Myelodysplastic syndromes (MDSs) represent a heterogeneous hematopoietic stem cell disorder.1 A precise estimation of the prognosis within the various MDS subgroups is essential for tailored therapeutic decisions. Especially, MDS patients with an isolated deletion of the long arm of chromosome 5 (del(5q)) represent a distinct subgroup regarding clinical outcome with a favorable prognosis in the majority of cases.2 Furthermore, they present with characteristic cytomorphological features, such as hypolobated megakaryocytes, macrocytic anemia and a normal or increased peripheral platelet count.3 Recently, flow cytometry (FCM) has been shown to serve as a valuable additional diagnostic and prognostic tool, especially to separate between unilineage and multilineage dysplasia.4, 5 Besides, it is known that abnormal antigen expression on myeloid progenitor cells (myPCs) is associated with a poor outcome.6, 7 In fact, aberrant CD7 expression on myPC of anemic lower-risk MDS patients predicts for a significantly lower response rate to erythropoiesis-stimulating agent (ESA) therapy irrespective of comparable other clinical predictive markers (erythropoietin level, transfusion burden).8 The pathophysiological background for this observation is still unknown. Notably, it has not been shown so far whether these distinct immunophenotypic characteristics correlate with presence and extent of clonal hematopoiesis, which in turn might not be responsive to growth factor stimulation. Therefore, in this study we separated different hematopoietic cell compartments of del(5q) MDS patients by fluorescence-activated cell sorting (FACS) and quantified the respective distribution of clonal burden with interphase fluorescence in situ hybridization (iFISH).
In total, 41 bone marrow samples (IPSS-R very low/low/int=22, high/very high=19) of 30 MDS patients with del(5q) were investigated (Supplementary Table 1). Before cell sorting, on a FACS-Aria-II (BD, San Jose, CA, USA), samples were immunophenotyped to ensure the presence of the cell populations to be sorted using a preparation, gating and analysis procedure according to IMDSFlow Working Group guidelines.4 Thus, the following cell populations were defined and sorted: myPC— SSClowCD45dimCD34+CD117+, myPC with/without CD56 expression; granulopoiesis (GP)—SSC++CD45dim and their respective maturation stages (CD13+CD16−, CD13−CD16−, CD13+CD16+); and nucleated red cells (NRC)— CD45−CD235a+CD71(+)++ (Supplementary Figures 1A and E). A range of 5000–50 000 cells were sorted for the subsequent iFISH analyses. Del(5q) was detected using the LSI EGR1/D5S23,D5S721 Dual Color Probe (Abbott, Wiesbaden, Germany). A detailed description of all methods is given as Supplementary Information at Leukemia's website.
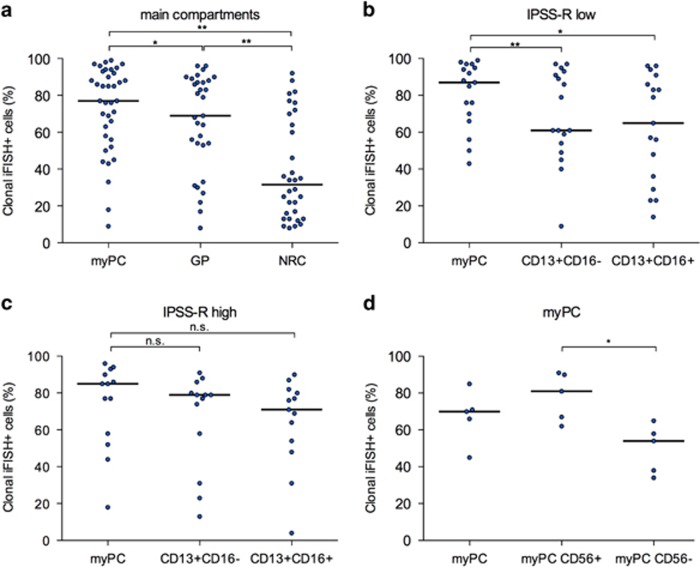
First, the distribution of clonal cells in the main hematopoietic compartments was analyzed. As a result, clonal iFISH+ cells could be detected in all subsets with the highest frequency in myPC (77%) followed by GP (69%) and NRC (31%), respectively (P<0.020; Figure 1a). Braulke et al.9 described a high clonal burden in MACS-sorted CD34+ cells in peripheral blood and proposed this method for therapeutic monitoring of patients. Furthermore, Tehranchi et al.10 reported that the malignant clone is dominant and even persists in the progenitor cell compartment when patients enter complete remission after lenalidomide treatment. These findings together with our current data confirm the high frequency of clonal cells predominant in the myPC again providing evidence for MDS being a stem cell disease.
Figure 1.
Frequency of clonal iFISH+ cells. (a) Main cell compartments (myPC, n=37; GP, n=31; NRC, n=31; myPC vs GP, P=0.015; myPC vs NRC, P<0.001; GP vs NRC, P<0.001). Paired comparison of clonal iFISH+ cells in myPC and maturing subpopulations of GP according to IPSS-R risk groups: (b) very low/low/int, n=17 (myPC vs CD13+CD16−, P=0.004; myPC vs CD13+CD16+, P=0.009); (c) high/very high, n=13 (not significant). (d) Paired comparison of myPC according to aberrant CD56 expression (n=5, P=0.043). *P<0.05; **P<0.005.
Next, we evaluated immunophenotypically characterized maturation stages of GP. We observed that the clonal burden declined significantly with maturation with the lowest frequency of del(5q) cells in the CD13+CD16+ most mature compartment (P=0.023; data not shown). In addition, we aimed to investigate whether there is a correlation between disease stage by IPSS-R (very low/low/int vs high/very high) and clonal burden in the cellular subpopulations. In fact, myPC presented with a comparable high clonality independent from IPSS-R risk category (Figures 1b and c). Remarkably, the comparison of myPC and GP resulted in a significantly lower frequency of clonal iFISH+ cells in the mature and immature GP in del(5q) MDS with lower IPSS-R (myPC: 87% vs mature and immature GP: 65% and 61%, respectively), whereas in higher-risk MDS, myPC and GP showed similar high percentages of clonality. This points toward a proliferation and differentiation advantage of the cytogenetically abnormal clone, which becomes even more prominent with the evolution into more advanced clinical stages. The high clonality within the GP is not surprising but has not been so far investigated in del(5q) MDS. As GP represents a considerable part of nucleated cells, it also mainly reflects the clonal burden in the whole bone marrow. Especially in lower-risk MDS, GP seems to be an easily accessible target for therapy monitoring. This thesis is supported by Lübbert et al.11 who investigated the GP in four MDS patients during decitabine treatment, using density gradient cell separation. Dependent on the success of therapy they reported a reappearance of normal GP as well as a modest differentiation of clonal GP.
Although to a lesser extent than myPC and GP, nucleated red cells (NRCs) showed a distinctive clonal involvement, as well. The extent of clonality within erythropoiesis was wide spread within the entire MDS cohort (Figure 1a). Especially in lower-risk MDS with anemia and dysplastic alterations of erythropoiesis as one of the leading symptoms, NRC might also serve as a compartment worth to be considered for therapy monitoring. In the future, an immunophenotypic separation of NRC, for example, in CD71+CD117+CD105+ immature NRC vs the more mature counterpart (CD105−), might allow new insights in terms of the extent of clonality within the different maturation stages of erythropoiesis.
A step further, we addressed the question whether abnormal antigen expression on myPC is associated with the frequency of clonality. Notably, sorted myPC with aberrant CD56 expression included significantly more iFISH+ cells compared with the immunophenotypically inconspicuous counterpart in the same samples (81% vs 54%, P=0.043; Figure 1d). Of note, CD56 expression on myPC per se was not associated with a more advanced clinical score, as only three of the five sorted samples belonged to patients with a high/very high IPSS-R score and/or del(5q) as part of a complex karyotype.
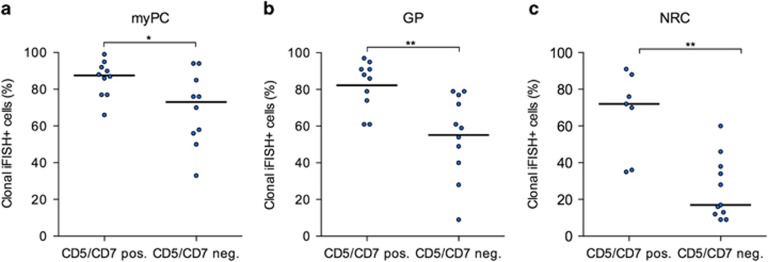
Furthermore, Westers et al.8 found an association between abnormal antigen expression on myPC, for example, CD7, CD56 and CD11b, and resistance to ESA treatment in transfusion-dependent lower-risk MDS. Although mechanistically not well understood at this time, an extension of the Nordic score adding abnormal antigen expression on myPC besides erythropoietin levels was proposed. Rigolin et al.12 detected a higher percentage of cytogenetically abnormal metaphases in patients nonresponsive to erythropoietin. Bardet et al.13 point to the importance of aberrant CD7 expression in MDS diagnostics, too. Therefore, we investigated the clonal involvement in a subgroup of predominantly lower-risk MDS harboring del(5q) plus an aberrant CD5/CD7 expression on myPC and compared it with a cohort with matched IPSS-R but without this aberrant antigen expression (Supplementary Table 1). Remarkably, the frequency of clonal cells was significantly higher in all of the FACS-sorted main populations (myPC, GP and NRC) compared with the respective controls (Figures 2a and c). This difference was most prominent in NRC (72% vs 17%, P=0.002) compared with GP (88% vs 56%, P=0.003) and myPC (88% vs 73%, P=0.035). In addition, we found slightly higher serum erythropoietin levels in patients with aberrant CD5/CD7 expression compared with the control group (355 vs 78 U/l) while transfusion burden did not differ (Supplementary Table 1). These results provide for the first time some explanation on the clonal level for the above mentioned theory of phenotypic alterations (for example, CD5 on myPC) and subsequent resistance to ESA. Owing to these findings it might be feasible to incorporate the knowledge on the phenotypically abnormal myPC together with the high clonal burden in NRC in future trial design, for example, combining lenalidomide and hypomethylating agents.
Figure 2.
Frequency of clonal iFISH+ cells in MDS patients with aberrant CD5 and/or CD7 expression on myPC. (a) myPC (n=10/10, P=0.035); (b) GP (n=9/10, P=0.003); (c) NRC (n=7/11, P=0.002). *P<0.05; **P<0.005.
Finally, it is known that the presence of a TP53 mutation in del(5q) MDS is associated with lenalidomide treatment failure.14 In order to associate our findings with the respective clonality on the molecular level, we compared the iFISH results with the mutation load of five patients presenting with a del(5q) and a concomitant TP53 mutation. Considering the main cell compartments, we observed a similar mutational load of TP53 within myPC and NRC (50% and 49%, respectively), whereas GP showed a lower mutation load (29%). Interestingly, in all FACS-sorted compartments, the clonal burden assessed by mutational analysis of TP53 was lower compared with the amount of cells harboring a del(5q) as assessed by iFISH. Significance was reached for the GP (GP: 29% vs 83%, n=5, P=0.043; myPC: 50% vs 85%, n=4; NRC: 49% vs 77%, n=3). This very high percentage of iFISH+ cells could be attributed to the presence of an aberrant CD5/CD7 expression per se in four of the five patients, but points also toward del(5q) as early event in the pathogenesis of MDS compared with the TP53 mutation. These data support the paradigm that mTP53 is a late event in the evolution of MDS as well as confirms the heterogeneous character of this disease.15
In summary, our data show a correlation of specific aberrant phenotypes detected by FCM and the respective proportion of clonal cells within different hematopoietic subpopulations. The data help to improve our understanding of MDS biology and warrant further evaluation of FCM in diagnostics and treatment monitoring of MDS.
Acknowledgments
We appreciate the excellent technical assistance of Jana Bornhäuser, Cornelia Hoffmann, Claudia Klotsche, Cathleen Rüger and Catrin Theuser. The work was in part supported by SFB 655 ‘cells into tissues and the Jose Carreras foundation (DJCLS R13/15)'.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- 1Malcovati L, Hellström-Lindberg E, Bowen D, Ades L, Cermak J, del Canizo C et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood 2013; 122: 2943–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Giagounidis AAN, Germing U, Haase S, Hildebrandt B, Schlegelberger B, Schoch C et al. Clinical, morphological, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del(5q) including band q31. Leukemia 2004; 18: 113–119. [DOI] [PubMed] [Google Scholar]
- 3Brunning D, Orazi A, Germing U. Myelodysplastic syndromes. In: Swerlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th edn. International Agency for Research on Cancer: Lyon, France, 2008, pp 88–107. [Google Scholar]
- 4Westers TM, Ireland R, Kern W, Alhan C, Balleisen JS, Bettelheim P et al. Standardization of flow cytometry in myelodysplastic syndromes: a report from an international consortium and the European LeukemiaNet Working Group. Leukemia 2012; 26: 1730–1741. [DOI] [PubMed] [Google Scholar]
- 5Della Porta MG, Picone C, Pascutto C, Malcovati L, Tamura H, Handa H et al. Multicenter validation of a reproducible flow cytometric score for the diagnosis of low-grade myelodysplastic syndromes: results of a European LeukemiaNET study. Haematologica 2012; 97: 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Alhan C, Westers TM, van der Helm LH, Eeltink C, Huls G, Witte BI et al. Absence of aberrant myeloid progenitors by flow cytometry is associated with favourable response to azacitidine in higher risk myelodysplastic syndromes. Cytometry B Clin Cytom 2014; 86: 207–215. [DOI] [PubMed] [Google Scholar]
- 7Satoh C, Tamura H, Yamashita T, Tsuji T, Dan K, Ogata K. Aggressive characteristics of myeloblasts expressing CD7 in myelodysplastic syndromes. Leuk Res 2009; 33: 326–331. [DOI] [PubMed] [Google Scholar]
- 8Westers TM, Alhan C, Chamuleau ME, van der Vorst MJ, Eeltink C, Ossenkoppele GJ et al. Aberrant immunophenotype of blasts in myelodysplastic syndromes is a clinically relevant biomarker in predicting response to growth factor treatment. Blood 2010; 115: 1779–1784. [DOI] [PubMed] [Google Scholar]
- 9Braulke F, Schanz J, Jung K, Shirneshan K, Schulte K, Schuetze C et al. FISH analysis of circulating CD34+ cells as a new tool for genetic monitoring in MDS: verification of the method and application to 27 MDS patients. Leuk Res 2010; 34: 1296–1301. [DOI] [PubMed] [Google Scholar]
- 10Tehranchi R, Woll PS, Anderson K, Buza-vidas N, Mizukami T, Mead AJ et al. Persistent malignant stem cells in del(5q) myelodysplasia in remission. N Engl J Med 2010; 363: 1025–1037. [DOI] [PubMed] [Google Scholar]
- 11Lübbert M, Daskalakis M, Kunzmann R, Engelhardt M, Guo Y, Wijermans P. Nonclonal neutrophil responses after successful treatment of myelodysplasia with low-dose 5-aza-2'-deoxycytidine (decitabine). Leuk Res 2004; 28: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 12Rigolin GM, Della Porta M, Bigoni R, Cavazzini F, Ciccone M, Bardi A et al. rHuEpo administration in patients with low-risk myelodysplastic syndromes: evaluation of erythroid precursors' response by fluorescence in situ hybridization on May-Grunwald-Giemsa-stained bone marrow samples. Br J Haematol 2002; 119: 652–659. [DOI] [PubMed] [Google Scholar]
- 13Bardet V, Wagner-Ballon O, Guy J, Morvan C, Debord C, Trimoreau F et al. Multicentric study underlining the interest of adding CD5, CD7 and CD56 expression assessment to the flow cytometric Ogata score in myelodysplastic syndromes and myelodysplasic/myeloproliferative neoplasms. Haematologica 2015; 100: 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Mallo M, del Rey M, Ibáñez M, Calasanz MJ, Arenillas L, Larráyoz MJ et al. Response to lenalidomide in myelodysplastic syndromes with del(5q): Influence of cytogenetics and mutations. Br J Haematol 2013; 162: 74–86. [DOI] [PubMed] [Google Scholar]
- 15Woll PS, Kjällquist U, Chowdhury O, Doolittle H, Wedge DC, Thongjuea S et al. Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell 2014; 25: 794–808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.