Abstract
A quarter of patients with essential thrombocythemia or primary myelofibrosis carry a driver mutation of CALR, the calreticulin gene. A 52-bp deletion (type 1) and a 5-bp insertion (type 2 mutation) are the most frequent variants. These indels might differentially impair the calcium binding activity of mutant calreticulin. We studied the relationship between mutation subtype and biological/clinical features of the disease. Thirty-two different types of CALR variants were identified in 311 patients. Based on their predicted effect on calreticulin C-terminal, mutations were classified as: (i) type 1-like (65%); (ii) type 2-like (32%); and (iii) other types (3%). Corresponding CALR mutants had significantly different estimated isoelectric points. Patients with type 1 mutation, but not those with type 2, showed abnormal cytosolic calcium signals in cultured megakaryocytes. Type 1-like mutations were mainly associated with a myelofibrosis phenotype and a significantly higher risk of myelofibrotic transformation in essential thrombocythemia. Type 2-like CALR mutations were preferentially associated with an essential thrombocythemia phenotype, low risk of thrombosis despite very-high platelet counts and indolent clinical course. Thus, mutation subtype contributes to determining clinical phenotype and outcomes in CALR-mutant myeloproliferative neoplasms. CALR variants that markedly impair the calcium binding activity of mutant calreticulin are mainly associated with a myelofibrosis phenotype.
Introduction
Philadelphia-negative myeloproliferative neoplasms (MPN) include polycythemia vera, essential thrombocythemia (ET) and primary myelofibrosis (PMF).1 Our understanding of the genetic basis of these disorders began in 2005, when the JAK2 (V617F) mutation was identified in patients with polycythemia vera, ET or PMF.2, 3 Mutations of JAK2 exon 12(refs 4, 5) and MPL exon 10(refs 6, 7) were subsequently detected in subsets of patients, while subclonal driver mutations in other genes were found to be associated with disease progression.8, 9, 10, 11 In 2013, somatic mutations in the gene CALR, encoding calreticulin, were detected in most patients with ET or PMF with unmutated JAK2 and MPL.12, 13
Although polycythemia vera is a condition almost exclusively associated with gain-of-function mutations of JAK2, the genetic basis of disease is more heterogeneous in ET and in PMF:14 JAK2 (V617F) is present in ~60–65% of cases, MPL exon 10 mutations in about 5%, and CALR exon 9 indels in ~20–25% of cases.15, 16, 17, 18 We recently found that the remaining 5–10% of cases do not represent a homogeneous disease entity, and that some of them carry novel MPL and JAK2 mutations.19
CALR-mutant MPN have distinctive clinical features.14 Patients with CALR-mutant ET have very high platelet counts but a relatively low risk of thrombosis, at least lower than that of patients with JAK2-mutant ET.15 Patients with CALR-mutant PMF have an indolent clinical course, and better survival compared with PMF patients carrying JAK2 (V617F) or an MPL exon 10 mutation.17, 18
More than 50 different indels in CALR exon 9 have been found, but a 52- bp deletion (type 1) and a 5- bp insertion (type 2 mutation) are the most common variants.12, 15, 18 These indels generate a novel C-terminus of the mutant protein in which the negatively charged amino acids are variably replaced by neutral and positively charged amino acids. The 52 bp deletion eliminates almost all negatively charged amino acids, whereas the 5 bp insertion retains approximately half the negatively charged amino acids.12 This suggests that impaired Ca2+-binding activity might have a role in cells expressing a mutant calreticulin, and that the different calreticulin mutants may differ considerably in terms of Ca2+-binding activity. Calcium signaling regulates many different cellular processes, and intracellular Ca2+ mobilization from endoplasmic reticulum (ER) into the cytoplasm triggers extracellular Ca2+ inflow in non-excitable cells,20 a mechanism termed store-operated Ca2+ entry (SOCE).21 Interestingly, both Ca2+ mobilization from intracellular stores and extracellular Ca2+ entry regulate human megakaryocyte (Mk) functions.22
Recently, Marty et al.23 investigated the effect of the type 1 and type 2 CALR mutant expression by retroviral mouse modeling. CALRdel52 (type 1 mutant) expressing mice rapidly developed marked thrombocytosis and then progressed to a condition similar to myelofibrosis. By contrast, CALRins5 (type 2 mutant) expressing mice had a mild ET phenotype with low propensity to disease progression. These observations in mouse models suggest that the different CALR mutations might have different clinical effects in humans. In this work, we studied patients with CALR-mutant ET or PMF to examine the relationship between mutations subtypes, Mk cytosolic Ca2+ signals and clinical outcomes.
Patients and methods
Study population
This study was approved by the institutional Ethics Committee (Comitato di Bioetica, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy). The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000, and samples were obtained after patients had provided written informed consent.
We identified 1282 consecutive patients (572 males, 710 females; median age 50 years, range 15–92 years) diagnosed with ET or PMF between 1982 and 2014 at Fondazione IRCCS Policlinico San Matteo, Italy, for which at least one DNA sample was available (Table 1). According to the 2008 WHO criteria,1 adopted for reclassification of patients, 908 out of 1282 subjects had ET (all from the Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, and University of Pavia, Italy) and 374 had PMF (205 from the Department of Hematology Oncology and 169 from the Center for the Study of Myelofibrosis, Fondazione IRCCS Policlinico San Matteo Pavia, Italy). ET patients included the 642 subjects previously reported by Rumi et al.,15 while PMF patients included 358 subjects previously described.18 Overall, 799 (62%) patients carried JAK2 (V617F) and 311 (24%) had a CALR indel (Table 1). Patients with MPL mutation or nonmutated JAK2/MPL/CALR were excluded from subsequent analyses.
Table 1. Initial study population including 1282 patients diagnosed with essential thrombocythemia or primary myelofibrosis at Fondazione IRCCS Policlinico San Matteo, Italy, between 1982 and 2014.
| Myeloproliferative neoplasm | JAK2 (V617F) mutated (%) | MPL exon 10 mutated (%) | CALR exon 9 mutated (%) | Nonmutated JAK2/MPL/CALR (%) | All genotypes |
|---|---|---|---|---|---|
| Essential thrombocythemia | 567 (62%) | 36 (4%) | 216 (24%) | 89 (10%) | 908 |
| Primary myelofibrosis | 232 (62%) | 20 (5%) | 95 (26%) | 27 (7%) | 374 |
| All patients | 799 (62%) | 56 (5%) | 311 (24%) | 116 (9%) | 1282 |
For the assessment of bone marrow fibrosis, paraffin sections were stained with Gomori's silver impregnation technique, and fibrosis was assessed semi-quantitatively following the European consensus guidelines.24 Post-ET myelofibrosis was diagnosed according to the criteria of the International Working Group of Myelofibrosis Research and Treatment,25 while evolution into acute myeloid leukemia was defined according to the WHO criteria.1 Thrombotic events were defined as described in detail elsewhere.26
JAK2, CALR and MPL mutation analysis
Granulocyte JAK2 (V617F) mutation status and mutant allele burden were assessed using a quantitative PCR (qPCR)-based allelic discrimination assay on a Rotor-Gene 6000 real-time analyzer (Qiagen, Hilden, Germany).27 MPL exon 10 mutations were detected using a high-resolution melt assay or Sanger sequencing,28 and CALR exon 9 mutations were studied as previously described.15
In vitro megakaryocyte differentiation from human peripheral blood hematopoietic progenitors
Blood samples were obtained from healthy subjects and patients with MPNs. CD45+ hematopoietic progenitor cells from peripheral blood were separated by immunomagnetic bead selection (Miltenyi Biotec, Bologna, Italy), as previously described.29 Cells were cultured for 14 days, in Stem Span medium (STEMCELL Technologies Inc, Vancouver, BC, Canada) supplemented with 10 ng/ml TPO, IL-6 and IL-11 at 37 °C in a 5% CO2 fully humidified atmosphere.
Measurement of intracellular Ca2+ concentration
At day 14 of culture, the Mk population was enriched through a bovine serum albumin gradient as previously described,30 12-mm glass cover-slips were coated with 100 μg/ml fibrinogen, overnight at 4 °C, 1 × 105 Mks were then harvested and allowed to adhere at 37 °C and 5% CO2 for 16 h. Then, Mks were loaded with 4 μm fura-2 AM (Molecular Probes Europe BV, Leiden, The Netherlands) in physiological salt solution (NaCl 150 mm, KCl 6 mm, CaCl2 1.5 mm, MgCl2 1 mm, glucose 10 mm, HEPES 10 mm, pH 7.4) for additional 30 min at 37 °C and 5% CO2. After being washed in physiological salt solution, the cover-slip was fixed to the bottom of a Petri dish and the cells were observed using an upright epifluorescence Axiolab microscope (Carl Zeiss, Arese, Italy), equipped with a Zeiss X63 Achroplan objective (water-immersion, 2.0 mm working distance, 0.9 numerical aperture). Mks were excited alternately at 340 and 380 nm, and the emitted light was detected at 510 nm. A first neutral density filter (1 or 0.3 optical density) reduced the overall intensity of the excitation light and a second neutral density filter (0.3 optical density) was coupled to the 380 nm filter to approach the intensity of the 340 nm light. The excitation filters were mounted on a filter wheel (Lambda 10; Sutter Instrument, Novato, CA, USA). Custom software, working in the LINUX environment, was used to drive the camera (Extended-ISIS Camera; Photonic Science, Robertsbridge, UK) and the filter wheel and to measure and plot the fluorescence from rectangular regions of interest each enclosing every Mk present within the analyzed field. Analysis of Ca2+ signals was performed according to previously published methods.22
Statistical analysis
Numerical variables have been summarized by their median and range, and categorical variables by count and relative frequency (%) of each category. Comparisons of quantitative variables between two groups of patients were carried out by the non-parametric Wilcoxon rank-sum test. The Kruskal–Wallis test was applied when comparing more than two groups. Association between categorical variables (two-way tables) was tested by the Fisher's exact test. Overall survival (OS) was estimated using the Kaplan–Meier product limit method, and hazard ratios were estimated by Cox proportional hazard univariate and multivariate regression models. The cumulative incidence of leukemic transformation, that of thrombotic events and that of fibrotic evolution were estimated with a competing risk approach, considering death for any cause as a competing event. Cumulative incidence curves were estimated with the Kalbfleisch & Prentice method,31 while the effect of mutation type was estimated by applying the Fine & Gray regression model.32
All P-values were considered statistically significant when smaller than 0.05 (two-tailed). Statistical analyses were performed using Stata 12.1 (StataCorp LP, College Station, TX, USA) software.
Results
Different types of CALR exon 9 mutations and their categorization
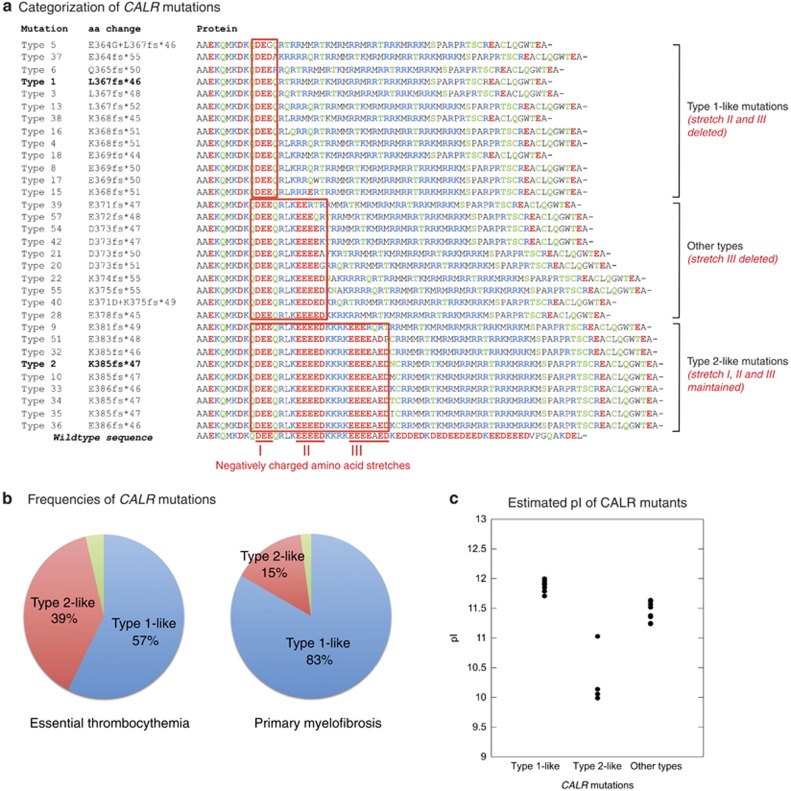
Thirty-two different types of CALR lesions were identified (Figure 1a): 175 (56%) patients had type 1 mutation, 86 (28%) had type 2, and 50 (16%) carried other indels. The frequency of type 1 mutation was significantly higher in PMF than in ET (75% vs 48%, P<0.001).
Figure 1.
Types and categorization of the 32 different CALR mutations found in 311 patients with essential thrombocythemia or primary myelofibrosis. (a) Alignment of C-domain in wild-type and mutant CALR proteins. Mutation denomination, as previously defined,15 is indicated on the left (type 1 and type 2 variants are in bold). The amino acid sequence starts from codon A352: acidic, basic and neutral residues are in red, blue and green, respectively. All the variants involved three different stretches of negatively charged amino acids, here defined as I, II and III, and highlighted in red in the wild-type sequence. Type 1-like mutations predict deletion of stretches II and III (as happens with the L367fs*46 or type 1 mutation), while type 2-like mutations predict conservation of all the three stretches (as happens with the K385fs*47 or type 2 mutation); other types involve deletion of stretches III exclusively. (b) Distribution of categorized CALR mutations in 216 patients with ET and 95 with PMF (type 1-like mutations in blue, type 2-like in red, and other types in green). The frequency of type 1-like mutations was significantly higher in PMF than in ET (83% and 57%, P<0.001). (c) Values for isoelectric point (pI) in mutant CALR peptides starting from codon A352. Estimates were performed using the Scripps Institute's online Protein Calculator v.3.3 (http://protcalc.sourceforge.net). Mutants had higher pI values (9.99–12.00) than wild-type sequence (4.11). The pI values were significantly different in the three categories (P<0.001), and were significantly higher in type 1-like than in type 2-like mutants (P<0.001).
As shown in Figure 1a, the observed variants had different predicted effect on three different stretches (here defined as I, II and III) of negatively charged amino acids of the wild-type sequence. CALR variants could therefore be categorized as follows: (i) type 1-like mutations (n=203, 65% of all lesions), which predict deletion of stretch II and III, as happens with the L367fs*46 or type 1 mutation; (ii) type 2-like mutations (n=98, 32% of all lesions), which have no impact on the above mentioned stretches, as happens with the K385fs*47 or type 2 mutation; and (iii) other types (n=10, 3% of all lesions), involving deletion of stretch III exclusively. The frequency of type 1-like mutations was significantly higher in PMF than in ET (83% vs 57%, P<0.001, Figure 1b).
Estimated isoelectric point of CALR mutants
The isoelectric point (pI) is the pH at which a protein carries no net electrical charge, and its value depends on the aminoacid composition. We therefore assessed the pI of mutant CALR peptides starting from codon A352 using the Scripps Institute's online Protein Calculator (http://protcalc.sourceforge.net). As shown in Figure 1c, markedly higher pI values (ranging from 9.99 to 12.00) were estimated in CALR mutants compared with the wild-type sequence, whose estimated pI is 4.11. Moreover, pI values were significantly different in three groups of categorized mutations, and were significantly higher in type 1-like than in type 2-like mutants (P<0.001).
Cytosolic Ca2+ signals in cultured megakaryocytes from patients with myeloproliferative neoplasms
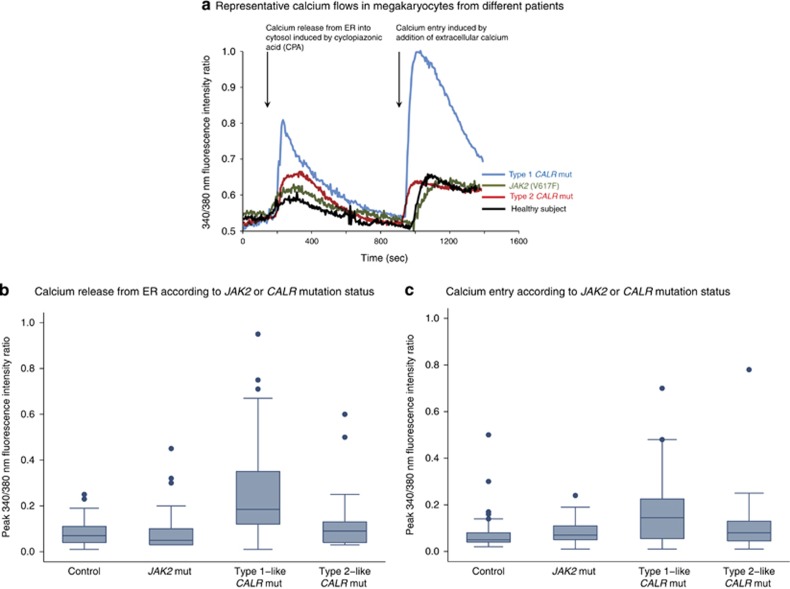
CALR mutants may affect both ER Ca2+ levels and SOCE, this latter likely being the most important pathway for Ca2+ entry in Mks.22 To evaluate cytoplasmic Ca2+ mobilization in human Mks from MPN patients carrying JAK2 (V617F), type 1 or type 2 CALR mutation, we exposed cells to cyclopiazonic acid (10 μm).22 Cyclopiazonic acid specifically blocks the sarco-ER Ca2+ ATPase activity, thereby preventing Ca2+ sequestration into the stores and leading to their depletion.33 This maneuver is routinely employed to assess ER Ca2+ levels and, consequently, to activate SOCE in non-excitable cells.33 As illustrated in Figure 2a, sequential fluorescence measurements showed that cyclopiazonic acid-mediated store depletion, in the absence of extracellular Ca2+, induced a transient increase in cytosolic Ca2+ concentration because of passive emptying of ER Ca2+ stores (calcium release). Thereafter, Ca2+ levels dropped to the baseline as the plasma membrane extruded Ca2+ from the cytosol. At this stage, ER Ca2+ stores are fully depleted. Therefore, the subsequent restoration of extracellular Ca2+ induced a second increase in cytosolic Ca2+ concentration due to SOCE activation (calcium entry).
Figure 2.
Fluorescence-based measurements of Ca2+ flows in cultured megakaryocytes. (a) Representative examples of fluorescence-based measurements of Ca2+ flows in cultured megakaryocytes from a healthy subject and from patients with a myeloproliferative neoplasm carrying different driver mutations. Intracellular Ca2+ pools were depleted, in absence of extracellular Ca2+, by cyclopiazonic acid, an inhibitor of the sarco-endoplasmic reticulum Ca2+-ATPase. This treatment evoked a transient rise in intracellular Ca2+ concentration because of the emptying of Ca2+ stores (Ca2+ release); thereafter, Ca2+ levels dropped to the baseline. When extracellular Ca2+ concentration was restored to 1.5 mm, this drove a second increase in intracellular Ca2+ levels due to activated influx through plasma membrane channels (Ca2+ entry). (b) Measurements of calcium release in cultured megakaryocytes. Sequential fluorescence-based measurements of Ca2+ flow in cultured megakaryocytes from 16 subjects were performed: four healthy subjects as controls, four JAK2 (V617F)-mutated patients (two ET and two PMF), four type 1-like CALR-mutated cases (two ET and two PMF, all carrying the type 1 mutation), and four type 2-like CALR-mutated patients (two ET and two PMF, all carrying the type 2 mutation). Overall, each molecular group consisted of 60 measurements, and in each patient at least eight megakaryocytes were analyzed. Data are shown in a box plot depicting the upper and lower values (lowest and highest horizontal line, respectively), lower and upper quartile with median value (box), and outside values (dots). Patients with type 1-like CALR mutation had higher values than both normal controls and patients with either JAK2 or type 2-like CALR mutation (P<0.001). (c) Measurements of calcium entry in cultured megakaryocytes from patients of b. Patients with type 1-like CALR mutation had higher values than normal controls and patients with either JAK2 or type 2-like CALR mutation (P<0.001).
Mks from patients with type 1 CALR mutation displayed the greatest cytosolic Ca2+ oscillations (defined as peak fluorescence ratio) in terms of both Ca2+ release from ER (Figure 2b) and Ca2+ entry (Figure 2c). Although there was considerable variability, peak values for both Ca2+ release and Ca2+ entry were significantly higher in patients with type 1 CALR mutation than in patients with JAK2 (V617F) or type 2 CALR mutation, or in healthy subjects (P<0.001 in all comparisons).
Relationship between driver somatic mutation and clinical phenotype
Tables 2 and 3 report clinical and laboratory features at diagnosis in ET and PMF patients, respectively, stratified according to their driver mutation.
Table 2. Main clinical and hematological features of patients with essential thrombocythemia stratified according to their driver mutation.
| JAK2 (V617) | Type 1-like CALR mutation | Type 2-like CALR mutation |
Comparisons (P-value) |
|||
|---|---|---|---|---|---|---|
| JAK2 vs type 1-like CALR mut | JAK2 vs type 2-like CALR mut | Type 1-like vs type 2-like CALR mut | ||||
| Patient no. | 567 | 124 | 84 | |||
| Age at onset, years, median (range) | 50 (15–92) | 45 (15–88) | 40 (19–91) | 0.094 | <0.001 | 0.049 |
| Hemoglobin, g/dl, median (range) | 14.3 (10–17.7) | 13.8 (10.7–17.6) | 13.8 (9.2–16.5) | 0.002 | <0.001 | 0.411 |
| WBC count, x109/l, median (range) | 9.2 (3.8–62.2) | 7.9 (4–17.5) | 8.1 (4.3–17.9) | <0.001 | <0.001 | 0.945 |
| PLT count, x109/l, median (range) | 700 (456–2148) | 832 (502–3000) | 982 (500–2670) | <0.001 | <0.001 | 0.027 |
| Patients with thrombosis at diagnosis, no. (%) | 48 (8%) | 7 (6%) | 0 | 0.293 | 0.006 | 0.027 |
Abbreviations: PLT, platelet; WBC, white blood cell.
Table 3. Main clinical and hematological features of patients with primary myelofibrosis stratified according to their driver mutation.
| JAK2 (V617F) | Type 1-like CALR mutation | Type 2-like CALR mutation |
Comparisons (P-value) |
|||
|---|---|---|---|---|---|---|
| JAK2 vs type 1-like CALR mut | JAK2 vs type 2-like CALR mut | Type 1-like vs type 2-like CALR mut | ||||
| Patient no. | 232 | 79 | 14 | |||
| Age at onset, years, median (range) | 60 (18–86) | 47 (27–75) | 54 (24–76) | <0.001 | 0.139 | 0.422 |
| Hemoglobin, g/dl, median (range) | 12.2 (5.5–17.9) | 11.8 (7.1–15.7) | 12.3 (7.1–15.9) | 0.316 | 0.952 | 0.375 |
| WBC count, x109/l, median (range) | 9.7 (1.6–54) | 7.6 (2.2–27) | 8.6 (5.4–13.5) | 0.008 | 0.444 | 0.257 |
| PLT count, x109/l, median (range) | 350 (38–1963) | 492 (89–1679) | 745 (46–1463) | <0.001 | 0.012 | 0.288 |
| Circulating CD34+ cells, x106/l, median (range)a | 16.1 (0.8–1190.2) | 34.1 (0.2–1902) | 25.2 (1.7–974.7) | 0.030 | 0.496 | 0.924 |
| IPSS risk group, no. (%) | 0.115 | 0.558 | 0.647 | |||
| Low | 87 (43.5%) | 42 (60%) | 7 (53.8%) | |||
| Intermediate 1 | 58 (29%) | 13 (18.6%) | 3 (23.1%) | |||
| Intermediate 2 | 34 (17%) | 10 (14.3%) | 3 (23.1%) | |||
| High | 21 (10.5%) | 5 (7.1%) | 0 | |||
Abbreviations: IPSS, International Prognostic Score System; PLT, platelet; WBC, white blood cell.
Data were available for 114 patients carrying JAK2 (V617F), 41 with type 1-like CALR mutation, and 6 with type 2-like CALR mutation.
Compared with those carrying JAK2 (V617F) or type 1-like CALR mutation, ET patients with type 2-like CALR mutation were younger, and had higher platelet count but lower incidence of thrombosis at diagnosis. Within PMF patients, no significant differences were observed between patients with type 1-like CALR and those with type 2-like CALR mutation (Table 3). Compared with patients carrying JAK2 (V617F), those with type 1-like CALR mutation were younger and had higher values for circulating CD34-positive cells.
In PMF patients, there was no significant difference between the three genetic categories with respect to the International Prognostic Score System risk.
Impact of driver mutation on clinical outcome
The median follow-up of the study cohort was 4.7 years (range 0–31 years).
In ET patients, death occurred in 25 out of 567 patients with JAK2 (V617F) (4.4%), 2 out of 124 with a type 1-like CALR mutation (1.6%) and 2 out of 84 of those carrying a type 2-like CALR indel (2.4%). The OS at 10 years was 98.6% (95% confidence interval (CI), 90.7–99.8) and 98% (95% CI, 86.6–99.7) in patients with a type 1-like or type 2-like CALR mutation, respectively, and 94% (95% CI, 90–96.4) in those with the JAK2 (V617F), with no significant difference between groups.
In PMF patients, death occurred in 12 out of 79 patients with type 1-like CALR mutation (15.2%), 4 out of 14 with type 2-like CALR mutation (28.6%), and 58 out of 232 with JAK2 (V617F) (25%). Median OS was 17.7 years in type 1-like CALR-mutated, 15.5 years in type 2-like CALR-mutated and 12.8 years in JAK2-mutated patients, with a survival rate at 10 years of 85.7% (95% CI, 71.7–93.1), 84.4% (95% CI, 50.4–95.9) and 58.6% (48.3–67.6), respectively. Patients carrying a type 1-like CALR mutation had a better OS compared with those carrying JAK2 (V617F) (JAK2 vs type 1-like: hazards ratio (HR) 2.4, 95% CI 1.3–4.6, P=0.007), while there was no difference between patients with type 1-like and those with type 2-like CALR mutation (type 2-like vs type 1-like: HR 1.4, 95% CI 0.4–4.4 P=0.562), and between patients with JAK2 (V617F) and those with type 2-like CALR mutation (JAK2 vs type 2-like: HR 1.7, 95% CI 0.6-4.8, P=0.294). These results were confirmed after adjusting for International Prognostic Score System, with a better OS for patients with type 1-like CALR mutation compared with those with JAK2 (V617F) (JAK2 vs type 1-like: HR 3.4, 95% CI 1.4–8.3, P=0.009), and no difference between patients with type 1-like and those with type 2-like CALR mutation (type 2-like vs type 1-like: HR 2.3, 95% CI 0.6–8.3, P=0.213), as well as between patients with type 2-like CALR mutation and those with JAK2 (V617F) (JAK2 vs type 2-like: HR 1.5, 95% CI 0.5–4.5, P=0.491).
Leukemic transformation was observed in 16 patients with ET and in 46 with PMF. In ET, the 10-year cumulative incidence of leukemic transformation was 0% in patients with type 1-like CALR mutation, 0% in those with type 2-like CALR mutation, and 5.4% (95% CI, 3.2–8.4) in those with JAK2 (V617F), with no significant difference among the three molecular subgroups. In PMF, the 10-year cumulative incidence of leukemic transformation was 19.2% (95% CI, 12.2–27.5) in patients with JAK2 (V617F), 12.2% (95% CI, 4.2–24.8) in those with type 1-like CALR mutation, and 7.1% (95% CI, 0.5–27.5) in those with type 2-like CALR mutation; there was no significant difference between these subgroups.
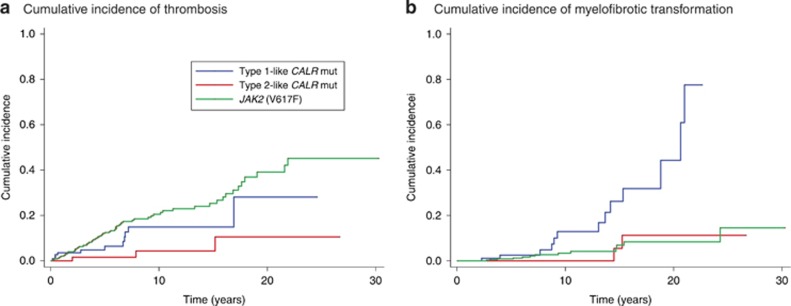
In ET patients, we evaluated the impact of driver mutation on the risk of thrombotic events and myelofibrotic transformation. The 10-year cumulative incidence of thrombotic events was 14.9% (95% CI, 7.2–25.2) in patients with type 1-like CALR mutation, 4.3% (95% CI, 0.7–13.4) in those with type 2-like CALR mutation, and 20.6% (95% CI, 15.8–25.7) in those with JAK2 (V617F) (Figure 3a). Patients carrying JAK2 (V617F) had a higher risk of thrombosis compared with those with type 2-like CALR mutation (subdistribution hazard ratio (SHR) 5.3, 95% CI, 1.7–16.8, P=0.004).
Figure 3.
Cumulative incidence of thrombosis and myelofibrotic transformation in patients with essential thrombocythemia carrying JAK2 (V617F), type 1-like CALR mutation, or type 2-like CALR mutation. (a) The 10-year cumulative incidence of thrombotic events was 20.6% (95% CI, 15.8–25.7) in patients with JAK2 (V617F), 14.9% (95% CI, 7.2–25.2) in those with type 1-like CALR mutation and 4.3% (95% CI, 0.7–13.4) in those with type 2-like CALR mutation. ET patients carrying JAK2 (V617F) had a higher risk of thrombosis compared with those carrying type 2-like CALR mutation (SHR 5.3, 95% CI, 1.7–16.8, P=0.004), while there was no significant difference compared with those carrying type 1-like CALR mutation (SHR 1.6, 95% CI, 0.9–3.1, P=0.135). Patients with type 2-like CALR mutation showed a trend towards a lower risk of thrombosis compared with those carrying type 1-like CALR mutation (SHR 0.3, 95% CI 0.1–1.1, P=0.067). To compare the estimates of this study with those of our previous work,15 we calculated also values at 15 years. The 15-year cumulative incidence of thrombotic events was 25.3% (95% CI, 19.3–31.7) in patients with JAK2 (V617F), 14.9% (95% CI, 7.2–25.2) in those with type 1-like CALR mutation, and 4.3% (95% CI, 0.7–13.4) in those with type 2-like CALR mutation. (b) The 10-year cumulative incidence of myelofibrotic transformation was 12.9% (95% CI, 4.9–24.8) in patients with type 1-like CALR mutation, 3.3% (95% CI, 1.5–6.4) in those carrying JAK2 (V617F), and 0% in those carrying type 2-like CALR mutation. ET patients carrying type 1-like CALR mutation showed a higher risk of myelofibrotic transformation compared with both those carrying type 2-like CALR mutation (SHR 7.8, 95% CI 1.8–34.4, P=0.007) and those with JAK2 (V617F) (SHR 5.4, 95% CI 2.5–11.6, P<0.001), while no difference was observed between JAK2 mutated and type 2-like CALR mutated patients (SHR 1.4, 95% CI 0.3–6.3, P=0.627). The 15-year cumulative incidence of myelofibrotic transformation was 26.2% (95% CI, 11.8–43.2) in patients with type 1-like CALR mutation, 6.9% (95% CI, 3.2–12.5) in those carrying JAK2 (V617F) and 5.4% (95% CI, 0.4–22.0) in those carrying type 2-like CALR mutation.
The 10-year cumulative incidence of myelofibrotic transformation was 12.9% (95% CI, 4.9–24.8) in ET patients with type 1-like CALR mutation, 0% in those with type 2-like CALR mutation and 3.3% (95% CI, 1.5–6.4) in those carrying JAK2 (V617F) (Figure 3b). ET patients with type 1-like CALR mutation showed a higher risk of myelofibrotic transformation compared with either those with type 2-like CALR mutation (SHR 7.8, 95% CI 1.8–34.4, P=0.007) or those carrying JAK2 (V617F) (SHR 5.4, 95% CI 2.5–11.6, P<0.001), while no difference was observed between patients with JAK2 mutant ET and those with type 2-like CALR mutant ET (SHR 1.4, 95% CI 0.3–6.3, P=0.627).
Discussion
Somatic mutations of CALR exon 9 are found almost exclusively in patients with myeloid neoplasms associated with thrombocytosis, that is, ET, PMF and refractory anemia with ring sideroblasts associated with marked thrombocytosis.12 This observation and the finding that CALR-mutant MPNs are characterized by a gene signature associated with activated JAK2 signaling34 strongly suggests that the mutant calreticulin activates the JAK-STAT pathway through the thrombopoietin receptor, thereby leading to excessive platelet production. As shown in Tables 2 and 3, we found only minor differences in platelet counts between patients with ET or PMF carrying different CALR mutation subtypes, suggesting that there should be no major differences between these subtypes in terms of abnormal MPL-JAK-STAT signaling.
Calreticulin binds Ca2+ ions in the ER, rendering these ions inactive. In our initial work on CALR mutation, we already suggested that loss of calcium binding activity of mutant calreticulin and higher levels of cytoplasmic calcium might contribute to the pathogenesis of MPN.12 A bioinformatic analysis of CALR mutations has indeed shown that only +1 frameshift mutations (that is, those observed in MPN patients) exhibit a positively charged C-terminus.35 Interestingly, type 1 CALR mutation and more generally exon 9 deletions create mutant proteins with more altered calcium binding activity.35, 36 Since cytoplasmic Ca2+ levels regulate human Mk22 and platelet function,37 we reasoned that different CALR mutants might differentially contribute to the pathogenesis of MPN.
We first categorized the different CALR indels into three subgroups based on their predicted effect on three different stretches of negatively charged amino acids of the wild-type sequence (Figure 1). We then estimated the pI value of each mutant CALR peptide starting from codon A352 to validate our categorization of CALR mutants. As shown in Figure 1c, pI values were significantly different between the three groups of CALR mutation, while they were homogeneous within each group, thus supporting our categorization of CALR mutants.
The three categories showed significantly different frequencies in patients with ET compared with patients with PMF. In particular, the high proportion of PMF patients with type 1-like CALR mutation suggests that the MPN associated with this driver mutation is very likely to develop bone marrow fibrosis. By contrast, the MPN associated with type 2-like CALR mutation is less likely to undergo myelofibrotic transformation, and indeed only 15% of patients with CALR-mutant myelofibrosis carried this mutation subtype.
The two major subtypes of mutant calreticulin had different effects on calcium flows in cultured Mks (Figure 2). More specifically, the largest cytosolic Ca2+ mobilization and SOCE were found in Mks from patients with type 1 CALR mutation, that is, a mutation subtype in which the negatively charged amino acids of C-terminal region of calreticulin are almost completely replaced by both neutral and positively charged amino acids (Figure 1a). Accordingly, type 1 CALR mutants are also those with the highest estimated pls (Figure 1c). The larger ER-dependent Ca2+ release observed in Mks from these patients is fully consistent with the impairment of the ER Ca2+-storage ability caused by the loss of the ER Ca2+-binding residues. In the presence of a Ca2+-mobilizing stimulus, such as cyclopiazonic acid, Mks derived from type 1 CALR mutated patients release a significantly higher amount of Ca2+ as compared with cells obtained from patients with JAK2 (V617F) or type 2 CALR mutation (Figure 2b).
The higher Ca2+ release associated to type 1 mutation was associated with an increase in SOCE amplitude. Whether the higher SOCE magnitude could be due to the higher ER Ca2+ mobilization or to the end tail modifications of calreticulin mutants still remains to be understood.38 In principle, a larger ER Ca2+ depletion should lead to a larger SOCE activation. However, it is known that partial dissociation between Ca2+ mobilization and SOCE activation may occur.39 Given that type 1, but not type 2 mutation results in the loss of stretches II and III at the C-terminus of calreticulin, which might be involved in SOCE development,40 the differences observed between the two mutants could be because of their different ability to interact with SOCE machinery. Therefore, our hypothesis is that del52 may result in a lesser inhibition of the SOCE amplitude, thus resulting in the second higher peak of cytosolic Ca2+.
CALR mutation subtype had a profound impact on clinical outcomes of patients with ET, as illustrated in Figure 3. Despite the fact that these individuals had the highest values for platelet count (Table 2), patients with type 2-like CALR mutation had the lowest risk of thrombosis, significantly lower than that of patients carrying JAK2 (V617F) (Figure 3a). This observation reinforces the opinion that JAK2 (V617F) represents the most thrombogenic driver mutation in MPN, and that the platelet count does not represent per se a predictive factor for thrombosis.41
We previously reported that the cumulative incidence of myelofibrotic transformation was not significantly different between JAK2-mutant and CALR-mutant ET.15 This is true if the whole population of CALR-mutant patients is considered. However, by taking into account CALR variant subtypes, we have now found a significantly higher risk of myelofibrotic transformation in ET patients carrying type 1-like than in those carrying type 2-like CALR mutation. Since type 1-like CALR mutation is much more frequent in patients with PMF than in those with ET (Figure 1b), these observations support a unifying model in which CALR-mutant ET and CALR-mutant PMF represent different phenotypes/stages in the evolution of a CALR-mutant MPN.14 The initial phenotype is necessarily ET (isolated thrombocytosis), since overproduction of platelets is faster than deposition of reticulin fibers in the bone marrow. Development of bone marrow fibrosis occurs with time, more actively in patients with type 1-like than in those with type 2-like CALR mutation, and this leads to myelofibrosis. This process has been clearly demonstrated in retroviral mouse models, in which CALR mutants were found to be able and sufficient to induce a thrombocytosis progressing to myelofibrosis, thus mimicking the natural history of MPN patients:23 in particular, progression to a myelofibrosis was much more common in CALRdel52- than in CALRins5-expressing mice. Whether this difference in risk of myelofibrotic transformation is related to different degrees of abnormal cytosolic Ca2+ flows in Mks remains to be demonstrated, but our findings strongly support this notion. In the minority of ET patients with type 2-like CALR mutation who progress to myelofibrosis, this transformation is likely related to the occurrence of cooperating subclonal mutations.42
With respect to patients with PMF, we did not find any difference in terms of OS between patients carrying type 1-like and those carrying type 2-like CALR mutation. Tefferi et al.43 previously reported survival data on 440 patients with PMF stratified by their JAK2 and CALR mutation status. CALR mutations were sub-stratified into type 1/type 1-like and type 2/type 2-like, based on the helical propensity of their mutant CALR. Compared with JAK2-mutated cases, OS was longer in patients with type 1/type 1-like but not in those with type 2/type 2-like CALR mutation. Although this study suggests a better prognosis for patients with type 1 CALR mutation, Cabagnols et al44 found that type 1 CALR mutation is associated with a shorter survival in PMF. All these studies—including ours—have analyzed relatively small patient populations, and larger studies that take into account also subclonal mutations are needed to define the prognostic significance of mutation subtypes in CALR-mutant PMF.
In conclusion, this study shows differential clinical effects of mutation subtypes in CALR-mutant MPNs. Type 2-like CALR mutations are mainly associated with an ET phenotype, low risk of thrombosis and indolent clinical course, while type 1-like mutations are mainly associated with a myelofibrosis phenotype and a high risk of progression from ET to myelofibrosis. The different clinical outcomes should be taken into account in clinical-decision making.
Acknowledgments
This study was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC), Fondo per gli investimenti della ricerca di base (FIRB, project no. RBAP11CZLK) and Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR, PRIN 2010-2011) to MC; from Italian Ministry of Health (GR-2010-2312855) and AIRC (MFAG-2014-15672) to ERu; and from Fondazione Cariplo (2010-0807) to AB. In particular, MC acknowledges funding from the AIRC Special Program 'Molecular Clinical Oncology 5 per mille' (AIRC Gruppo Italiano Malattie Mieloproliferative (AGIMM), project no. 1005).
Author contributions
MC, DP and ERu conceived this study, collected and analyzed data and wrote the manuscript; CADB, VA, FM and AB performed megakaryocyte studies; CC, ESA, ICC, MB, ERo, CA, VR and GB collected clinical data; CM and MCR did molecular investigations; VVF and EF did statistical analyses; EB studied bone marrow biopsies.
The authors declare no conflict of interest.
References
- 1Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC: Lyon, 2008.
- 2James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005; 434: 1144–1148. [DOI] [PubMed] [Google Scholar]
- 3Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005; 352: 1779–1790. [DOI] [PubMed] [Google Scholar]
- 4Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007; 356: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Passamonti F, Elena C, Schnittger S, Skoda RC, Green AR, Girodon F et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011; 117: 2813–2816. [DOI] [PubMed] [Google Scholar]
- 6Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006; 3: e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Rumi E, Pietra D, Guglielmelli P, Bordoni R, Casetti I, Milanesi C et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013; 121: 4388–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Carbuccia N, Murati A, Trouplin V, Brecqueville M, Adelaide J, Rey J et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia 2009; 23: 2183–2186. [DOI] [PubMed] [Google Scholar]
- 9Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 2010; 42: 722–726. [DOI] [PubMed] [Google Scholar]
- 10Harutyunyan A, Klampfl T, Cazzola M, Kralovics R. p53 lesions in leukemic transformation. N Engl J Med 2011; 364: 488–490. [DOI] [PubMed] [Google Scholar]
- 11Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A et al. Mutations and prognosis in primary myelofibrosis. Leukemia 2013; 27: 1861–1869. [DOI] [PubMed] [Google Scholar]
- 12Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013; 369: 2379–2390. [DOI] [PubMed] [Google Scholar]
- 13Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013; 369: 2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood 2014; 123: 3714–3719. [DOI] [PubMed] [Google Scholar]
- 15Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014; 123: 1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014; 123: 1552–1555. [DOI] [PubMed] [Google Scholar]
- 17Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia 2014; 28: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 18Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martinez-Trillos A, Casetti I et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood 2014; 124: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Milosevic Feenstra JD, Nivarthi H, Gisslinger H, Leroy E, Rumi E, Chachoua I et al. Whole exome sequencing identifies novel MPL and JAK2 mutations in triple negative myeloproliferative neoplasms. Blood 2015; e-pub ahead of print 30 September 2015. [DOI] [PMC free article] [PubMed]
- 20Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 2003; 4: 517–529. [DOI] [PubMed] [Google Scholar]
- 21Parekh AB. Store-operated CRAC channels: function in health and disease. Nat Revs Drug Discov 2010; 9: 399–410. [DOI] [PubMed] [Google Scholar]
- 22Di Buduo CA, Moccia F, Battiston M, De Marco L, Mazzucato M, Moratti R et al. The importance of calcium in the regulation of megakaryocyte function. Haematologica 2014; 99: 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Marty C, Harini N, Pecquet C, Chachoua I, Gryshkova V, Villeval JL et al. Calr mutants retroviral mouse models lead to a myeloproliferative neoplasm mimicking an essential thrombocythemia progressing to a myelofibrosis. Blood 2014; 124: 157.25013158 [Google Scholar]
- 24Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 2005; 90: 1128–1132. [PubMed] [Google Scholar]
- 25Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 2008; 22: 437–438. [DOI] [PubMed] [Google Scholar]
- 26Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013; 368: 22–33. [DOI] [PubMed] [Google Scholar]
- 27Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 2010; 24: 1574–1579. [DOI] [PubMed] [Google Scholar]
- 28Pietra D, Brisci A, Rumi E, Boggi S, Elena C, Pietrelli A et al. Deep sequencing reveals double mutations in cis of MPL exon 10 in myeloproliferative neoplasms. Haematologica 2011; 96: 607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Balduini A, Badalucco S, Pugliano MT, Baev D, De Silvestri A, Cattaneo M et al. In vitro megakaryocyte differentiation and proplatelet formation in Ph-negative classical myeloproliferative neoplasms: distinct patterns in the different clinical phenotypes. PLoS One 2011; 6: e21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Balduini A, Malara A, Pecci A, Badalucco S, Bozzi V, Pallotta I et al. Proplatelet formation in heterozygous Bernard-Soulier syndrome type Bolzano. J Thromb Haemost 2009; 7: 478–484. [DOI] [PubMed] [Google Scholar]
- 31Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Wiley: New YorK, 1980. [Google Scholar]
- 32Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 33Dragoni S, Laforenza U, Bonetti E, Reforgiato M, Poletto V, Lodola F et al. Enhanced expression of Stim, Orai, and TRPC transcripts and proteins in endothelial progenitor cells isolated from patients with primary myelofibrosis. PLoS One 2014; 9: e91099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood 2014; 123: e123–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Eder-Azanza L, Navarro D, Aranaz P, Novo FJ, Cross NC, Vizmanos JL. Bioinformatic analyses of CALR mutations in myeloproliferative neoplasms support a role in signaling. Leukemia 2014; 28: 2106–2109. [DOI] [PubMed] [Google Scholar]
- 36Shivarov V, Ivanova M, Tiu RV. Mutated calreticulin retains structurally disordered C terminus that cannot bind Ca(2+): some mechanistic and therapeutic implications. Blood Cancer J 2014; 4: e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Grosse J, Braun A, Varga-Szabo D, Beyersdorf N, Schneider B, Zeitlmann L et al. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J Clin Invest 2007; 117: 3540–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Parekh AB, Putney JW Jr.. Store-operated calcium channels. Physiol Rev 2005; 85: 757–810. [DOI] [PubMed] [Google Scholar]
- 39Parekh AB, Fleig A, Penner R. The store-operated calcium current I(CRAC): nonlinear activation by InsP3 and dissociation from calcium release. Cell 1997; 89: 973–980. [DOI] [PubMed] [Google Scholar]
- 40Fasolato C, Pizzo P, Pozzan T. Delayed activation of the store-operated calcium current induced by calreticulin overexpression in RBL-1 cells. Mol Biol Cell 1998; 9: 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood 2012; 120: 5128–5133, quiz 5252. [DOI] [PubMed] [Google Scholar]
- 42Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 2014; 123: 2220–2228. [DOI] [PubMed] [Google Scholar]
- 43Tefferi A, Lasho TL, Tischer A, Wassie EA, Finke CM, Belachew AA et al. The prognostic advantage of calreticulin mutations in myelofibrosis might be confined to type 1 or type 1-like CALR variants. Blood 2014; 124: 2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44Cabagnols X, Defour JP, Ugo V, Ianotto JC, Mossuz P, Mondet J et al. Differential association of calreticulin type 1 and type 2 mutations with myelofibrosis and essential thrombocytemia: relevance for disease evolution. Leukemia 2015; 29: 249–252. [DOI] [PubMed] [Google Scholar]