerance test to evaluate insulin sensitivity, glucose effectiveness, and free fatty acid dynamics. Insulin sensitivity was greater in the islet transplant and normal controls compared to the type 1 diabetes subjects, suggesting again that at least in this small cohort of thin (BMI, 22.5 ± 1.1 kg/m2) islet transplant recipients, the tacrolimus dose administered did not reduce insulin sensitivity (72).
Another mechanism by which CNI has been suggested to contribute to hyperglycemia is hypomagnesemia (73, 74). Hypomagnesemia alone is known to impact insulin signaling (75), is commonly associated with CNI treatment, and is associated with increased risk for PTDM. In a retrospective analysis of 254 renal transplant recipients, patients who developed PTDM had lower serum magnesium levels in the first month after transplant compared to non-PTDM recipients, and those with the lowest magnesium levels developed PTDM more rapidly (76). Hypomagnesemia was associated with PTDM independent of the immunosuppressant regimen used. In this study, tacrolimus was associated with PTDM, but the association disappeared when controlling for hypomagnesemia, suggesting that tacrolimus may increase the risk of PTDM, in part through the induction of hypomagnesemia. A smaller retrospective study revealed similar findings (77). A study in liver transplant recipients also suggested that early post-transplant hypomagnesemia was associated with increased PTDM (78). A small clinical trial of magnesium supplementation was conducted to determine the relative impact of hypomagnesemia on PTDM in CNI-treated kidney transplant recipients. Unfortunately, the results were inconclusive. Magnesium supplementation resulted in a lower fasting glucose without a change in insulin resistance, which might suggest that hypomagnesemia does contribute. However, because there was a significant difference in fasting glucose between the two groups at baseline, the results were not definitive and further studies are still needed (79).
In summary, CNIs have been associated with the risk of PTDM in kidney transplant recipients as a whole and in animal studies. Studies performed predominantly in vitro and in animal models suggest that CNIs may increase the risk of PTDM by reducing insulin secretion, increasing insulin resistance through both insulin signaling and noninsulin signaling mechanisms, or indirectly through its impact on magnesium concentration. However, small clinical studies of thin, euglycemic subjects did not demonstrate a significant impact of CNIs on insulin action, suggesting that pre-existing risk or susceptibility may be required.
Sirolimus, the first mTOR inhibitor to be used in solid organ transplantation, has also been associated with glucose intolerance after organ transplant. Soon after its introduction, patients treated with sirolimus were observed to have severe hypertriglyceridemia similar to that observed with other agents known to aggravate insulin resistance, raising suspicion that it could affect insulin action (80). Studies of data from the U.S. Renal Database suggest that sirolimus is independently associated with increased risk for diabetes after kidney transplant (25, 81, 82). A 10-year retrospective analysis of immunosuppression regimens also revealed that regimens that included sirolimus were more likely to develop diabetes after kidney transplantation (83). However, the impact of sirolimus may depend on individual or pre-existing susceptibility or may overall be less than that of the CNIs because case reports suggest that conversion from cyclosporine to sirolimus can improve glycemic control (84).
Several mechanisms have been proposed. Sirolimus treatment has been shown to reduce β-cell mass of human and rat islets through apoptosis (85). Sirolimus also has antiproliferative effects on multiple cells. In a pregnant mouse model, sirolimus impaired the proliferation of β-cells, so it may also impact β-cell replication in nonpregnant adults (86). Sirolimus has also been shown to impair proliferation of pancreatic ductal cells, which some believe serve as a precursor for the development of new islets (87).
However, the greatest impact of sirolimus on glucose metabolism may be on insulin signal transduction (88, 89) (Figure 3). The insulin receptor is composed of two extracellular α-subunits, containing insulin binding sites, and two intracellular β-subunits with tyrosine kinase activity. Insulin receptor binding triggers insulin receptor tyrosine autophosphorylation. This triggers phosphorylation of the docking protein family, known as the IRS proteins, which activates the phosphatidylinositol 3 kinase (PI3K) pathway and subsequent translocation of PI3K-dependent effector enzymes, such as phosphoinositide-dependent protein kinase 1 and Akt. After phosphorylation of Akt (at serine 473 and threonine 308), its activation is key to promotion of protein synthesis. This requires stimulation of the mTOR-containing complex (mTORC1) and its substrate p70 ribosomal protein S6 kinase. Sirolimus, also called rapamycin, binds to mTOR, and one of the important negative feedback mechanisms of this pathway is via protein kinase-dependent phosphorylation of IRS-1 at specific serine residues (307, 612, and 636, among others), leading to suppression of PI3K/Akt signaling.
mTOR exists in two multimeric complexes, mTORC1 and mTORC2 (90–92). mTORC1 is comprised of mTOR, raptor, mLST8/GβL, and PRAS40 (proline-rich Akt substrate 40). It regulates the phosphorylation of p70 ribosomal protein S6 kinase and promotes protein synthesis. mTORC2 consists of mTOR, mLST8/GβL, rictor, PROTOR, and mSin1 and it plays a role in phosphorylation of Akt at serine 473, an event required for optimal Akt activity.
In normal Sprague-Dawley rats, we showed that sirolimus treatment resulted in a dose-dependent increase in hyperglycemia accompanied by relative hyperinsulinemia and a dose-dependent increase in insulin secretion in response to an oral glucose challenge, both suggesting insulin resistance (56). Sirolimus suppresses insulin-stimulated Akt phosphorylation in the liver, fat, and muscle of male and female rats (54, 55). Sirolimus also disrupts the phosphorylation of mTORC2, reducing insulin-mediated suppression of hepatic gluconeogenesis (93), and can induce Yin Yang 1 dephosphorylation, which mediates suppression of insulin signaling genes, resulting in decreased insulin signaling (94). Finally, sirolimus has been shown in vitro to reduce β-cell function and islet mass (37, 55, 85, 86). Although low-dose sirolimus can inhibit mTOR1, responsible for its immunosuppressive effects, high-dose sirolimus may be required to inhibit mTOR2, which is linked to insulin resistance (93, 95). However, not all studies have reported an impact of sirolimus on insulin action or glucose metabolism. In a study of normal, thin, human controls given a single dose of oral sirolimus, insulin-mediated glucose uptake was increased under somatostatin-insulin clamp conditions used to simulate a postprandial environment, but not at baseline, and there was no impact on glucose production in either fasting or somatostatin clamp conditions (96). In pancreatectomized dogs made euglycemic with intrasplenic autoislet transplant, daily oral sirolimus treatment for 30 days (1 mg/kg/d) resulted in fasting and stimulated hyperinsulinemia although glucose uptake was increased and glucose concentration was normal, which was interpreted by the authors to suggest no significant impact on islet function. In this same model, daily im cyclosporine treatment for 30 days (12 mg/kg/d) also had no impact on glucose homeostasis. After allo-islet transplant of nonhuman primates treated with calcineurin-free, sirolimus immunosuppression, insulin secretion was reduced, as was glucagon response to arginine, although the impact of reduced islet mass cannot easily be discriminated from that of sirolimus treatment in this setting. Notably, at 2 to 3 weeks after transplant, insulin sensitivity was unchanged in these animals compared to their naive state before streptozotocin treatment (97, 98). Finally, studies 6 to 7 months after human islet transplant treated with low-dose tacrolimus and sirolimus immunosuppression also suggested normal insulin sensitivity at the liver and skeletal muscle (99).
It should be noted that these negative studies have been performed in animal models and human subjects with presumed normal insulin sensitivity, whether normal controls or islet transplant recipients, who are often thinner than most type 1 diabetes patients. The absence of an effect in these studies again raises the question of whether underlying susceptibility to type 2 diabetes risk is required, as would be more likely to be observed in the kidney transplant and many other organ transplant populations. Time on medication may also be a factor because studies done acutely have not shown an impact. Some studies have already alluded to a potential biphasic effect of time, where an increase in insulin sensitivity was observed acutely after sirolimus treatment in C2C12 myotubes (100), L6 skeletal muscle cells (101), and healthy humans (96), possibly mediated by disrupting an S6-mediated feedback loop (100), whereas chronic administration induces insulin resistance in vitro (in C2C12 myotubes) (100) and in vivo (56, 93, 102), most likely due to disruption of mTORC2 (93).
Everolimus is another available mTOR inhibitor. Although there are fewer studies evaluating its relative impact on glucose metabolism, its mechanism of action, there are no data to suggest that it has any substantially different impact on future diabetes risk than sirolimus.
D. Potential role of “stress,” inflammation, and infection
Acute and chronic systemic inflammation have long been shown to be risk factors for the development of type 2 diabetes (103). Solid organ transplantation is, by definition, an inflammatory state because of ongoing graft-host response due to acute and/or chronic rejection, reduced renal function common in this setting, and great incidence of and/or chronic infections associated with immunosuppression. Some of these potential causes of inflammation that may contribute to glucose intolerance and the development of PTDM are summarized below.
Acute and chronic rejection is associated with risk of PTDM after kidney transplant (104, 105). However, cause and effect are harder to prove because rejection is also generally treated with high-dose corticosteroids, which can also contribute to risk (18, 104, 106, 107).
In kidney transplant recipients, the source of the donor (deceased vs living) is a factor for PTDM. Deceased donor kidney allografts express higher levels of proinflammatory cytokines compared to living kidney donor grafts, whether related or unrelated, suggesting the graft injury that is more likely with a deceased donor is more important than relative histocompatibility to rejection. This proinflammatory response may contribute to the observation that kidney transplant recipients who receive a deceased donor graft rather than a living donor graft are also at greater risk for PTDM (108–111). In a retrospective analysis of 386 kidney transplant recipients, those who received deceased donor grafts were nearly four times more likely to develop PTDM than recipients of living donor grafts (15), as confirmed in other studies (112, 113). Although one commonly cited study did not find an association between graft type and PTDM, it was published before the International Consensus Guidelines were established for diagnosis of PTDM, and the only criterion described for PTDM was initiation of hypoglycemic agent(s) (14).
Infections are common after transplantation and are another source of inflammation. Two infections in particular have been observed to contribute to PTDM risk. One is hepatitis C virus (HCV), well established to be associated with the risk of diabetes in nontransplant patients (114). HCV infection is a common cause of chronic liver disease, the most common cause of cirrhosis requiring liver transplantation (115). Those receiving liver transplant for HCV-associated cirrhosis are known to have persistent HCV infection after transplant (116), and HCV has been consistently associated with an increased relative risk of PTDM (2.5- to 4-fold) after liver transplant (116–119). HCV infection after liver transplant is associated with increased insulin resistance, as measured by homeostasis model assessment of insulin resistance, even when controlling for BMI, medications, alcohol, or degree of liver fibrosis (120). The burden of infection is a factor because insulin resistance is reported to develop sooner in recipients with the highest HCV RNA levels (120).
HCV is also a risk factor for PTDM after kidney transplant. In a meta-analysis of 10 studies, Fabrizi et al (121) reported an increased risk of PTDM in kidney transplant recipients with HCV infection compared to HCV-negative recipients (odds ratio, 3.97; 95% confidence interval, 1.83–8.61). HCV was also associated with risk after controlling for known risk factors in another cohort of 306 kidney transplant recipients (122) and a prospective observational trial (odds ratio, 6.37; 95% confidence interval, 2.28–17.7) (123). As in liver transplant recipients, HCV-positive kidney recipients had reduced insulin sensitivity by minimal model estimate compared to matched HCV-negative patients (124). Further support for HCV as a risk factor for PTDM comes from small studies suggesting that treatment to clear HCV before kidney transplant may reduce the risk of PTDM after transplant (125, 126). In liver and kidney transplant recipients, the risk of PTDM is even higher in those who have HCV and are treated with tacrolimus-based immunosuppression, compared to either risk factor alone (40, 127, 128).
Cytomegalovirus (CMV) is another infection associated with PTDM. CMV infection is more common after transplant due to immunosuppression. In one retrospective study of kidney transplant recipients who underwent OGTT at 10 weeks after transplant, those known to be CMV-positive demonstrated greater PTDM than those who were not (129). However, other studies did not show that CMV increased the risk of PTDM (9, 130, 131). These differences may be explained by differences in the size of studies, immunosuppressant regimens used that may impact PTDM risk, and methods used to diagnosis PTDM. However, a meta-analysis of seven kidney transplant studies incorporating 1389 recipients suggested that overall, CMV-positive recipients were at greater risk for PTDM (132). In a prospective observational trial of recipients with or without CMV, CMV infection was an independent risk factor for PTDM, whether or not the recipients were symptomatic, as assessed by OGTT at 10 weeks after transplant (133). First-phase, second-phase, and area-under-the-curve insulin secretion were all lower in CMV-positive recipients (133). CMV infection has also been associated with increased risk for PTDM after liver transplant, although in few studies (78, 118). To date, CMV infection has not yet been suggested to be a significant contributing factor for PTDM after heart or lung transplant. Although several potential mechanisms for these CMV effects include CMV-induced or leukocyte-mediated damage or destruction of β-cells as well as proinflammatory cytokine production, none have been studied intensively (134).
E. Potential role of vitamin D or other factors
Vitamin D deficiency has been associated with increased risk of diabetes in the nontransplant population (135, 136). A randomized controlled trial is currently investigating vitamin D replacement as a method to prevent progression of prediabetes to diabetes (Vitamin D and type 2 diabetes study [D2d] Identifier NCT01942694). However, whether vitamin D deficiency is a risk factor for PTDM has not been established. A randomized controlled trial of vitamin D supplementation in kidney transplant recipients is ongoing in France, with development of PTDM being one of the primary end points of the study (137). Results from this study will hopefully shed light on the potential association between vitamin D deficiency and PTDM risk.
Statins are commonly used for treatment of hypercholesterolemia after transplant. Statins have also recently been shown to increase the incidence of new-onset diabetes outside of transplant groups. Statins have been associated with increased risk of impaired fasting glucose (IFG) and dysglycemia (IFG and/or PTDM) in renal allograft recipients (138). Patients treated with atorvastatin had more IFG and PTDM than those on fluvastatin (138). Statins have also been shown to increase the risk of PTDM in liver transplant recipients, especially in those with IFG before transplant (139).
There are fewer studies of PTDM risk outside kidney transplantation. Because there are more systematic studies of transplant patients, particularly in nonkidney transplant populations, other factors may still be identified, some of which may be specific to those groups.
IV. The Impact of PTDM on Transplant Outcomes
A. Introduction
Because diabetes and glucose intolerance alone have both been associated with adverse long-term outcomes after transplant in many groups, there is concern that PTDM may also impact outcomes after transplantation. These studies will necessarily depend, in part, on how and when PTDM is diagnosed. As noted previously, there was little consistency in the diagnosis of diabetes after transplant before the first International Consensus Guidelines were established in 2003, and there is still considerable variation in how centers screen for diabetes before and after transplant. Thus, many of the earliest studies of PTDM likely included patients who had unrecognized and previously untreated pretransplant diabetes (3). Because the diagnosis of PTDM was often transplant center-specific and often required a higher glucose threshold for a longer period of time than is used now, unrecognized or untreated hyperglycemia could itself impact outcomes.
The impact of PTDM on transplant outcomes will likely also vary between types of organ transplant. Age and BMI of candidates and recipients differ between organ transplant groups, in addition to relative frequency of other cardiovascular risk factors and immunosuppression regimen, all of which impact PTDM risk. There is a much larger literature available regarding the impact of PTDM on transplant outcomes after kidney than other transplant groups. Fewer reports are likely more a reflection of inadequate long-term data of patients with PTDM to assess outcomes than a lack of impact. However, with greater recognition of PTDM, earlier diagnosis, and improved treatment options and methods, the relative impact of PTDM on transplant outcomes may also improve over time. We have summarized available published studies by transplant group below.
B. Kidney transplant outcomes
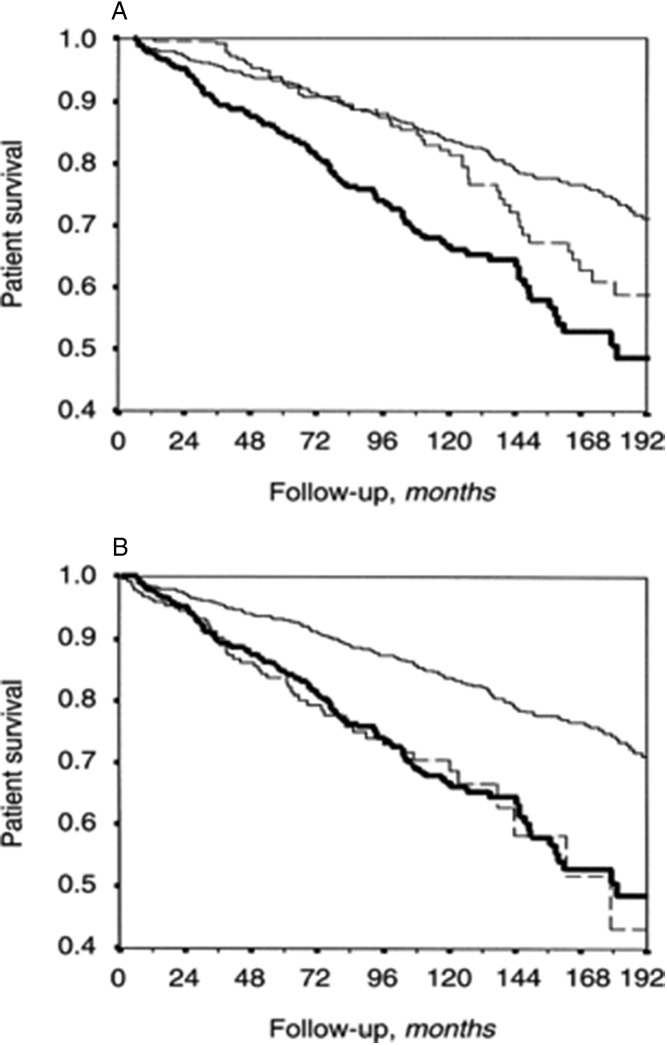
The first report to suggest that PTDM impacts survival showed that 1-year survival was 98% in those without PTDM vs 83% in those with PTDM (140). Age was also a factor in a retrospective study of 939 kidney transplant recipients wherein PTDM increased mortality in those less than 55 years of age, but not in those over 55, compared to non-PTDM (141). Awareness of the potential impact of post-transplant diabetes increased even more with the study by Cosio et al (142), who described a 2-fold increase in mortality (Figure 4) compared to nontransplant recipients, which was equal to that of pretransplant diabetes, already established to worsen outcome, and independent of other factors known to reduce survival, and was confirmed by Kaskiske et al and others (33, 34, 142, 143). The reduction in patient survival due to PTDM is largely attributable to increased cardiovascular disease events (33), as much as 3-fold even when controlling for other risk factors (144–146).
Figure 4.
Impact of PTDM on kidney transplant survival. Kaplan-Meier patient survival in the three study groups: no diabetes (thin line), pretransplant diabetes (thick line), and PTDM (dashed line). A, Survival was calculated from the day of transplantation in all three groups of patients. B, For PTDM, survival was calculated from the time of development of diabetes, and for the other two groups, from the day of transplantation. [Reprinted from F. G. Cosio et al: Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002;62:1440–1446 (142), with permission. © International Society of Nephrology.]
Most, but not all, studies also suggest that PTDM is associated with reduced graft survival, including a study of the United Renal Data System database (141, 147, 148). Although small studies suggest that PTDM is associated with higher acute rejection episode rates after kidney transplant (105, 149), it is difficult to determine whether PTDM causes rejection or whether the treatment of graft rejection, often requiring corticosteroids, results in greater risk for PTDM. However, the greatest impact of PTDM on graft survival is likely not due to increased rejection rates, but rather to death with a functioning graft (150). Only one study suggests PTDM to be a risk factor for death-censored renal graft failure (143).
Diabetes can predispose to many types of infection, as can immunosuppression. Thus, it is not surprising that PTDM has been shown to be associated with greater risk of sepsis and sepsis-related mortality (113, 148). PTDM is also a risk factor for developing CMV infection (15, 105).
There are inadequate data on whether PTDM increases long-term risk of diabetic microvascular complications. However, a large retrospective study of kidney transplant recipients with PTDM found microvascular complications to be common, so diabetic microvascular complications may develop more quickly with PTDM than nontransplant diabetes, or that many with PTDM had unrecognized, pre-existing diabetes for years prior to transplant (151).
C. Outcomes after other organ transplant groups
To date, there are few studies of outcomes after heart transplant in those with or without PTDM, and most have been retrospective. In one single-center retrospective study of heart transplant patients in Spain, PTDM was associated with a higher prevalence of hypertension (36 vs 23%; P = .001) and rejection (70 vs 45%; P = .001) compared to nondiabetics (152). In another single-center retrospective study of heart transplant patients, PTDM was associated with a greater incidence of post-transplant renal failure (P = .001) (153). There were no differences in overall survival in either study. PTDM has also been noted to increase the incidence of infection, but not survival, after heart transplantation in another study (154). In a Korean single-center study of heart transplants, the 5-year incidence of late infection (after 6 mo) was also higher in those with PTDM than those without (P = .031) (154), but there was no difference in the 5-year overall survival rate. In summary, in the few available retrospective studies, PTDM after heart transplantation does not affect survival, but it may increase hypertension, renal failure, infection, and rejection.
After liver transplant, a recent retrospective study of 438 recipients showed that PTDM was associated with increased risk of sepsis, chronic renal failure, and decreased survival, compared to those without PTDM (155). Both risk for and outcomes associated with HCV are also impacted by diabetes identified before or after transplant. One retrospective longitudinal cohort study of adult liver recipients with HCV showed that both pre-existing and post-transplant diabetes was associated with higher risk of HCV recurrence (≥stage 1 fibrosis), compared to nondiabetics (P = .006). Among these, better glycemic control (<150 mg/dL) resulted in lower rates of stage 2 fibrosis (84 vs 62%; P = .004) by multivariate analysis. Thus, diabetes (pre-existing and PTDM) has a particular impact on the development of HCV and HCV-related fibrosis, although glycemic control may reduce both the risk and severity of recurrence (156). A study in 160 HCV-positive liver transplant recipients found that insulin resistance alone impacts outcome; those with insulin resistance were twice as likely to develop fibrosis stage ≥ 3 by 5 years after transplant compared to those with normal insulin sensitivity (P = .01) (157). In another study of 163 HCV-positive liver transplant recipients, PTDM was a significant predictor for the development of severe fibrosis (P = .004) (158). HCV-related mortality was also increased in those with PTDM after liver transplant (P = .01) (117). In a larger study of 778 HCV-positive liver transplant recipients followed for a median of 6 years, those with PTDM or pretransplant diabetes also had increased HCV-related mortality (P = .036) and HCV-related graft loss (P = .026) compared with recipients with no PTDM or only transient hyperglycemia within 1 month of transplantation (159). Another prospective observational study of 165 liver transplant recipients showed that those with PTDM had a poorer survival and tumor-free survival in patients with hepatocellular carcinoma and a higher incidence of sepsis, fungal infection, chronic kidney diseases, and biliary complications (160). Also after liver transplant, PTDM and postoperative hyperglycemia (glucose > 200 mg/dL) increase acute allograft rejection and graft failure (11, 112, 159, 161). PTDM may reduce short-term survival (162) but does not consistently affect long-term survival after liver transplant (11, 117, 159, 161, 163).
Diabetes has been associated with decreased survival after lung transplant (164). However, in the one published study that included patients with PTDM, pretransplant diabetes was not differentiated from PTDM (165). Therefore, the impact of PTDM specifically on survival after lung transplant is unclear.
V. Treating Diabetes After Transplant
A. Treatment of the hospitalized patient
The treatment of diabetes in hospitalized transplant recipients requires attention to a multitude of factors that can impact glycemic control and influence the risk for adverse effects (Table 3). Methods to manage hyperglycemia vary among transplant centers and also vary according to whether the patient is immediately post-transplant or is being admitted in the post-transplant setting for another issue. Specific glycemic targets have not been established for patients in the immediate post-transplant setting. However, data from a study in kidney transplant recipients suggest that tight glycemic control (blood glucose goal, 70–110 mg/dL) is associated with increased hypoglycemia and may increase the risk for future rejection episodes compared to standard blood glucose control (blood glucose goal, 70–180 mg/dL) (166). Thus, for transplant recipients, it seems reasonable to follow current general practice guidelines for inpatient blood glucose goals (intensive care unit blood glucose goal, 140–180 mg/dL; non-intensive care unit premeal blood glucose goal, <140 mg/dL; random blood glucose goal, <180 mg/dL) (167).
Table 3.
Inpatient Treatment Considerations
| Clinical Scenario | Concerns | Treatment Considerations | Potential Problems |
|---|---|---|---|
| Immediate post-transplant | High-dose immunosuppression, pain, and stress are common | Frequently require iv insulin infusion protocol | Requires diligent monitoring of blood glucose |
| Often under observation in intensive care unit or require critical care | Hourly blood glucose monitoring initially | Frequent adjustment of insulin dose based on algorithm and/or anticipated dose changes to cover corticosteroids or other changes | |
| First week post-transplant | Increased nutritional intake | High-dose immunosuppression common | Insulin requirements may change daily due to renal function changes, increased nutritional intake |
| Steroid doses weaning | Transition to sc insulin when stable and/or starting oral intake | ||
| Rapid improvement in renal function (after kidney transplant) | Calculate sc insulin dose from last 8–24 h iv insulin requirement | ||
| Monitor blood glucose at least 4 times daily | |||
| Acute steroid bolus (eg, for acute rejection) | Increased insulin requirements | Consider NPH insulin for steroid bolus or, if very high-dose steroid, temporary iv insulin | If blood glucose rises significantly when on sc insulin, consider temporary iv insulin |
| Fluctuations in renal function, particularly, after kidney transplant | Transition back to previous insulin regimen once steroid complete, noting any changes in renal function | ||
| TPN or enteral feeding | Increased insulin requirements | Consider iv insulin as drip and/or in TPN bag | Adjust insulin dose for changes in TPN/tube feed rate or dextrose concentration |
| Once iv requirements are established and stable, switch to NPH insulin every 8 h plus fast-acting correction insulin every 4 to 6 h | Long-acting insulin held or decreased significantly if TPN or tube feeds stopped. |
Contributing factors and considerations to management of glucose during initial hospitalization.
In the immediate post-transplant setting, insulin therapy is generally required to manage postoperative hyperglycemia, especially given the requirement for high-dose immunosuppressants in this setting. Postoperative stress and pain likely also contribute to high insulin dose requirements (168). Kidney transplant recipients and any solid organ transplant recipient with varying kidney function will require diligent glucose monitoring in light of the impact of renal function on insulin metabolism (169). Immunosuppressant medications, in particular corticosteroids, will also significantly impact blood glucose values and insulin requirements. Nutritional status immediately after transplant can vary considerably and sometimes necessitate temporary supplementation with total parenteral nutrition (TPN) or enteral nutrition.
In light of high insulin requirements and frequent changes in immunosuppressant doses, renal function (in kidney transplant recipients), and nutritional intake, in the early postoperative period, iv insulin therapy is commonly utilized. Intravenous insulin algorithms allow insulin doses to be frequently titrated to better prevent significant glucose excursions. Intravenous insulin infusions have been studied in the post-transplant setting, and their use has been shown to be safe and efficacious (170, 171).
Once patients are on a stable nutritional regimen, they can be transitioned to a multiple-daily injectable insulin regimen, as recently reviewed (12). Subcutaneous insulin doses are generally calculated utilizing requirements from the iv insulin infusion, utilizing a formal basal/bolus regimen with correction insulin used as needed for persistent hyperglycemia. Additional fixed doses of long-acting insulin can be useful to cover intermittent doses of scheduled corticosteroids (eg, NPH, glargine, or detemir). TPN can increase insulin requirements that can be covered with a separate iv insulin drip, with or without the addition of insulin to the TPN solution. Once stable, iv insulin can be transitioned to long-acting insulin with correction insulin every 4 to 6 hours as needed, although there is safety in keeping some of the insulin requirements in the TPN to prevent hypoglycemia if the TPN were discontinued unexpectedly (172). However, it is essential to anticipate and change the insulin therapy, whether iv or sc, with noted interval improvement in insulin resistance, planned transition off TPN, or planned taper of immunosuppression. In summary, insulin requirements for hospitalized transplant recipients can vary considerably depending on the clinical scenario, requiring diligent blood glucose monitoring and frequent insulin titration (Table 3).
In general, as in the inpatient management of diabetes in nontransplant patients, oral hypoglycemic medications are typically avoided in hospitalized transplant recipients due to the risk of side effects, but also due to concerns over a lack of efficacy in this setting. In the event that a transplant patient requires very low-dose insulin therapy, consideration could be made to initiate oral hypoglycemic therapy at the time of discharge or at the follow-up clinic visit, keeping in mind the many caveats discussed in the next section.
B. Outpatient glucose management
Long-term glucose management of PTDM frequently requires insulin over time, particularly in those with the greatest obesity (37, 55, 57, 60, 141). However, some patients may be candidates for oral hypoglycemic medications alone or in combination with insulin therapy. Not all agents have been studied after transplant, and the available studies of both safety and efficacy are often very small. The potential risks that need to be considered are outlined in Table 4.
Table 4.
Non-Insulin Diabetes Treatments: Potential Considerations for Use in the Solid Organ Transplant Patient
| Agent | Safety or Efficacy Studies in Transplant Patients | Potential Considerations in Organ Transplant Patient |
|---|---|---|
| Metformin | Effective in stable KTX patients but contraindicated for many other TX groups, including during acute hospitalizations (177, 214) | Should not be used during acute hospitalization, with ↓ GFR, ↑ LFTs, CHF, or active, significant infection; and should be held for planned iv contrast procedure |
| Sulfonylureas | Efficacy is not well documented in transplant patients. Did not alter cyclosporine pharmacokinetics in a small study of KTX recipients with PTDM (215–218) | Increased risk of more frequent and more prolonged hypoglycemia with ↓ GFR |
| Repaglinide | Effective and safe with no interaction with CNIs in a small study of KTX recipients with PTDM (180) | Less risk of hypoglycemia with ↓ GFR than sulfonylureas |
| Thiazolidinediones (eg, pioglitazone) | Effective and safe in small studies of KTX recipients (177, 180, 183, 219, 220) | Known risk for weight gain, edema, heart failure, and reduced bone mass; contraindicated with known elevated liver function tests with the exception for known fatty liver disease including after liver transplant; contraindicated with known heart failure; unknown impact on risk for heart failure risk after transplant |
| α-Glucosidase inhibitors | No studies of safety or efficacy to date in organ transplant populations | Avoid with ↓ GFR; unlikely to be an effective single agent |
| GLP-1 agonists (exenatide, liraglutide, lixisenatide) | Liraglutide did not affect tacrolimus concentration in a very small study of KTX recipients (185) | Decreases bowel motility, which may impact absorption of immune suppression agents and has not yet been studied; should not use if GFR < 40 mL/min |
| DPP-4 inhibitors (sitagliptin, vildagliptin, saxagliptin, linagliptin, allogliptin) | Retrospective and small random controlled trials of KTX recipients show safety of several DPP-4 inhibitors (8, 181–184) | Reduce dose of all but linagliptin with ↓ GFR |
| SGLT-2 inhibitors (dapagliflozin, canagliflozin, empagliflozin) | Known to increase risk of genitourinary infections in those with previous history, which is a concern in immunocompromised transplant patients, known to cause volume dehydration and hypotension, which may also be a concern in these patients as well as recent reports of diabetic ketoacidosis raise concerns of safety for most transplant populations (186, 187) | Avoid until safety studies are performed |
Abbreviations: GFR, glomerular filtration rate; LFT, liver function test; CHF, congestive heart failure; TX, transplant; KTX, kidney transplant; SGLT-2, sodium glucose co-transporter-2.
Metformin is often the first-line therapy for treatment of type 2 diabetes in nontransplant recipients and has been used for treatment of PTDM (173). Animal studies suggest that metformin reduces immunosuppressant-induced exocrine (and possibly endocrine) cell apoptosis with subsequent improvement in glycemic control (54). Thus, some have suggested that metformin should be first-line therapy for PTDM after kidney transplant (174). However, because of the frequency of reduced kidney function, infections, and the use of contrast agents, metformin should be used carefully, particularly in kidney transplant recipients, due to the rare but potential risk of lactic acidosis with reduced renal function (175). Despite this concern, a recent review of 47 000 kidney transplant recipients in the Scientific Registry of Transplant Recipients suggests that 10% of transplant recipients filled at least one prescription for metformin, although nearly 40% of the metformin users had a serum creatinine level above the Food and Drug Administration-approved cutoff (176). No data were given on the incidence of lactic acidosis in this study, but there was no difference in patient or allograft survival between those who received metformin and those who did not, suggesting that metformin did not appear to negatively impact long-term outcomes. One other small, retrospective study of metformin use after kidney transplant showed no significant change in hemoglobin A1c (HbA1c) and no significant side effects over 16 months of use (177). To date, there are no published data on the use of metformin in other solid organ transplant groups.
Sulfonylureas are commonly utilized for PTDM, with little available safety or efficacy data (178, 179). Hypoglycemia can be significantly increased with renal insufficiency. Repaglinide has sometimes been preferred in patients with reduced kidney function because of its shorter half-life, although it stimulates insulin secretion similar to sulfonylureas. In a small observational trial, repaglinide was shown to be modestly efficacious in kidney transplant recipients with PTDM (180).
Dipeptidyl-peptidase-4 (DPP-4) inhibitors were developed to inhibit the primary enzyme responsible for the metabolism of glucagon-like peptide-1 (GLP-1), thereby prolonging the effects of endogenous GLP-1. In the nontransplant setting, DPP-4 inhibitors have a relatively low risk of hypoglycemia, are weight neutral, and can be used safely in patients who have only mild reductions in kidney function or if the dose is adjusted appropriately with more significant chronic kidney disease. Because of these factors, as well as evidence that they do not affect immunosuppressant levels, DPP-4 inhibitors are increasingly used for treatment of PTDM without significant safety concerns identified (179, 181, 182). Vildagliptin reduced 2-hour plasma glucose on OGTT as well as HbA1c in kidney transplant recipients with impaired glucose tolerance, as well as for PTDM in a randomized, double-blind, placebo-controlled, phase II trial (179, 183). Importantly, no differences in renal function or immunosuppressant levels were noted, and adverse effects were not different between the two groups. Another DPP-4 inhibitor, sitagliptin, has also been studied for safety and efficacy in a small case series and retrospective study (181, 182). Vildagliptin was also reported to be efficacious for pretransplant diabetes or PTDM after heart transplant (184). HbA1c was significantly reduced after 8 months of therapy, with no change in immunosuppressant levels or required change in immunosuppressant drug dose.
Although DPP-4 inhibitors have been used safely, there are less data and greater concern about the use of GLP-1 agonists, which can cause greater nausea and have a greater impact on gastric emptying. Both effects raise concerns about whether these agents might impact transplant outcomes by changing immunosuppressant absorption and/or immunosuppressant peak concentrations. To date, only one small case series of five subjects has reported on treatment with GLP-1 agonist in kidney transplant recipients (185). Three weeks of liraglutide therapy did not alter tacrolimus levels or dosing in these subjects, and no transplant-specific adverse effects were noted. There are no studies of exenatide, which is often associated with even greater nausea. Exenatide should not be used with creatinine clearance < 30 mL/min. Thus, this class of agents should be reserved for treatment only under a research protocol until their safety and efficacy in transplant recipients can be determined.
Similarly, the newest class of medications, sodium-glucose cotransporter 2 inhibitors, should be used with considerable caution in transplant recipients, if at all. They are known to cause volume depletion and increased risk for genitourinary infections, both of which may have greater consequences in the immunosuppressed transplant recipients, who commonly have reduced kidney function. Recent reports of diabetic ketoacidosis associated with these agents are just one more reason to avoid these agents in the transplant population until safety studies have been performed (186, 187).
Goals for glycemic control in transplant recipients are similar to other diabetes groups and should take into account duration of diabetes, comorbidities, risk for hypoglycemia, and life expectancy, allowing for less stringent glycemic control in those at high risk for hypoglycemia (188). The American Diabetes Association recommends a HbA1c goal of < 7% for patients outside of the transplant setting. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend a HbA1c goal of 7–7.5% for kidney transplant patients because of their risk of hypoglycemia due to reduced renal function and frequent history of heart disease (189). However, HbA1c alone is not adequate for assuring glucose control, particularly in the first year after transplant, because of blood loss and higher red blood cell turnover. Self-glucose monitoring is important for any transplant patient prescribed insulin or any agent that stimulates insulin secretion, directly or indirectly. There are no published guidelines for pre- or postmeal glucose goals for transplant patients separate from the American Diabetes Association (80–130 mg/dL). In the end, controlling glucose at whatever level can be achieved safely. Although caution must be used in those with reduced kidney function, glucose control remains a goal to prevent future microvascular complications because kidney transplant patients, in particular, can live another 20 years or more (190).
C. Preventing cardiovascular disease
As anyone who takes care of diabetes patients already knows, diabetes care extends beyond glucose management alone. Because cardiovascular events are the most common cause of mortality, smoking should be discouraged in all recipients. Although most programs require smoking cessation before transplant, some recipients return to smoking and may not report it unless asked. Dyslipidemia and hypertension are frequent if not ubiquitous accompaniments to diabetes of all types and are often exacerbated by many of the immunosuppressants, as well as reduced renal function, common to the post-transplant setting. Treatment must be mindful of drug-drug interactions because many of the immunosuppressants are metabolized through the same pathways as most of the lipid-lowering agents and some blood pressure medications.
Hypertension is common in many transplant groups before transplant. Blood pressure is further exacerbated by many immunosuppression regimens as well as fluctuations in renal function after transplant. General principles for treatment of hypertension after kidney transplant are outlined by both the US KDOQI guidelines, which recommend a blood pressure goal of < 140/80 mm Hg for both diabetic and nondiabetic patients with chronic kidney disease, and the international KDIGO guidelines, which recommend a more stringent < 130/80 mm Hg guideline, without specific trial evidence (191). Although written for kidney transplant management, these guidelines are often applied to other transplant groups. Treatment with an angiotensin-converting enzyme or angiotensin-receptor blocker is recommended as the first-line agent for treatment of blood pressure in most, with the latter preferred if hyperkalemia is a concern. Commonly, diuretics are used as the second agent, as in nontransplant patients. Calcium channel blockers are more likely to have drug-drug interactions with immunosuppressants and must be taken into consideration with other medications cleared by the same metabolic pathways, which also include lipid-lowering therapies.
Dyslipidemia is common, so management of lipids is often part of diabetes care related to transplant as well as in nontransplant populations. Fasting lipids should be measured routinely for all transplant recipients. Dyslipidemia is common in transplant candidates and can be exacerbated by immunosuppression and reduced renal function, as well as inadequate insulin action accompanying PTDM. Thus, control of PTDM is one of the strategies to improve lipid profile, as well as evaluating for other secondary causes, such as alcohol use and hypothyroidism. KDOQI guidelines for the treatment of lipids of kidney transplant recipients suggest statins as the first-line agent for treatment of hypercholesterolemia after excluding secondary causes (192), as do the new American Heart Association/American College of Cardiology guidelines for diabetes patients and the American Diabetes Association's revised guidelines for treatment of dyslipidemia in nontransplant patients (193, 194). Sirolimus and everolimus, in particular, can be associated with severe hypertriglyceridemia. Treatment with fish oil is well tolerated, and fibrates can be used safely in combination with immunosuppressants, but other combination therapies, such as statins with fibrates or niacin in recipients also receiving CNIs, should be used carefully because of the risk for drug-drug interactions, with case reports of rhabdomyolysis and severe liver injury (195). In addition to pharmacological treatments, changes in immunosuppression may also be required—from reducing the dose of, or switching completely away from, a mTOR inhibitor to another agent (196–198), or reducing the steroid dose (199).
D. Eye care, foot care, preventing infections, and reproductive health
Annual eye exams are as important or even more important to those with PTDM as well as nontransplant diabetes. Corticosteroids common to many transplant regimens can cause or accelerate cataracts. Immunosuppression also increases the risk of other eye infections.
Examining the feet should be a part of every diabetes visit, including and in particular the diabetic transplant patient. The primary goal of this examination is to identify concerns that could prevent future infections or injuries that could lead to amputation. “Bathroom surgery” should be discouraged, and patients should be referred for podiatry if they have difficulty with nail care, evidence of fungal infection, excessive dryness or moisture, or calluses. Evidence of skin irritation that could lead to skin breakdown should also prompt an evaluation of their shoe choice or inserts to prevent injury. Many transplant recipients have neuropathy at the time of transplant due to their other underlying disease, with or without PTDM, so all should be screened with monofilament testing to assess risk for injury. Because some may also have peripheral vascular disease, any injury to the skin, particularly in the presence of immunosuppression, can result in infection. All foot infections should be treated emergently because they can progress to deeper infections and osteomyelitis in the presence of immunosuppression. Foot education and early recognition of signs that could lead to future problems are key to preventing amputation.
Patients with diabetes are already at increased risk for many types of infection, which can be further exaggerated by immunosuppression. Transplant patients should get annual flu shots and the pneumonia vaccine, if it has been more than 5 years since they previously received the vaccine. If they have never had a pneumonia vaccine before transplant, if they develop PTDM, they should receive one upon diagnosis. When infections do occur in transplant patients, whether viral infections, bacterial or fungal infections of the skin, or pneumonia, they can accelerate even more rapidly than in a nondiabetic transplant patient. Any patient report of a possible infection should trigger a rapid request for assessment and treatment. Additionally, because HCV and CMV infections increase the risk for PTDM, they are more common among patients with PTDM and may require ongoing treatment. Unrecognized, pre-existing human papilloma virus infection can also accelerate the development of venereal warts after transplant that require treatment, as well.
Hypogonadism and infertility are common to many transplant candidates of reproductive age. Whereas amenorrhea often improves after transplant, many women in particular may still have menstrual irregularities after transplant (200), and recent evidence suggests that both tacrolimus and sirolimus may impact reproductive function (201).
Ideally, pregnancy should be planned, and the transplant team should be involved before, during, and after pregnancy involving transplant recipients. Women desiring pregnancy should be encouraged to wait until immunosuppression doses are stable and their risk of rejection is reduced, at least 6 months and preferably 1 year after transplant. If pregnancy is not desired, a discussion of birth control options should be offered early. Surgical methods of birth control are preferred because the risks of birth control pills (eg, thromboembolic disease) and intrauterine devices (eg, infection) may be greater in a transplant patient than a nontransplant patient. Thus, pretransplant counseling of women of reproductive age should include the potential need to consider birth control so that unexpected pregnancy does not occur.
In general, men have normal testosterone after transplant (200), but sirolimus, in particular, has been reported to reduce sperm count and in some cases alter gonadotropins, as well as reduce fertility rates (202, 203). It is currently unknown whether a male transplant recipient on immunosuppression can confer any other risk to a child that is conceived.
E. Importance of team-based care
One of the most important variables in transplant care, as a whole, including the management of pre-existing diabetes or PTDM, is coordination of care among team members. Although not specifically studied in a research protocol, communication among team members is increasingly recognized as important to ideal glucose management of any hospitalized patient, transplant or otherwise. The immediate transplant hospitalization is characterized by frequent twists and turns with the introduction of large doses of immunosuppressants; frequent stops, starts, and transitions in iv and oral nutritional therapies due to nausea, vomiting, or ileus; postoperative complications requiring surgery; risk and impact of infections; and changing renal function. Severe hypoglycemia is even more of a risk than hyperglycemia unless there is regular and intentional communication between and among all the nurses, diabetes educators, transplant surgeons, nutrition therapy personnel, nephrologists, infectious disease specialists, hospitalists, and endocrinologists, all of whom, and more, may be involved with the patient's care. Transition to home is another time when it is imperative that the discharge team clearly outlines the responsibilities of all caring for the patient and adjustments in therapies as they are made. Long-term care also often requires coordination between team members, time that is not often allowed for in the usual outpatient clinic. Education of the patient and his/her family is also important because the treatments are as complex as they are critical to their outcome. Often, the transplant nurse is the “point person” in the immediate post-transplant setting, the person the patient knows will get the right person involved when unexpected things happen. Long-term, however, this emphasis will shift because transplant clinics can no longer regularly see all transplant recipients for all their chronic care. The transplant team needs to make it very clear to everyone who the new team's “quarterback” will be when the patient's chronic care shifts to the endocrinologist, nephrologist, gastroenterologist, pulmonologist, or primary care physician.
VI. Prevention of PTDM
From the perspective of the patient as well as the provider, prevention of PTDM would reduce cost and improve patient quality as well as possibly quantity of life. This is being approached by evaluating new drugs for their relative ability to affect glucose metabolism and insulin action; studying new combinations of existing drugs, including altering the maximum doses or timing of changes for doses to reduce the impact on glucose intolerance; protection of islets with early insulin treatment immediately after transplant; and using agents that have been or are being studied for the prevention of diabetes in nontransplant groups.
Intensive lifestyle changes, reduced calorie intake, weight loss, and exercise are well established steps to prevent type 2 diabetes in nontransplant populations with long-term impact (204–206). Attention to preventing weight gain may be another strategy to prevent PTDM, although weight loss should not be encouraged immediately after transplant to allow time for proper wound healing. Although activity may be limited early because of pain or muscle loss, lifestyle modification, including dietitian referral, exercise program, and weight loss advice, are beneficial after kidney transplant for those with impaired glucose tolerance and should be incorporated into any prevention measure (207).
Metformin and thiazolidinediones, in particular, are effective in preventing type 2 diabetes in nontransplant populations (208, 209). However, there are few studies to demonstrate whether these medications can prevent PTDM. In animal studies, metformin can minimize immunosuppressant-induced hyperglycemia (54), and rosiglitazone prevents hyperglycemia secondary to sirolimus (210). However, the potential side effects of thiazolidinediones (eg, edema, heart failure, and bone loss) reduce enthusiasm for their use as a diabetes prevention agent in any transplant group. Prevention strategies are likely to be required early after transplant, and metformin can also be difficult to use in this time frame because of frequent hospitalizations, contrast procedures and infections, and reduced kidney function.
As a result, others are evaluating the potential role for DPP-4 inhibitors, sitagliptin, vildagliptin, or linagliptin, for prevention although they have not been proven to prevent diabetes in nontransplant populations. All are safe and efficacious in treating PTDM and have no adverse effect on immunosuppressant concentrations (182–184). Linagliptin is predominantly cleared independent of the kidney, so it does not require a change in dosing with changes in creatinine, making it the most attractive of the DPP-4 inhibitors for use after kidney transplant, in particular, for prevention or treatment.
Insulin has been used extensively for the treatment of diabetes and has been explored in the prevention of type 1 diabetes, as well as recently in the prevention of PTDM after kidney transplant (8). However, early introduction of insulin as a means to prevent diabetes is likely to have lower patient acceptance and compliance than oral agents.
At the time of this review, there are at least three active studies focused on lifestyle intervention (Comparing Glycaemic Benefits of Active vs Passive Lifestyle Intervention in Kidney Allograft Recipients [CAVIAR; NCT02233491]), basal insulin administration (A Clinical Trial to Prevent New Onset Diabetes After Transplantation [ITP-NODAT; NCT01683331]), and use of a DPP-4 inhibitor (Efficacy Study of Sitagliptin to Prevent New-onset Diabetes After Kidney Transplant [NCT01928199]) for the prevention of PTDM.
Many are hopeful that new immunosuppression medicines or new regimens could be designed to prevent or reduce PTDM. New regimens under discussion include changing the timing, dose, or combination of available agents, as well as “precision medicine” strategies where the regimen is tailored to the individual based on their specific genetic or other biomarkers. However, these strategies remain part of the research protocols. It is most important that until changes in immunosuppression regimens have been well studied for overall outcomes, individualized strategies aimed at potentially reducing PTDM should not be preferred over those established to have the best overall transplant outcomes, as reiterated by the recent International Consensus Guidelines Committee for PTDM (6).
Although prevention is important to the future, good glucose, blood pressure, and lipid management is critical to improve immediate outcomes now, as in other types of diabetes.
VII. Summary
As more patients receive solid organ transplants, live longer, and are more likely to present to transplant with a higher BMI, PTDM will continue to impact the care of solid organ transplant recipients. Establishing consensus criteria for the diagnosis of PTDM and increased glucose surveillance of recipients in and out of the hospital has led to earlier diagnosis and treatment. It is hoped that earlier diagnosis and treatment of PTDM will also improve long-term outcomes. Regardless, recipients should ideally receive regular care for PTDM not only to safely manage glucose and prevent diabetic complications, but also to prevent as well as treat infections that are more likely to occur in these immunosuppressed patients. Although efforts are being made to prevent diabetes and immunosuppression agents contribute to PTDM, it is important that the transplant team chooses the immunosuppression most likely to be effective for successful solid organ transplant, not to minimize risk of PTDM. Long-term outcomes for the patient are linked first and foremost to graft survival. Until PTDM can be reliably prevented, it will require management much like those with pretransplant diabetes. Management of any type of diabetes is more challenging after transplant than in nontransplant settings because of greater risk of fluctuating kidney function; varying types of nutritional supplementation that impact diabetes treatment regimens; and large variations in insulin resistance due to changes in immunosuppression, intermittent episodes of rejection, infection or need for interval procedures or surgery. The management is further complicated by the greater risk for drug-drug interactions between the immunosuppression agents and drugs needed for treatment of comorbidities of hypertension and hyperlipidemia. Despite these challenges, the management of the patient with PTDM has greatly improved. There is also hope for further improvements as more groups work on better strategies to prevent as well as treat PTDM for the benefit of all future transplant recipients.
Acknowledgments
This work was done in part by the faculty of the VA Nebraska-Western Iowa Health Care System and is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Biomedical Laboratory Research and Development.
Disclosure Summary: V.S. received a grant from Boehringer Ingelheim, Inc., for medication used in animal experiments evaluating immunosuppressant effects on insulin action. The contents of this review do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
- BMI
- body mass index
- CF
- cystic fibrosis
- CFRD
- CF-related diabetes
- CMV
- cytomegalovirus
- CNI
- calcineurin inhibitor
- DPP-4
- dipeptidyl-peptidase-4
- GLP-1
- glucagon-like peptide-1
- HbA1c
- hemoglobin A1c
- HCV
- hepatitis C virus
- IFG
- impaired fasting glucose
- IRS
- insulin receptor substrate
- mTOR
- mammalian target of rapamycin
- mTORC
- mTOR-containing complex
- NODAT
- new-onset diabetes after transplantation
- OGTT
- oral glucose tolerance test
- PCKD
- polycystic kidney disease
- PI3K
- phosphatidylinositol 3 kinase
- PTDM
- post-transplant diabetes mellitus
- SNP
- single nucleotide polymorphism
- SPK
- simultaneous pancreas-kidney
- TPN
- total parenteral nutrition.
References
- 1. Cosio FG, Hickson LJ, Griffin MD, Stegall MD, Kudva Y. Patient survival and cardiovascular risk after kidney transplantation: the challenge of diabetes. Am J Transplant. 2008;8:593–599. [DOI] [PubMed] [Google Scholar]
- 2. Morales JM, Marcén R, del Castillo D, et al. Risk factors for graft loss and mortality after renal transplantation according to recipient age: a prospective multicentre study. Nephrol Dial Transplant. 2012;27(suppl 4):iv39–iv46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davidson J, Wilkinson A, Dantal J, et al. New-onset diabetes after transplantation: 2003 International Consensus Guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75(10 suppl):SS3–SS24. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association. 2 Classification and diagnosis of diabetes. Diabetes Care. 2015;38(suppl 1):S8–S16. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. Report of a WHO/IDF Consultation. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 6. Sharif A, Hecking M, de Vries AP, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armstrong KA, Prins JB, Beller EM, et al. Should an oral glucose tolerance test be performed routinely in all renal transplant recipients? Clin J Am Soc Nephrol. 2006;1:100–108. [DOI] [PubMed] [Google Scholar]
- 8. Hecking M, Haidinger M, Döller D, et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol. 2012;23:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sulanc E, Lane JT, Puumala SE, Groggel GC, Wrenshall LE, Stevens RB. New-onset diabetes after kidney transplantation: an application of 2003 international guidelines. Transplantation. 2005;80:945–952. [DOI] [PubMed] [Google Scholar]
- 10. Shabir S, Jham S, Harper L, Ball S, Borrows R, Sharif A. Validity of glycated haemoglobin to diagnose new onset diabetes after transplantation. Transpl Int. 2013;26:315–321. [DOI] [PubMed] [Google Scholar]
- 11. John PR, Thuluvath PJ. Outcome of patients with new-onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl. 2002;8:708–713. [DOI] [PubMed] [Google Scholar]
- 12. Boerner B, Shivaswamy V, Goldner W, Larsen J. Management of the hospitalized transplant patient. Curr Diab Rep. 2015;15:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caillard S, Eprinchard L, Perrin P, et al. Incidence and risk factors of glucose metabolism disorders in kidney transplant recipients: role of systematic screening by oral glucose tolerance test. Transplantation. 2011;91:757–764. [DOI] [PubMed] [Google Scholar]
- 14. Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM. Post-transplant diabetes mellitus: increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int. 2001;59:732–737. [DOI] [PubMed] [Google Scholar]
- 15. Gourishankar S, Jhangri GS, Tonelli M, Wales LH, Cockfield SM. Development of diabetes mellitus following kidney transplantation: a Canadian experience. Am J Transplant. 2004;4:1876–1882. [DOI] [PubMed] [Google Scholar]
- 16. Boerner BP, Shivaswamy V, Desouza CV, Larsen JL. Diabetes and cardiovascular disease following kidney transplantation. Curr Diabetes Rev. 2011;7:221–234. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Hutchinson II, Shah T, Min DI. Genetic and clinical risk factors of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation. 2011;91:1114–1119. [DOI] [PubMed] [Google Scholar]
- 18. Ghisdal L, Baron C, Le Meur Y, et al. TCF7L2 polymorphism associates with new-onset diabetes after transplantation. J Am Soc Nephrol. 2009;20:2459–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parvizi Z, Azarpira N, Kohan L, Darai M, Kazemi K, Parvizi MM. Association between E23K variant in KCNJ11 gene and new-onset diabetes after liver transplantation. Mol Biol Rep. 2014;41:6063–6069. [DOI] [PubMed] [Google Scholar]
- 20. Tavira B, Coto E, Torres A, et al. Association between a common KCNJ11 polymorphism (rs5219) and new-onset posttransplant diabetes in patients treated with tacrolimus. Mol Genet Metab. 2012;105:525–527. [DOI] [PubMed] [Google Scholar]
- 21. Kurzawski M, Dziewanowski K, Łapczuk J, Wajda A, Droździk M. Analysis of common type 2 diabetes mellitus genetic risk factors in new-onset diabetes after transplantation in kidney transplant patients medicated with tacrolimus. Eur J Clin Pharmacol. 2012;68:1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tavira B, Coto E, Díaz-Corte C, et al. KCNQ1 gene variants and risk of new-onset diabetes in tacrolimus-treated renal-transplanted patients. Clin Transplant. 2011;25:E284–E291. [DOI] [PubMed] [Google Scholar]
- 23. Kim YG, Ihm CG, Lee TW, et al. Association of genetic polymorphisms of interleukins with new-onset diabetes after transplantation in renal transplantation. Transplantation. 2012;93:900–907. [DOI] [PubMed] [Google Scholar]
- 24. Chen Y, Sampaio MS, Yang JW, Min D, Hutchinson IV. Genetic polymorphisms of the transcription factor NFATc4 and development of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation. 2012;93:325–330. [DOI] [PubMed] [Google Scholar]
- 25. Nicoletto BB, Souza GC, Fonseca NK, et al. Association between 276G/T adiponectin gene polymorphism and new-onset diabetes after kidney transplantation. Transplantation. 2013;96:1059–1064. [DOI] [PubMed] [Google Scholar]
- 26. de Mattos AM, Olyaei AJ, Prather JC, Golconda MS, Barry JM, Norman DJ. Autosomal-dominant polycystic kidney disease as a risk factor for diabetes mellitus following renal transplantation. Kidney Int. 2005;67:714–720. [DOI] [PubMed] [Google Scholar]
- 27. Ducloux D, Motte G, Vautrin P, Bresson-Vautrin C, Rebibou JM, Chalopin JM. Polycystic kidney disease as a risk factor for post-transplant diabetes mellitus. Nephrol Dial Transplant. 1999;14:1244–1246. [DOI] [PubMed] [Google Scholar]
- 28. Hamer RA, Chow CL, Ong AC, McKane WS. Polycystic kidney disease is a risk factor for new-onset diabetes after transplantation. Transplantation. 2007;83:36–40. [DOI] [PubMed] [Google Scholar]
- 29. Ruderman I, Masterson R, Yates C, Gorelik A, Cohney SJ, Walker RG. New onset diabetes after kidney transplantation in autosomal dominant polycystic kidney disease: a retrospective cohort study. Nephrology (Carlton). 2012;17:89–96. [DOI] [PubMed] [Google Scholar]
- 30. Pietrzak-Nowacka M, Safranow K, Bińczak-Kuleta A, Rózański J, Ciechanowski K, Ciechanowicz A. Association of C49620T ABCC8 polymorphism with anthropometric and metabolic parameters in patients with autosomal dominant polycystic kidney disease: a preliminary study. Nefrologia. 2012;32:153–159. [DOI] [PubMed] [Google Scholar]
- 31. Menezes LF, Zhou F, Patterson AD, et al. Network analysis of a Pkd1-mouse model of autosomal dominant polycystic kidney disease identifies HNF4α as a disease modifier. PLoS Genet. 2012;8:e1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ye X, Kuo HT, Sampaio MS, Jiang Y, Bunnapradist S. Risk factors for development of new-onset diabetes mellitus after transplant in adult lung transplant recipients. Clin Transplant. 2011;25:885–891. [DOI] [PubMed] [Google Scholar]
- 33. Wauters RP, Cosio FG, Suarez Fernandez ML, Kudva Y, Shah P, Torres VE. Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation. 2012;94:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mathew JT, Rao M, Job V, Ratnaswamy S, Jacob CK. Post-transplant hyperglycaemia: a study of risk factors. Nephrol Dial Transplant. 2003;18:164–171. [DOI] [PubMed] [Google Scholar]
- 35. Joss N, Staatz CE, Thomson AH, Jardine AG. Predictors of new onset diabetes after renal transplantation. Clin Transplant. 2007;21:136–143. [DOI] [PubMed] [Google Scholar]
- 36. Pirsch JD, Henning AK, First MR, et al. New-onset diabetes after transplantation: results from a double-blind early corticosteroid withdrawal trial. Am J Transplant. 2015;15:1982–1990. [DOI] [PubMed] [Google Scholar]
- 37. Larsen JL, Bennett RG, Burkman T, et al. Tacrolimus and sirolimus cause insulin resistance in normal Sprague Dawley rats. Transplantation. 2006;82:466–470. [DOI] [PubMed] [Google Scholar]
- 38. Shapiro R, Jordan M, Fung J, et al. Kidney transplantation under FK 506 immunosuppression. Transplant Proc. 1991;23:920–923. [PMC free article] [PubMed] [Google Scholar]
- 39. Mieles L, Gordon RD, Mintz D, Toussaint RM, Imventarza O, Starzl TE. Glycemia and insulin need following FK 506 rescue therapy in liver transplant recipients. Transplant Proc. 1991;23:949–953. [PMC free article] [PubMed] [Google Scholar]
- 40. Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol. 2002;13:1374–1380. [DOI] [PubMed] [Google Scholar]
- 41. Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 Kidney Transplant Study Group. Transplantation. 1998;65:515–523. [DOI] [PubMed] [Google Scholar]
- 42. Woodward RS, Schnitzler MA, Baty J, et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003;3:590–598. [DOI] [PubMed] [Google Scholar]
- 43. Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–1514. [DOI] [PubMed] [Google Scholar]
- 44. Porrini E, Moreno JM, Osuna A, et al. Prediabetes in patients receiving tacrolimus in the first year after kidney transplantation: a prospective and multicenter study. Transplantation. 2008;85:1133–1138. [DOI] [PubMed] [Google Scholar]
- 45. Shihab FS, Waid TH, Conti DJ, et al. Conversion from cyclosporine to tacrolimus in patients at risk for chronic renal allograft failure: 60-month results of the CRAF Study. Transplantation. 2008;85:1261–1269. [DOI] [PubMed] [Google Scholar]
- 46. Robertson RP, Franklin G, Nelson L. Intravenous glucose tolerance and pancreatic islet beta-cell function in patients with multiple sclerosis during 2-yr treatment with cyclosporin. Diabetes. 1989;38:58–64. [DOI] [PubMed] [Google Scholar]
- 47. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group. Lancet. 1994;344:423–428. [PubMed] [Google Scholar]
- 48. Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997;63:977–983. [DOI] [PubMed] [Google Scholar]
- 49. Boots JM, van Duijnhoven EM, Christiaans MH, Wolffenbuttel BH, van Hooff JP. Glucose metabolism in renal transplant recipients on tacrolimus: the effect of steroid withdrawal and tacrolimus trough level reduction. J Am Soc Nephrol. 2002;13:221–227. [DOI] [PubMed] [Google Scholar]
- 50. Crutchlow MF, Bloom RD. Transplant-associated hyperglycemia: a new look at an old problem. Clin J Am Soc Nephrol. 2007;2:343–355. [DOI] [PubMed] [Google Scholar]
- 51. Duijnhoven EM, Boots JM, Christiaans MH, Wolffenbuttel BH, Van Hooff JP. Influence of tacrolimus on glucose metabolism before and after renal transplantation: a prospective study. J Am Soc Nephrol. 2001;12:583–588. [DOI] [PubMed] [Google Scholar]
- 52. Fuji S, Kim SW, Mori S, et al. Decreased insulin secretion in patients receiving tacrolimus as GVHD prophylaxis after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2010;45:405–406. [DOI] [PubMed] [Google Scholar]
- 53. Hjelmesaeth J, Jenssen T, Hagen M, Egeland T, Hartmann A. Determinants of insulin secretion after renal transplantation. Metabolism. 2003;52:573–578. [DOI] [PubMed] [Google Scholar]
- 54. Shivaswamy V, Bennett RG, Clure CC, Larsen JL, Hamel FG. Metformin improves immunosuppressant induced hyperglycemia and exocrine apoptosis in rats. Transplantation. 2013;95:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fraenkel M, Ketzinel-Gilad M, Ariav Y, et al. mTOR inhibition by rapamycin prevents β-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–957. [DOI] [PubMed] [Google Scholar]
- 56. Shivaswamy V, Bennett RG, Clure CC, et al. Tacrolimus and sirolimus have distinct effects on insulin signaling in male and female rats. Transl Res. 2014;163:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oetjen E, Baun D, Beimesche S, et al. Inhibition of human insulin gene transcription by the immunosuppressive drugs cyclosporin A and tacrolimus in primary, mature islets of transgenic mice. Mol Pharmacol. 2003;63:1289–1295. [DOI] [PubMed] [Google Scholar]
- 58. Radu RG, Fujimoto S, Mukai E, et al. Tacrolimus suppresses glucose-induced insulin release from pancreatic islets by reducing glucokinase activity. Am J Physiol Endocrinol Metab. 2005;288:E365–E371. [DOI] [PubMed] [Google Scholar]
- 59. Redmon JB, Olson LK, Armstrong MB, Greene MJ, Robertson RP. Effects of tacrolimus (FK506) on human insulin gene expression, insulin mRNA levels, and insulin secretion in HIT-T15 cells. J Clin Invest. 1996;98:2786–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Johnson JD, Ao Z, Ao P, et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18:833–845. [DOI] [PubMed] [Google Scholar]
- 61. Soleimanpour SA, Crutchlow MF, Ferrari AM, et al. Calcineurin signaling regulates human islet β-cell survival. J Biol Chem. 2010;285:40050–40059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Demozay D, Tsunekawa S, Briaud I, Shah R, Rhodes CJ. Specific glucose-induced control of insulin receptor substrate-2 expression is mediated via Ca2+-dependent calcineurin/NFAT signaling in primary pancreatic islet β-cells. Diabetes. 2011;60:2892–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Drachenberg CB, Klassen DK, Weir MR, et al. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68:396–402. [DOI] [PubMed] [Google Scholar]
- 64. Wyzgał J, Paczek L, Sańko-Resmer J, et al. Insulin resistance in kidney allograft recipients treated with calcineurin inhibitors. Ann Transplant. 2007;12:26–29. [PubMed] [Google Scholar]
- 65. Sui W, Zou H, Zou G, et al. Clinical study of the risk factors of insulin resistance and metabolic syndrome after kidney transplantation. Transpl Immunol. 2008;20:95–98. [DOI] [PubMed] [Google Scholar]
- 66. Chen QJ, Li J, Zuo SR, et al. Tacrolimus decreases insulin sensitivity without reducing fasting insulin concentration: a 2-year follow-up study in kidney transplant recipients. Ren Fail. 2015;37:601–606. [DOI] [PubMed] [Google Scholar]
- 67. Pereira MJ, Palming J, Rizell M, et al. Cyclosporine A and tacrolimus reduce the amount of GLUT4 at the cell surface in human adipocytes: increased endocytosis as a potential mechanism for the diabetogenic effects of immunosuppressive agents. J Clin Endocrinol Metab 2014;99:E1885–E1894. [DOI] [PubMed] [Google Scholar]
- 68. Fuhrmann A, Lopes P, Sereno J, et al. Molecular mechanisms underlying the effects of cyclosporin A and sirolimus on glucose and lipid metabolism in liver, skeletal muscle and adipose tissue in an in vivo rat model. Biochem Pharmacol. 2014;88:216–228. [DOI] [PubMed] [Google Scholar]
- 69. Øzbay LA, Møller N, Juhl C, et al. Calcineurin inhibitors acutely improve insulin sensitivity without affecting insulin secretion in healthy human volunteers. Br J Clin Pharmacol. 2012;73:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rickels MR, Mueller R, Teff KL, Naji A. β-Cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants. J Clin Endocrinol Metab. 2010;95:1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cottrell DA. Normalization of insulin sensitivity and glucose homeostasis in type I diabetic pancreas transplant recipients: a 48-month cross-sectional study–a clinical research center study. J Clin Endocrinol Metab. 1996;81:3513–3519. [DOI] [PubMed] [Google Scholar]
- 72. Rickels MR, Naji A, Teff KL. Insulin sensitivity, glucose effectiveness, and free fatty acid dynamics after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab. 2006;91:2138–2144. [DOI] [PubMed] [Google Scholar]
- 73. Navaneethan SD, Sankarasubbaiyan S, Gross MD, Jeevanantham V, Monk RD. Tacrolimus-associated hypomagnesemia in renal transplant recipients. Transplant Proc. 2006;38:1320–1322. [DOI] [PubMed] [Google Scholar]
- 74. Vannini SD, Mazzola BL, Rodoni L, et al. Permanently reduced plasma ionized magnesium among renal transplant recipients on cyclosporine. Transpl Int. 1999;12:244–249. [DOI] [PubMed] [Google Scholar]
- 75. Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2:366–373. [DOI] [PubMed] [Google Scholar]
- 76. Van Laecke S, Van Biesen W, Verbeke F, De Bacquer D, Peeters P, Vanholder R. Posttransplantation hypomagnesemia and its relation with immunosuppression as predictors of new-onset diabetes after transplantation. Am J Transplant. 2009;9:2140–2149. [DOI] [PubMed] [Google Scholar]
- 77. Garg N, Weinberg J, Ghai S, et al. Lower magnesium level associated with new-onset diabetes and pre-diabetes after kidney transplantation. J Nephrol. 2014;27:339–344. [DOI] [PubMed] [Google Scholar]
- 78. Van Laecke S, Desideri F, Geerts A, et al. Hypomagnesemia and the risk of new-onset diabetes after liver transplantation. Liver Transpl. 2010;16:1278–1287. [DOI] [PubMed] [Google Scholar]
- 79. Van Laecke S, Nagler EV, Taes Y, Van Biesen W, Peeters P, Vanholder R. The effect of magnesium supplements on early post-transplantation glucose metabolism: a randomized controlled trial. Transpl Int. 2014;27:895–902. [DOI] [PubMed] [Google Scholar]
- 80. Hakeam HA, Al-Jedai AH, Raza SM, Hamawi K. Sirolimus induced dyslipidemia in tacrolimus based vs. tacrolimus free immunosuppressive regimens in renal transplant recipients. Ann Transplant. 2008;13:46–53. [PubMed] [Google Scholar]
- 81. Flechner SM, Glyda M, Cockfield S, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant. 2011;11:1633–1644. [DOI] [PubMed] [Google Scholar]
- 82. Johnston O, Rose CL, Webster AC, Gill JS. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol. 2008;19:1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gyurus E, Kaposztas Z, Kahan BD. Sirolimus therapy predisposes to new-onset diabetes mellitus after renal transplantation: a long-term analysis of various treatment regimens. Transplant Proc. 2011;43:1583–1592. [DOI] [PubMed] [Google Scholar]
- 84. Veroux M, Tallarita T, Corona D, et al. Conversion to sirolimus therapy in kidney transplant recipients with new onset diabetes mellitus after transplantation. Clin Dev Immunol. 2013;2013:496974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bell E, Cao X, Moibi JA, et al. Rapamycin has a deleterious effect on MIN-6 cells and rat and human islets. Diabetes. 2003;52:2731–2739. [DOI] [PubMed] [Google Scholar]
- 86. Zahr E, Molano RD, Pileggi A, et al. Rapamycin impairs in vivo proliferation of islet β-cells. Transplantation. 2007;84:1576–1583. [DOI] [PubMed] [Google Scholar]
- 87. Bussiere CT, Lakey JR, Shapiro AM, Korbutt GS. The impact of the mTOR inhibitor sirolimus on the proliferation and function of pancreatic islets and ductal cells. Diabetologia. 2006;49:2341–2349. [DOI] [PubMed] [Google Scholar]
- 88. Arvisais E, Hou X, Wyatt TA, et al. Prostaglandin F2α represses IGF-I-stimulated IRS1/phosphatidylinositol-3-kinase/AKT signaling in the corpus luteum: role of ERK and P70 ribosomal S6 kinase. Mol Endocrinol. 2010;24:632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hashimoto R, Sakai K, Matsumoto H, Iwashita M. Tumor necrosis factor-α (TNF-α) inhibits insulin-like growth factor-I (IGF-I) activities in human trophoblast cell cultures through IGF-I/insulin hybrid receptors. Endocr J. 2010;57:193–200. [DOI] [PubMed] [Google Scholar]
- 90. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. [DOI] [PubMed] [Google Scholar]
- 91. Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. [DOI] [PubMed] [Google Scholar]
- 92. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. [DOI] [PubMed] [Google Scholar]
- 93. Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Blättler SM, Cunningham JT, Verdeguer F, et al. Yin Yang 1 deficiency in skeletal muscle protects against rapamycin-induced diabetic-like symptoms through activation of insulin/IGF signaling. Cell Metab. 2012;15:505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Krebs M, Brunmair B, Brehm A, et al. The mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–1607. [DOI] [PubMed] [Google Scholar]
- 97. Kneteman NM, Lakey JR, Wagner T, Finegood D. The metabolic impact of rapamycin (sirolimus) in chronic canine islet graft recipients. Transplantation. 1996;61:1206–1210. [DOI] [PubMed] [Google Scholar]
- 98. Soleimanpour SA, Hirshberg B, Bunnell DJ, et al. Metabolic function of a suboptimal transplanted islet mass in nonhuman primates on rapamycin monotherapy. Cell Transplant. 2012;21:1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rickels MR, Kong SM, Fuller C, et al. Improvement in insulin sensitivity after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab. 2013;98:E1780–E1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ye L, Varamini B, Lamming DW, Sabatini DM, Baur JA. Rapamycin has a biphasic effect on insulin sensitivity in C2C12 myotubes due to sequential disruption of mTORC1 and mTORC2. Front Genet. 2012;3:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. [DOI] [PubMed] [Google Scholar]
- 102. Houde VP, Brûlé S, Festuccia WT, et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 104. Schweer T, Gwinner W, Scheffner I, Schwarz A, Haller H, Blume C. High impact of rejection therapy on the incidence of post-transplant diabetes mellitus after kidney transplantation. Clin Transplant. 2014;28:512–519. [DOI] [PubMed] [Google Scholar]
- 105. Siraj ES, Abacan C, Chinnappa P, Wojtowicz J, Braun W. Risk factors and outcomes associated with posttransplant diabetes mellitus in kidney transplant recipients. Transplant Proc. 2010;42:1685–1689. [DOI] [PubMed] [Google Scholar]
- 106. Maes BD, Kuypers D, Messiaen T, et al. Posttransplantation diabetes mellitus in FK-506-treated renal transplant recipients: analysis of incidence and risk factors. Transplantation. 2001;72:1655–1661. [DOI] [PubMed] [Google Scholar]
- 107. Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. 2002;25:583–592. [DOI] [PubMed] [Google Scholar]
- 108. de Vries DK, Lindeman JH, Ringers J, Reinders ME, Rabelink TJ, Schaapherder AF. Donor brain death predisposes human kidney grafts to a proinflammatory reaction after transplantation. Am J Transplant. 2011;11:1064–1070. [DOI] [PubMed] [Google Scholar]
- 109. Koo DD, Welsh KI, McLaren AJ, Roake JA, Morris PJ, Fuggle SV. Cadaver versus living donor kidneys: impact of donor factors on antigen induction before transplantation. Kidney Int. 1999;56:1551–1559. [DOI] [PubMed] [Google Scholar]
- 110. Naesens M, Li L, Ying L, et al. Expression of complement components differs between kidney allografts from living and deceased donors. J Am Soc Nephrol. 2009;20:1839–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pratschke J, Wilhelm MJ, Laskowski I, et al. Influence of donor brain death on chronic rejection of renal transplants in rats. J Am Soc Nephrol. 2001;12:2474–2481. [DOI] [PubMed] [Google Scholar]
- 112. Mazali FC, Lalli CA, Alves-Filho G, Mazzali M. Posttransplant diabetes mellitus: incidence and risk factors. Transplant Proc. 2008;40:764–766. [DOI] [PubMed] [Google Scholar]
- 113. Sumrani NB, Delaney V, Ding ZK, et al. Diabetes mellitus after renal transplantation in the cyclosporine era–an analysis of risk factors. Transplantation. 1991;51:343–347. [DOI] [PubMed] [Google Scholar]
- 114. Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J Gastroenterol. 2012;18:1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Verna EC, Brown RS., Jr Hepatitis C virus and liver transplantation. Clin Liver Dis. 2006;10:919–940. [DOI] [PubMed] [Google Scholar]
- 116. Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14(suppl 2):S36–S44. [DOI] [PubMed] [Google Scholar]
- 117. Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, et al. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066–1072. [DOI] [PubMed] [Google Scholar]
- 118. Chen T, Jia H, Li J, Chen X, Zhou H, Tian H. New onset diabetes mellitus after liver transplantation and hepatitis C virus infection: meta-analysis of clinical studies. Transpl Int. 2009;22:408–415. [DOI] [PubMed] [Google Scholar]
- 119. Driscoll CJ, Cashion AK, Hathaway DK, et al. Posttransplant diabetes mellitus in liver transplant recipients. Prog Transplant. 2006;16:110–116. [DOI] [PubMed] [Google Scholar]
- 120. Delgado-Borrego A, Liu YS, Jordan SH, et al. Prospective study of liver transplant recipients with HCV infection: evidence for a causal relationship between HCV and insulin resistance. Liver Transpl. 2008;14:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. Am J Transplant. 2005;5:2433–2440. [DOI] [PubMed] [Google Scholar]
- 122. Shah T, Kasravi A, Huang E, et al. Risk factors for development of new-onset diabetes mellitus after kidney transplantation. Transplantation. 2006;82:1673–1676. [DOI] [PubMed] [Google Scholar]
- 123. Prasad N, Gurjer D, Bhadauria D, et al. Is basiliximab induction, a novel risk factor for new onset diabetes after transplantation for living donor renal allograft recipients? Nephrology (Carlton). 2014;19:244–250. [DOI] [PubMed] [Google Scholar]
- 124. Baid-Agrawal S, Frei U, Reinke P, et al. Impaired insulin sensitivity as an underlying mechanism linking hepatitis C and posttransplant diabetes mellitus in kidney recipients. Am J Transplant. 2009;9:2777–2784. [DOI] [PubMed] [Google Scholar]
- 125. Gürsoy M, Köksal R, Karavelioğlu D, et al. Pretransplantation α-interferon therapy and the effect of hepatitis C virus infection on kidney allograft recipients. Transplant Proc. 2000;32:580–582. [DOI] [PubMed] [Google Scholar]
- 126. Kamar N, Mariat C, Delahousse M, et al. Diabetes mellitus after kidney transplantation: a French multicentre observational study. Nephrol Dial Transplant. 2007;22:1986–1993. [DOI] [PubMed] [Google Scholar]
- 127. Saliba F, Lakehal M, Pageaux GP, et al. Risk factors for new-onset diabetes mellitus following liver transplantation and impact of hepatitis C infection: an observational multicenter study. Liver Transpl. 2007;13:136–144. [DOI] [PubMed] [Google Scholar]
- 128. Sánchez-Pérez B, Aranda Narváez JM, Santoyo Santoyo J, et al. Influence of immunosuppression and effect of hepatitis C virus on new onset of diabetes mellitus in liver transplant recipients. Transplant Proc. 2008;40:2994–2996. [DOI] [PubMed] [Google Scholar]
- 129. Hjelmesaeth J, Hartmann A, Kofstad J, et al. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation. 1997;64:979–983. [DOI] [PubMed] [Google Scholar]
- 130. Abou-Ayache R, Büchler M, Le Pogamp P, et al. The influence of cytomegalovirus infections on patient and renal graft outcome: a 3-year, multicenter, observational study (Post-ECTAZ Study). Transplant Proc. 2011;43:2630–2635. [DOI] [PubMed] [Google Scholar]
- 131. Marin M, Renoult E, Bondor CI, Kessler M. Factors influencing the onset of diabetes mellitus after kidney transplantation: a single French center experience. Transplant Proc. 2005;37:1851–1856. [DOI] [PubMed] [Google Scholar]
- 132. Einollahi B, Motalebi M, Salesi M, Ebrahimi M, Taghipour M. The impact of cytomegalovirus infection on new-onset diabetes mellitus after kidney transplantation: a review on current findings. J Nephropathol. 2014;3:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hjelmesaeth J, Sagedal S, Hartmann A, et al. Asymptomatic cytomegalovirus infection is associated with increased risk of new-onset diabetes mellitus and impaired insulin release after renal transplantation. Diabetologia. 2004;47:1550–1556. [DOI] [PubMed] [Google Scholar]
- 134. Hjelmesaeth J, Müller F, Jenssen T, Rollag H, Sagedal S, Hartmann A. Is there a link between cytomegalovirus infection and new-onset posttransplantation diabetes mellitus? Potential mechanisms of virus induced β-cell damage. Nephrol Dial Transplant. 2005;20:2311–2315. [DOI] [PubMed] [Google Scholar]
- 135. Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59:381–391. [DOI] [PubMed] [Google Scholar]
- 136. Song Y, Wang L, Pittas AG, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Courbebaisse M, Alberti C, Colas S, et al. VITamin D supplementation in renAL transplant recipients (VITALE): a prospective, multicentre, double-blind, randomized trial of vitamin D estimating the benefit and safety of vitamin D3 treatment at a dose of 100,000 UI compared with a dose of 12,000 UI in renal transplant recipients: study protocol for a double-blind, randomized, controlled trial. Trials. 2014;15:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Choe EY, Wang HJ, Kwon O, et al. HMG CoA reductase inhibitor treatment induces dysglycemia in renal allograft recipients. Transplantation. 2014;97:419–425. [DOI] [PubMed] [Google Scholar]
- 139. Cho Y, Lee MJ, Choe EY, et al. Statin therapy is associated with the development of new-onset diabetes after transplantation in liver recipients with high fasting plasma glucose levels. Liver Transpl. 2014;20:557–563. [DOI] [PubMed] [Google Scholar]
- 140. Boudreaux JP, McHugh L, Canafax DM, et al. The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation. 1987;44:376–381. [DOI] [PubMed] [Google Scholar]
- 141. Revanur VK, Jardine AG, Kingsmore DB, Jaques BC, Hamilton DH, Jindal RM. Influence of diabetes mellitus on patient and graft survival in recipients of kidney transplantation. Clin Transplant. 2001;15:89–94. [DOI] [PubMed] [Google Scholar]
- 142. Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation. IV. Impact of post-transplant diabetes. Kidney Int. 2002;62:1440–1446. [DOI] [PubMed] [Google Scholar]
- 143. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. [DOI] [PubMed] [Google Scholar]
- 144. Cosio FG, Kudva Y, van der Velde M, et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415–2421. [DOI] [PubMed] [Google Scholar]
- 145. Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69:588–595. [DOI] [PubMed] [Google Scholar]
- 146. Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16:496–506. [DOI] [PubMed] [Google Scholar]
- 147. Kuo HT, Sampaio MS, Vincenti F, Bunnapradist S. Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis. 2010;56:1127–1139. [DOI] [PubMed] [Google Scholar]
- 148. Miles AM, Sumrani N, Horowitz R, et al. Diabetes mellitus after renal transplantation: as deleterious as non-transplant-associated diabetes? Transplantation. 1998;65:380–384. [DOI] [PubMed] [Google Scholar]
- 149. Vesco L, Busson M, Bedrossian J, Bitker MO, Hiesse C, Lang P. Diabetes mellitus after renal transplantation: characteristics, outcome, and risk factors. Transplantation. 1996;61:1475–1478. [DOI] [PubMed] [Google Scholar]
- 150. Cole EH, Johnston O, Rose CL, Gill JS. Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin J Am Soc Nephrol. 2008;3:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Burroughs TE, Swindle J, Takemoto S, et al. Diabetic complications associated with new-onset diabetes mellitus in renal transplant recipients. Transplantation. 2007;83:1027–1034. [DOI] [PubMed] [Google Scholar]
- 152. Moro JA, Martínez-Dolz L, Almenar L, et al. Impact of diabetes mellitus on heart transplant recipients [in Spanish]. Rev Esp Cardiol. 2006;59:1033–1037. [DOI] [PubMed] [Google Scholar]
- 153. Mogollón Jiménez MV, Sobrino Márquez JM, Arizón Muñoz JM, et al. Incidence and importance of de novo diabetes mellitus after heart transplantation. Transplant Proc. 2008;40:3053–3055. [DOI] [PubMed] [Google Scholar]
- 154. Cho MS, Choi HI, Kim IO, et al. The clinical course and outcomes of post-transplantation diabetes mellitus after heart transplantation. J Korean Med Sci. 2012;27:1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lv C, Zhang Y, Chen X, et al. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes. 2015;7:881–890. [DOI] [PubMed] [Google Scholar]
- 156. Morbitzer KA, Taber DJ, Pilch NA, et al. The impact of diabetes mellitus and glycemic control on clinical outcomes following liver transplant for hepatitis C. Clin Transplant. 2014;28:862–868. [DOI] [PubMed] [Google Scholar]
- 157. Veldt BJ, Poterucha JJ, Watt KD, et al. Insulin resistance, serum adipokines and risk of fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2009;9:1406–1413. [DOI] [PubMed] [Google Scholar]
- 158. Foxton MR, Quaglia A, Muiesan P, et al. The impact of diabetes mellitus on fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2006;6:1922–1929. [DOI] [PubMed] [Google Scholar]
- 159. Moon JI, Barbeito R, Faradji RN, Gaynor JJ, Tzakis AG. Negative impact of new-onset diabetes mellitus on patient and graft survival after liver transplantation: long-term follow up. Transplantation. 2006;82:1625–1628. [DOI] [PubMed] [Google Scholar]
- 160. Xu X, Ling Q, He ZL, Gao F, Zheng SS. Post-transplant diabetes mellitus in liver transplantation: Hangzhou experience. Hepatobiliary Pancreat Dis Int. 2008;7:465–470. [PubMed] [Google Scholar]
- 161. Navasa M, Bustamante J, Marroni C, et al. Diabetes mellitus after liver transplantation: prevalence and predictive factors. J Hepatol. 1996;25:64–71. [DOI] [PubMed] [Google Scholar]
- 162. Trail KC, McCashland TM, Larsen JL, et al. Morbidity in patients with posttransplant diabetes mellitus following orthotopic liver transplantation. Liver Transpl Surg. 1996;2:276–283. [DOI] [PubMed] [Google Scholar]
- 163. Trail KC, Stratta RJ, Larsen JL, et al. Results of liver transplantation in diabetic recipients. Surgery. 1993;114:650–656; discussion 656–658. [PubMed] [Google Scholar]
- 164. Hackman KL, Bailey MJ, Snell GI, Bach LA. Diabetes is a major risk factor for mortality after lung transplantation. Am J Transplant. 2014;14:438–445. [DOI] [PubMed] [Google Scholar]
- 165. Hackman KL, Snell GI, Bach LA. Prevalence and predictors of diabetes after lung transplantation: a prospective, longitudinal study. Diabetes Care. 2014;37:2919–2925. [DOI] [PubMed] [Google Scholar]
- 166. Hermayer KL, Egidi MF, Finch NJ, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes. J Clin Endocrinol Metab. 2012;97:4399–4406. [DOI] [PubMed] [Google Scholar]
- 167. American Diabetes Association. 13 Diabetes care in the hospital, nursing home, and skilled nursing facility. Diabetes Care. 2015;38(suppl):S80–S85. [DOI] [PubMed] [Google Scholar]
- 168. Pei D, Chen TW, Kuo YL, et al. The effect of surgical stress on insulin sensitivity, glucose effectiveness and acute insulin response to glucose load. J Endocrinol Invest. 2003;26:397–402. [DOI] [PubMed] [Google Scholar]
- 169. Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984;27:351–357. [DOI] [PubMed] [Google Scholar]
- 170. Garcia C, Wallia A, Gupta S, et al. Intensive glycemic control after heart transplantation is safe and effective for diabetic and non-diabetic patients. Clin Transplant. 2013;27:444–454. [DOI] [PubMed] [Google Scholar]
- 171. Keegan MT, Vrchota JM, Haala PM, Timm JV. Safety and effectiveness of intensive insulin protocol use in post-operative liver transplant recipients. Transplant Proc. 2010;42:2617–2624. [DOI] [PubMed] [Google Scholar]
- 172. Roehl KA, Lach K, Coltman AE, et al. Predictors of insulin requirements among hospitalized adults receiving parenteral nutrition. JPEN J Parenter Enteral Nutr. 2013;37:755–762. [DOI] [PubMed] [Google Scholar]
- 173. American Diabetes Association. 7 Approaches to glycemic treatment. Diabetes Care. 2015;38(suppl 1):S41–S48. [DOI] [PubMed] [Google Scholar]
- 174. Sharif A. Should metformin be our antiglycemic agent of choice post-transplantation? Am J Transplant. 2011;11:1376–1381. [DOI] [PubMed] [Google Scholar]
- 175. Larsen JL. Potential risks of metformin in transplant patients. Am J Transplant. 2012;12:795; author reply 796. [DOI] [PubMed] [Google Scholar]
- 176. Stephen J, Anderson-Haag TL, Gustafson S, Snyder JJ, Kasiske BL, Israni AK. Metformin use in kidney transplant recipients in the United States: an observational study. Am J Nephrol. 2014;40:546–553. [DOI] [PubMed] [Google Scholar]
- 177. Kurian B, Joshi R, Helmuth A. Effectiveness and long-term safety of thiazolidinediones and metformin in renal transplant recipients. Endocr Pract. 2008;14:979–984. [DOI] [PubMed] [Google Scholar]
- 178. Baldwin D, Jr, Duffin KE. Rosiglitazone treatment of diabetes mellitus after solid organ transplantation. Transplantation. 2004;77:1009–1014. [DOI] [PubMed] [Google Scholar]
- 179. Haidinger M, Antlanger M, Kopecky C, Kovarik JJ, Säemann MD, Werzowa J. Post-transplantation diabetes mellitus: evaluation of treatment strategies. Clin Transplant. 2015;29:415–424. [DOI] [PubMed] [Google Scholar]
- 180. Türk T, Pietruck F, Dolff S, et al. Repaglinide in the management of new-onset diabetes mellitus after renal transplantation. Am J Transplant. 2006;6:842–846. [DOI] [PubMed] [Google Scholar]
- 181. Boerner BP, Miles CD, Shivaswamy V. Efficacy and safety of sitagliptin for the treatment of new-onset diabetes after renal transplantation. Int J Endocrinol. 2014;2014:617638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lane JT, Odegaard DE, Haire CE, Collier DS, Wrenshall LE, Stevens RB. Sitagliptin therapy in kidney transplant recipients with new-onset diabetes after transplantation. Transplantation. 2011;92:e56–e57. [DOI] [PubMed] [Google Scholar]
- 183. Werzowa J, Hecking M, Haidinger M, et al. Vildagliptin and pioglitazone in patients with impaired glucose tolerance after kidney transplantation: a randomized, placebo-controlled clinical trial. Transplantation. 2013;95:456–462. [DOI] [PubMed] [Google Scholar]
- 184. Gueler I, Mueller S, Helmschrott M, et al. Effects of vildagliptin (Galvus) therapy in patients with type 2 diabetes mellitus after heart transplantation. Drug Des Devel Ther. 2013;7:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pinelli NR, Patel A, Salinitri FD. Coadministration of liraglutide with tacrolimus in kidney transplant recipients: a case series. Diabetes Care. 2013;36:e171–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77:299–311. [DOI] [PubMed] [Google Scholar]
- 190. American Diabetes Association. 6 Glycemic targets. Diabetes Care. 2015;38(suppl 1):S33–S40. [DOI] [PubMed] [Google Scholar]
- 191. Taler SJ, Agarwal R, Bakris GL, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 2013;62:201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Bia M, Adey DB, Bloom RD, Chan L, Kulkarni S, Tomlanovich S. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis. 2010;56:189–218. [DOI] [PubMed] [Google Scholar]
- 193. American Diabetes Association. 8 Cardiovascular disease and risk management. Diabetes Care. 2015;38(suppl):S49–S57. [DOI] [PubMed] [Google Scholar]
- 194. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 195. Kasiske B, Cosio FG, Beto J, et al. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: a report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am J Transplant. 2004;4(suppl 7):13–53. [DOI] [PubMed] [Google Scholar]
- 196. Brattström C, Wilczek H, Tydén G, Böttiger Y, Säwe J, Groth CG. Hyperlipidemia in renal transplant recipients treated with sirolimus (rapamycin). Transplantation. 1998;65:1272–1274. [DOI] [PubMed] [Google Scholar]
- 197. Chueh SC, Kahan BD. Dyslipidemia in renal transplant recipients treated with a sirolimus and cyclosporine-based immunosuppressive regimen: incidence, risk factors, progression, and prognosis. Transplantation. 2003;76:375–382. [DOI] [PubMed] [Google Scholar]
- 198. Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation. 2003;75:1213–1220. [DOI] [PubMed] [Google Scholar]
- 199. Gruessner RW, Sutherland DE, Parr E, Humar A, Gruessner AC. A prospective, randomized, open-label study of steroid withdrawal in pancreas transplantation–a preliminary report with 6-month follow-up. Transplant Proc. 2001;33:1663–1664. [DOI] [PubMed] [Google Scholar]
- 200. Mack-Shipman LR, Ratanasuwan T, Leone JP, et al. Reproductive hormones after pancreas transplantation. Transplantation. 2000;70:1180–1183. [DOI] [PubMed] [Google Scholar]
- 201. Shivaswamy V, Ochsner L, Maroni D, et al. Tacrolimus and sirolimus induce reproductive abnormalities in female rats. Transplantation. 2011;91:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Deutsch MA, Kaczmarek I, Huber S, et al. Sirolimus-associated infertility: case report and literature review of possible mechanisms. Am J Transplant. 2007;7:2414–2421. [DOI] [PubMed] [Google Scholar]
- 203. Zuber J, Anglicheau D, Elie C, et al. Sirolimus may reduce fertility in male renal transplant recipients. Am J Transplant. 2008;8:1471–1479. [DOI] [PubMed] [Google Scholar]
- 204. Knowler WC, Fowler SE, Hamman RF, et al. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–1789. [DOI] [PubMed] [Google Scholar]
- 206. Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. [DOI] [PubMed] [Google Scholar]
- 207. Sharif A, Moore R, Baboolal K. Influence of lifestyle modification in renal transplant recipients with postprandial hyperglycemia. Transplantation. 2008;85:353–358. [DOI] [PubMed] [Google Scholar]
- 208. Hecking M, Werzowa J, Haidinger M, et al. Novel views on new-onset diabetes after transplantation: development, prevention and treatment. Nephrol Dial Transplant. 2013;28:550–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Therasse A, Wallia A, Molitch ME. Management of post-transplant diabetes. Curr Diab Rep. 2013;13:121–129. [DOI] [PubMed] [Google Scholar]
- 210. Festuccia WT, Blanchard PG, Belchior T, et al. PPARγ activation attenuates glucose intolerance induced by mTOR inhibition with rapamycin in rats. Am J Physiol Endocrinol Metab. 2014;306:E1046–E1054. [DOI] [PubMed] [Google Scholar]
- 211. Valderhaug TG, Jenssen T, Hartmann A, et al. Fasting plasma glucose and glycosylated hemoglobin in the screening for diabetes mellitus after renal transplantation. Transplantation. 2009;88:429–434. [DOI] [PubMed] [Google Scholar]
- 212. Ye X, Kuo HT, Sampaio MS, Jiang Y, Reddy P, Bunnapradist S. Risk factors for development of new-onset diabetes mellitus in adult heart transplant recipients. Transplantation. 2010;89:1526–1532. [DOI] [PubMed] [Google Scholar]
- 213. Pageaux GP, Faure S, Bouyabrine H, Bismuth M, Assenat E. Long-term outcomes of liver transplantation: diabetes mellitus. Liver Transpl. 2009;15(suppl 2):S79–S82. [DOI] [PubMed] [Google Scholar]
- 214. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. [DOI] [PubMed] [Google Scholar]
- 215. Del Prato S, Aragona M, Coppelli A. Sulfonylureas and hypoglycaemia. Diabetes Nutr Metab. 2002;15:444–450; discussion 450–451. [PubMed] [Google Scholar]
- 216. Krepinsky J, Ingram AJ, Clase CM. Prolonged sulfonylurea-induced hypoglycemia in diabetic patients with end-stage renal disease. Am J Kidney Dis. 2000;35:500–505. [DOI] [PubMed] [Google Scholar]
- 217. Sagedal S, Asberg A, Hartmann A, Bergan S, Berg KJ. Glipizide treatment of post-transplant diabetes does not interfere with cyclosporine pharmacokinetics in renal allograft recipients. Clin Transplant. 1998;12:553–556. [PubMed] [Google Scholar]
- 218. Tuerk TR, Bandur S, Nuernberger J, et al. Gliquidone therapy of new-onset diabetes mellitus after kidney transplantation. Clin Nephrol. 2008;70:26–32. [DOI] [PubMed] [Google Scholar]
- 219. Luther P, Baldwin D., Jr Pioglitazone in the management of diabetes mellitus after transplantation. Am J Transplant. 2004;4:2135–2138. [DOI] [PubMed] [Google Scholar]
- 220. Pietruck F, Kribben A, Van TN, et al. Rosiglitazone is a safe and effective treatment option of new-onset diabetes mellitus after renal transplantation. Transpl Int. 2005;18:483–486. [DOI] [PubMed] [Google Scholar]