Abstract
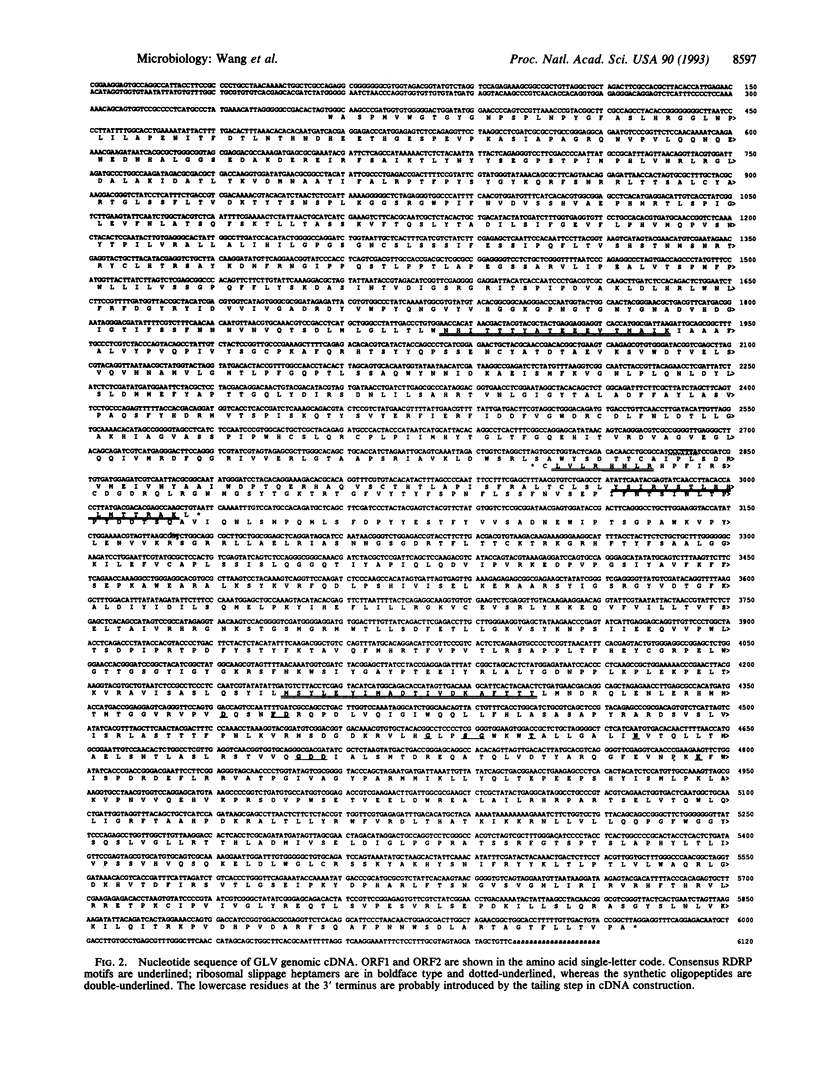
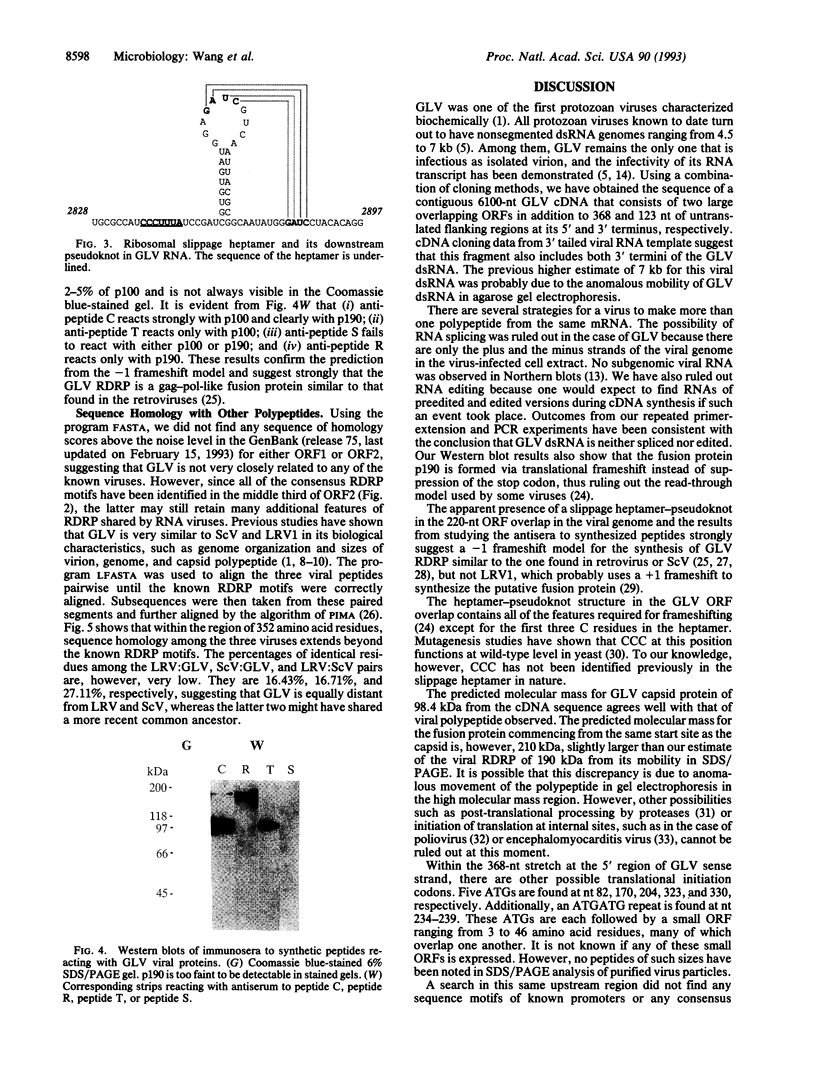
Giardiavirus is a small, nonenveloped virus comprising a monopartite double-stranded RNA genome, a major protein of 100 kDa, and a less abundant polypeptide of 190 kDa. It can be isolated from the culture supernatant of Giardia lamblia, a parasitic flagellate in human and other mammals, and efficiently infects other virus-free G. lamblia. A single-stranded copy of the viral RNA can be electroporated into uninfected G. lamblia cells to complete the viral replication cycle. Giardiavirus genomic cDNA of 6100 nt was constructed and its sequence revealed the presence of two large open reading frames that are separated by a -1 frameshift and share an overlap of 220 nt. The 3' open reading frame contains all consensus RNA-dependent RNA polymerase sequence motifs. A heptamer-pseudoknot structure similar to those found at ribosomal slippage sites in retroviruses and yeast killer virus was identified within this overlap. Immunostudies using antisera against synthesized peptides from four regions in the two open reading frames indicated that the 100- and 190-kDa viral proteins share a common domain in the amino-terminal region. But the 190-kDa protein makes a -1 switch of its reading frame beyond the presumed slippage heptamer and is therefore a -1 frameshift fusion protein similar to the gag-pol fusion protein found in retroviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D. The biology of Giardia spp. Microbiol Rev. 1991 Dec;55(4):706–732. doi: 10.1128/mr.55.4.706-732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J. A. Virus-like particles of yeast. Annu Rev Microbiol. 1980;34:49–68. doi: 10.1146/annurev.mi.34.100180.000405. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan A. M., Donahue T. F. Sequence and structural features associated with translational initiator regions in yeast--a review. Gene. 1987;59(1):1–18. doi: 10.1016/0378-1119(87)90261-7. [DOI] [PubMed] [Google Scholar]
- Diamond M. E., Dowhanick J. J., Nemeroff M. E., Pietras D. F., Tu C. L., Bruenn J. A. Overlapping genes in a yeast double-stranded RNA virus. J Virol. 1989 Sep;63(9):3983–3990. doi: 10.1128/jvi.63.9.3983-3990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfine E. S., Wang C. C. Transfection of the Giardia lamblia double-stranded RNA virus into giardia lamblia by electroporation of a single-stranded RNA copy of the viral genome. Mol Cell Biol. 1990 Jul;10(7):3659–3662. doi: 10.1128/mcb.10.7.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfine E. S., White T. C., Wang A. L., Wang C. C. A single-stranded RNA copy of the Giardia lamblia virus double-stranded RNA genome is present in the infected Giardia lamblia. Nucleic Acids Res. 1989 Sep 25;17(18):7453–7467. doi: 10.1093/nar/17.18.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U. Second-strand cDNA synthesis: mRNA fragments as primers. Methods Enzymol. 1987;152:330–335. doi: 10.1016/0076-6879(87)52038-9. [DOI] [PubMed] [Google Scholar]
- Icho T., Wickner R. B. The double-stranded RNA genome of yeast virus L-A encodes its own putative RNA polymerase by fusing two open reading frames. J Biol Chem. 1989 Apr 25;264(12):6716–6723. [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemke P. A. Viruses of eucaryotic microorganisms. Annu Rev Microbiol. 1976;30:105–145. doi: 10.1146/annurev.mi.30.100176.000541. [DOI] [PubMed] [Google Scholar]
- Lundblad V., Blackburn E. H. RNA-dependent polymerase motifs in EST1: tentative identification of a protein component of an essential yeast telomerase. Cell. 1990 Feb 23;60(4):529–530. doi: 10.1016/0092-8674(90)90653-v. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Wang A. L., Wang C. C. Identification of Giardia lamblia isolates susceptible and resistant to infection by the double-stranded RNA virus. Exp Parasitol. 1988 Jun;66(1):118–123. doi: 10.1016/0014-4894(88)90056-2. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Wang A. L., Wang C. C. Purification and characterization of the Giardia lamblia double-stranded RNA virus. Mol Biochem Parasitol. 1988 Apr;28(3):189–195. doi: 10.1016/0166-6851(88)90003-5. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C. Proteolytic processing of picornaviral polyprotein. Annu Rev Microbiol. 1990;44:603–623. doi: 10.1146/annurev.mi.44.100190.003131. [DOI] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Stuart K. D., Weeks R., Guilbride L., Myler P. J. Molecular organization of Leishmania RNA virus 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8596–8600. doi: 10.1073/pnas.89.18.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr P. I., Aline R. F., Jr, Smiley B. L., Scholler J., Keithly J., Stuart K. LR1: a candidate RNA virus of Leishmania. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9572–9575. doi: 10.1073/pnas.85.24.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Miller R. L., Wang C. C. Antibodies to the Giardia lamblia double-stranded RNA virus major protein can block the viral infection. Mol Biochem Parasitol. 1988 Sep;30(3):225–232. doi: 10.1016/0166-6851(88)90091-6. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol. 1986 Dec;21(3):269–276. doi: 10.1016/0166-6851(86)90132-5. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Viruses of the protozoa. Annu Rev Microbiol. 1991;45:251–263. doi: 10.1146/annurev.mi.45.100191.001343. [DOI] [PubMed] [Google Scholar]
- White T. C., Wang C. C. RNA dependent RNA polymerase activity associated with the double-stranded RNA virus of Giardia lamblia. Nucleic Acids Res. 1990 Feb 11;18(3):553–559. doi: 10.1093/nar/18.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer G., Comeau A. M., Furlong D. B., Wirth D. F., Patterson J. L. Characterization of a RNA virus from the parasite Leishmania. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5979–5982. doi: 10.1073/pnas.86.15.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dam E. B., Pleij C. W., Bosch L. RNA pseudoknots: translational frameshifting and readthrough on viral RNAs. Virus Genes. 1990 Jul;4(2):121–136. doi: 10.1007/BF00678404. [DOI] [PMC free article] [PubMed] [Google Scholar]




