Abstract
Nitric oxide synthase (NOS) contributes to sweating and cutaneous vasodilation during exercise in younger adults. We hypothesized that endothelial NOS (eNOS) and neuronal NOS (nNOS) mediate NOS-dependent sweating, whereas eNOS induces NOS-dependent cutaneous vasodilation in younger adults exercising in the heat. Further, aging may upregulate inducible NOS (iNOS), which may attenuate sweating and cutaneous vasodilator responses. We hypothesized that iNOS inhibition would augment sweating and cutaneous vasodilation in exercising older adults. Physically active younger (n = 12, 23 ± 4 yr) and older (n = 12, 60 ± 6 yr) adults performed two 30-min bouts of cycling at a fixed rate of metabolic heat production (400 W) in the heat (35°C). Sweat rate and cutaneous vascular conductance (CVC) were evaluated at four intradermal microdialysis sites with: 1) lactated Ringer (control), 2) nNOS inhibitor (nNOS-I, NPLA), 3) iNOS inhibitor (iNOS-I, 1400W), or 4) eNOS inhibitor (eNOS-I, LNAA). In younger adults during both exercise bouts, all inhibitors decreased sweating relative to control, albeit a lower sweat rate was observed at iNOS-I compared with eNOS-I and nNOS-I sites (all P < 0.05). CVC at the eNOS-I site was lower than control in younger adults throughout the intermittent exercise protocol (all P < 0.05). In older adults, there were no differences between control and iNOS-I sites for sweating and CVC during both exercise bouts (all P > 0.05). We show that iNOS and eNOS are the main contributors to NOS-dependent sweating and cutaneous vasodilation, respectively, in physically active younger adults exercising in the heat, and that iNOS inhibition does not alter sweating or cutaneous vasodilation in exercising physically active older adults.
Keywords: evaporation, endothelium, microcirculation, nitric oxide, aging
nitric oxide (NO) synthase (NOS) exists in three isoforms: neuronal (nNOS), inducible (iNOS), and endothelial (eNOS), each of which has been observed in human skin (3, 43). Studies have shown that NOS contributes to cutaneous vasodilation in younger adults during exercise (9, 28, 50). Recently, McNamara et al. (29) demonstrated an exclusive role for eNOS, and not nNOS, in mediating NOS-dependent cutaneous vasodilation during moderate-intensity exercise that induced increases in core body temperature of up to 0.8°C in a non-heat-stressed environment (∼23–24°C). However, it is unknown if eNOS alone mediates cutaneous vasodilation during exercise in a hot environment (e.g., 35°C) where mean skin temperature is elevated. Further, NOS contributes to sweating in younger adults during exercise with increases in core body temperature of 0.6–1.5°C (9, 27, 28, 45, 50), albeit the NOS isoform(s) that mediates this response is currently unknown. Given that both eNOS (40) and nNOS (55) exist in human eccrine sweat glands, these NOS isoforms may be important mediators of sweat production during exercise.
Adults as young as 40 yr of age exhibit attenuated whole body evaporative heat loss (and therefore sweating response) during exercise, resulting in markedly greater increases in body heat storage compared with their younger counterparts (23–25, 46), although this impairment is not always observed locally (25, 46). In line with the age-related attenuation of whole body heat dissipation, NOS-dependent sweating (11, 45) and cutaneous vasodilation (44) are diminished in older adults during heat stress. This reduced NOS contribution can occur even in active older adults who can exhibit similar local heat loss responses to those seen in younger adults, as evidenced by diminished NOS-dependent sweating (45). This diminished NOS-dependent mechanism may be associated with age-related increases in expression and/or activity of iNOS (36). iNOS can upregulate arginase (38), which is capable of metabolizing l-arginine (a precursor of NO), resulting in reduced NO bioavailability. Furthermore, iNOS can produce excessive amounts of NO (compared with nNOS and eNOS), which can promote binding of NO with superoxide, forming peroxynitrite. Peroxynitrite can subsequently reduce NO bioavailability by uncoupling NOS. This activity may be augmented in aged skin where levels of superoxide are elevated relative to young skin (48). Altogether, increases in iNOS expression and/or activation associated with aging may modulate sweating and cutaneous vasodilation during exercise.
The purpose of this study was to 1) examine the relative contribution of the different NOS isoforms in the regulation of sweating and cutaneous vasodilation in younger adults during exercise in the heat; and 2) determine if age-related changes in iNOS function contribute to sweating and cutaneous vasodilation in physically active older adults. We hypothesized that during exercise in the heat, eNOS and nNOS contribute to NOS-dependent sweating and that eNOS underlies NOS-dependent cutaneous vasodilation in younger adults. Furthermore, we surmised that iNOS inhibition would augment the sweating and cutaneous vasodilatory responses to exercise in physically active older adults.
MATERIALS AND METHODS
Ethical approval.
This study was approved by the University of Ottawa Health Sciences and Science Research Ethics Board and conformed to the guidelines set forth by the Declaration of Helsinki. Verbal and written informed consent were obtained from all volunteers prior to their participation in the study.
Subjects.
Twelve younger (range: 19–31 yr, 8 men and 4 women) and older (range: 53–73 yr, 6 men and 6 women), habitually active (2–6 days/wk, ≥30 min of exercise/day) adults participated in this study. Subjects were excluded if they had a history of cystic fibrosis transmembrane conductance regulator mutations, skin disorders, uncontrolled hypertension, heart disease, diabetes, autonomic disorders, or cigarette smoking. All younger females were taking contraceptives (two females were using an intrauterine device and two females were using oral contraceptives) and participated in the experimental session during the placebo phase. All older females were postmenopausal and were not on hormonal replacement therapy. Two of nine older subjects were taking prescription medications; one subject was taking 10 mg of Ezetimibe (cholesterol absorption inhibitor) and 0.4 mg of Tamsulosin (alpha-adrenergic blocker), whereas the other subject, who was hypertensive and was taking 5 mg of Cilazapril (angiotensin-converting enzyme inhibitor) and 25 mg of hydrochlorothiazide (diuretic). The two subjects refrained from taking the above medications for >48 h before the experiment, which was determined to be a sufficient period of 5 half-lives for the plasma concentration of Ezetimibe (34), Tamsulosin (51), Cilazapril (47), and hydrochlorothiazide (14). Furthermore, since the pattern of responses of sweating and cutaneous vasodilation in the two subjects did not differ from the rest of the older adults, we included them in data analysis. Additionally, since we did not see a clear sex difference in the pattern of responses of sweating and cutaneous vasodilation, we combined males and females for data analysis in both age groups. The characteristics (age, height, body mass, body surface area, body fat, and peak oxygen uptake), determined during a preliminary session as described below, are presented in Table 1.
Table 1.
Subject characteristics
| Younger | Older | |
|---|---|---|
| No. of subjects, n (male/female) | 12 (8/4) | 12 (6/6) |
| Age, yr | 23 ± 4 | 60 ± 6† |
| Height, m | 1.72 ± 0.08 | 1.70 ± 0.07 |
| Body mass, kg | 73.9 ± 13.6 | 74.2 ± 10.5 |
| Body surface area, m2 | 1.86 ± 0.16 | 1.86 ± 0.15 |
| Body fat, % | 18.6 ± 7.4 (n = 11) | 27.7 ± 9.5 (n = 10)† |
| Peak oxygen uptake, l/min | 3.1 ± 0.7 | 2.6 ± 0.6 |
| Peak oxygen uptake, ml·kg−1·min−1 | 43.0 ± 7.7 | 35.7 ± 9.1† |
| Peak heart rate, beats/min | 192 ± 10 | 155 ± 13† |
All values are expressed as means ± SD. One younger female and two older female data were missing for body fat.
P < 0.05 vs. younger.
Preliminary session.
All subjects abstained from taking prescribed (>48 h before) and/or over-the-counter (>24 h before) medications (including nonsteroidal anti-inflammatory drugs, vitamins, and minerals) prior to arriving to the laboratory. Subjects also refrained from alcohol, caffeine, and heavy physical activity at least 12 h before the preliminary session, and did not consume any food 2 h before and throughout the session. Body height, mass, surface area, and density as well as peak oxygen uptake were determined during the preliminary session. Body height was measured using an eye-level physician stadiometer (model 2391, Detecto Scale, Webb City, MO), while body mass was measured using a digital weight scale platform (model CBU150X, Mettler Toledo, Schwerzenbach, Switzerland) with a weighing terminal (model IND560, Mettler Toledo). Body surface area was subsequently calculated from the measurements of body height and mass (7). Body density was measured using the hydrostatic weighing technique, and used to estimate body fat percentage (41). To determine peak oxygen uptake and heart rate, the subjects performed an incremental cycling protocol until exhaustion at a pedaling rate of ∼60–90 revolutions/min on a recumbent cycle ergometer (Corival Recumbent, Lode, Groningen, Netherlands). The starting workload for the first 1 min was set at 60 W for older adults and 100 W for young adults and was increased at a rate of 20 W/min until the subject could no longer maintain a pedaling rate of >50 revolutions/min. During the incremental exercise, breath-by-breath oxygen uptake was measured by an automated gas analyzer (Medgraphics Ultima, Medical Graphics, St Paul, MN). Peak oxygen uptake was taken as the highest average oxygen uptake measured over 30 s. Peak respiratory exchange ratio during the incremental cycling was >1.1 in all subjects, suggesting that peak oxygen uptake was obtained. For older subjects, a qualified technician continuously monitored them via electrocardiogram. In both younger and older adults, resting and peak heart rate (near the end of incremental cycling) were determined to calculate heart rate reserve during the experimental session (see Measurements for more details).
Experimental procedures.
As with the above preliminary session, all subjects abstained from prescribed (>48 h before) and/or over-the-counter (>24 h before) medications, alcohol, caffeine, heavy exercise (>12 h before), and food consumption (>2 h before and throughout the experimental session). Shortly after arriving to the laboratory on the day of the experimental session, subjects changed into shorts and running shoes (and sports bras for women). Following a measurement of body mass, subjects were then seated in a recumbent position in an experimental room (∼23°C) and instrumented with four microdialysis fibers (30-kDa cutoff, 10-mm membrane) (MD2000, Bioanalytical Systems, West Lafayette, IN) in the dermal layer of the skin on the dorsal side of the left forearm. A 25-gauge needle was first inserted into the unanesthetized skin using aseptic technique, with the entry and exit points separated by ∼2.5 cm. The microdialysis fiber was then threaded through the lumen of the needle, after which the needle was withdrawn leaving the fiber in place. Each microdialysis fiber was separated from adjacent fibers by at least 4 cm and was secured with surgical tape. Thereafter, the subjects entered a thermal chamber (Can-Trol Environmental Systems, Markham, ON, Canada) regulated to an ambient air temperature of 35°C and a relative humidity of 20%, and rested on a recumbent cycle ergometer (Corival Recumbent, Lode). At least 20 min after the fiber placement, perfusion of the microdialysis fibers with the pharmacological agents began. Fibers were continuously perfused in a counterbalanced manner with 1) lactated Ringer (control), 2) 5 mM Nω-propyl-l-arginine (NPLA, Tocris, Ellisville, MO), nNOS inhibitor (nNOS-I), 3) 0.1 mM N-3-aminomethyl-benzyl-acetamidine (1400W, Sigma-Aldrich, St. Louis, MO), iNOS inhibitor (iNOS-I), or 4) 5 mM Nω-amino-l-arginine (LNAA, Cayman Chemical, Ann Arbor, MI), eNOS inhibitor (eNOS-I). These concentrations were determined based on previous studies in which intradermal microdialysis was employed in human skin (3, 16, 21, 29, 43). NOS isoform inhibitory specificity for NPLA (56), 1400W (13), and LNAA (1) has been previously confirmed. Although LNAA is considered a nonselective NOS inhibitor, it has 10-fold selectivity for eNOS over nNOS (53). This increased affinity of LNAA for eNOS relative to nNOS is indirectly supported by a previous work demonstrating that LNAA does not modulate cutaneous vasodilation during whole body passive heating induced by a water-perfused suit, a response which is in part mediated by nNOS-dependent mechanisms (21).
A microinfusion pump (model 400, CMA Microdialysis, Solna, Sweden) was used to continuously perfuse each drug at a rate of 4.0 μl/min for at least 60 min to ensure the establishment of each blockade. We previously showed that this 60 min plus ∼20 min between fiber placement and the start of drug infusion is sufficient for the hyperemia associated with insertion of the fibers to subside (i.e., >60 min) (10). The drug perfusion continued for the entire experimental protocol (2 h and 10 min) until the maximal cutaneous vasodilation procedure began (see below).
Given that the instrumentation period was performed in the thermal chamber, subjects were exposed to an ambient heat stress (35°C, 20% relative humidity) >60 min prior to 10 min of baseline data collection. Having this pre-heat exposure enabled us to evaluate how passive heat stress at rest, associated with high ambient temperature, affects NOS-dependent mechanisms. Thereafter, subjects performed two successive 30-min bouts of recumbent cycling at a fixed rate of metabolic heat production (400 W). Exercise intensity was defined as an absolute heat load to ensure a similar thermal drive for whole-body heat loss in all subjects (12). The external work rate was similar between younger (74 ± 5 W) and older (72 ± 2 W) adults (P = 0.51). Also, relative exercise intensity did not differ between younger (49 ± 7% peak oxygen uptake) and older (57 ± 6% peak oxygen uptake) adults (P = 0.08). The first and second bouts of exercise were followed by a 20- and 40-min recovery period, respectively. Two bouts of exercise were employed to determine whether the mechanisms underlying cutaneous blood flow and sweating differed between the initial and subsequent exercise bouts. A 20-min recovery was employed after the first exercise to affirm previous findings demonstrating that there is a marked suppression in the heat loss responses such that both cutaneous blood flow and sweating return to or near baseline resting levels within 20 min despite a sustained elevation in core body temperature (22). Following the second exercise bout, an extended 40-min recovery was used to determine if the attenuated heat loss responses remained intact as typically observed during a prolonged recovery period.
Following the second 40-min recovery period, 50 mM sodium nitroprusside (SNP; Sigma-Aldrich) was administered at a rate of 6.0 μl/min for 20–30 min to elicit maximal cutaneous vasodilation, as defined by a plateau in cutaneous blood flow for at least 2 min. Thereafter, a final measurement of body mass was obtained.
Measurements.
Sweat capsules were placed directly over the center of each microdialysis membrane and affixed to the skin with adhesive rings and topical skin glue (Collodion HV, Mavidon Medical products, Lake Worth, FL). For 9 younger and older adults, we used a sweat capsule covering a skin surface area of 2.8 cm2. To obtain insight into the potential effect of sweat capsule size on sweating data, we employed a smaller capsule (1.1 cm2) for three younger and three older adults. Since the patterns of sweating response did not differ between the two sweat capsules, we combined all sweating data for data analysis. Dry compressed air from gas tanks located in the thermal chamber was supplied to each capsule at a rate of 1.0 l/min (for a capsule with 2.8 cm2 area) or 0.2 l/min (for a smaller capsule with 1.1 cm2 area). The water content of the effluent air was measured with a capacitance hygrometer (model HMT333, Vaisala, Helsinki, Finland). Long vinyl tubes were used for connections between the gas tank (located in the chamber) and the sweat capsule, and between the sweat capsule and the hygrometer, to allow internal gas temperature to be equilibrated to near room temperature (∼35°C) before reaching the sweat capsule (inlet) and the hygrometer (outlet). Local forearm sweat rate was calculated every 5 s based on the difference in water content between influent and effluent air, multiplied by the flow rate, and normalized for the skin surface area under the capsule (expressed in mg·min−1·cm−2).
Cutaneous red blood cell flux expressed in perfusion units, which is an index of cutaneous blood flow, was locally measured at a sampling rate of 32 Hz with laser-Doppler flowmetry (PeriFlux System 5000, Perimed, Stockholm, Sweden). An integrated laser-Doppler flowmetry probe with a 7-laser array (model 413, Perimed) was housed in the center of each sweat capsule directly over each microdialysis fiber, allowing for the simultaneous measurement of both local forearm sweat rate and cutaneous red blood cell flux at the each skin site. Manual auscultation was performed using a validated mercury column sphygmomanometer (Baumanometer Standby model, WA Baum, Copiague, NY) to obtain systolic and diastolic blood pressures every 5 min. Mean arterial pressure was then calculated as diastolic arterial pressure plus one-third the difference between systolic and diastolic pressures (i.e., pulse pressure). Cutaneous vascular conductance (CVC) was calculated as cutaneous red blood cell flux divided by mean arterial pressure. CVC data were represented as percentage of maximum obtained during the maximal cutaneous vasodilation procedure (expressed as %max) to minimize the effect of site-to-site heterogeneity in the level of cutaneous blood flow (31).
Heart rate was recorded every 5 min using a heart rate monitor (RS400, Polar Electro, Kempele, Finland). Heart rate responses were presented as both absolute values and as a percentage of each subject's heart rate reserve (difference in resting and peak heart rates). Due to technical difficulties, heart rate was not recorded for one younger subject.
As an index of core body temperature, esophageal temperature was measured with a general purpose thermocouple temperature probe (Mallinckrodt Medical, St. Louis, MO). The temperature probe was inserted 40 cm past the entrance of the nostril while the subject simultaneously ingested water (∼200 ml) through a straw. Skin temperature was measured using thermocouples (Concept Engineering, Old Saybrook, CT) attached to the skin with adhesive rings and surgical tape. Mean skin temperature was calculated according to proportions determined by Hardy and Dubois (15) based on local skin temperature measurements at the six sites [upper back (21%), chest (21%), biceps (19%), quadriceps (9.5%), hamstring (9.5%), and front calf (20%)]. Core body and skin temperature data were collected at a sampling rate of 15 s using a data-acquisition module (model 34970A; Agilent Technologies Canada, Mississauga, ON, Canada) and simultaneously displayed and recorded in spreadsheet format on a personal computer with LabVIEW software (Version 7.0, National Instruments, Austin, TX). Due to technical difficulties, esophageal temperature was not recorded for two younger and two older subjects.
Metabolic rate was determined using indirect calorimetry (32). Expired gas was analyzed for oxygen (error of ± 0.01%) and carbon dioxide (error of ± 0.02%) concentrations using electrochemical gas analyzers (AMETEK model S-3A/1 and CD3A, Applied Electrochemistry, Pittsburgh, PA). Approximately 20 min before the start of baseline data collection, gas mixtures of known concentrations were used to calibrate gas analyzers and a 3-liter syringe was used to calibrate the turbine ventilometer. The subjects wore a face mask (model 7600 V2, Hans-Rudolph, Kansas City, MO) attached to two-way T-shape nonrebreathing valve (model 2700, Hans-Rudolph). Oxygen uptake and respiratory exchange ratio were obtained every 30 s and were used to calculate metabolic rate (22, 32). Metabolic heat load was estimated from metabolic rate minus external work (i.e., work rate during cycling).
Data analysis.
Baseline resting values were obtained by averaging measurements performed over >5 min. Values at the start of intermittent exercise (time 0) were obtained during the last 5 min before exercise commenced. Local forearm sweat rate and CVC as well as core body and mean skin temperature data acquired during the exercise and recovery periods were obtained by averaging measurements made over the last 5 min of each 10-min interval. Heart rate and blood pressure data acquired during the exercise and recovery periods were obtained by averaging the two measurements made over each 10-min interval. nNOS-, iNOS-, and eNOS-dependent sweating were evaluated as the difference in sweat rate (Δsweat rate) between the control and the nNOS-I, iNOS-I, and eNOS-I skin sites, respectively. Likewise, nNOS-, iNOS-, and eNOS-dependent cutaneous vasodilation were also evaluated relative to control. Maximal CVC values, induced by SNP administration at the end of the experimental protocol, were determined from averaging CVC data over at least 2 min once a plateau was established.
Statistical analysis.
For statistical purposes, the exercise/recovery cycles were defined based on the following time periods: 1) 0 to 30 min: exercise 1; 2) 30 to 50 min: recovery 1; 3) 50 to 80 min: exercise 2; and 4) 80 to 120 min: recovery 2. Local forearm sweat rate and CVC were analyzed using a two-way repeated-measures analysis of variance (ANOVA) separately performed in each group. The two factors for ANOVA analysis were time (14 levels: rest, 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, and 120 min) and treatment site (four levels: control, nNOS-I, iNOS-I, and eNOS-I). To evaluate age-related changes in sweating and cutaneous vasodilation, local forearm sweat rate and CVC at the control site were also analyzed using a two-way mixed-model ANOVA with the factors of time (14 levels: rest, 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, and 120 min) and age (2 levels: younger and older). Body temperature (i.e., core body and skin temperature) and cardiovascular (i.e., mean arterial pressure and heart rate) variables were analyzed using a two-way mixed-model ANOVA with the factors of time (6 levels: rest, exercise 1 at 30 min, recovery 1 at 20 min, exercise 2 at 30 min, recovery 2 at 20 min and 40 min) and age (2 levels: younger and older). Moreover, local forearm absolute maximal CVC (expressed in perfusion units/mmHg) attained during the SNP infusion was analyzed with a two-way mixed-model ANOVA with the factors of treatment site (4 levels: control, nNOS-I, iNOS-I, and eNOS-I) and age (2 levels: younger and older). When a significant main effect was observed, post hoc multiple comparisons were carried out using Student-Newman-Keuls procedure for comparison between treatment sites, and Student's paired t-tests corrected with a Holm-Bonferroni procedure for between-time comparisons. Student's unpaired t-tests were also used for all between-group comparisons including data presented in Figs. 2 and 4 and Tables 1 and 2. Pearson's product-moment correlation coefficients were used to determine if chronological age is associated with the magnitude of each NOS isoform contribution to sweating and cutaneous vasodilation in exercising older adults. Furthermore, the Pearson's product-moment correlation coefficients were also employed to determine if the magnitude of iNOS-dependent sweating is related to the magnitude of eNOS- and nNOS-dependent sweating in younger and older adults. All correlative analyses were performed using individual data presented in Figs. 2 and 4. The level of significance for all analyses was set at P ≤ 0.05. All values are reported as means ± 95% confidence interval unless otherwise indicated.
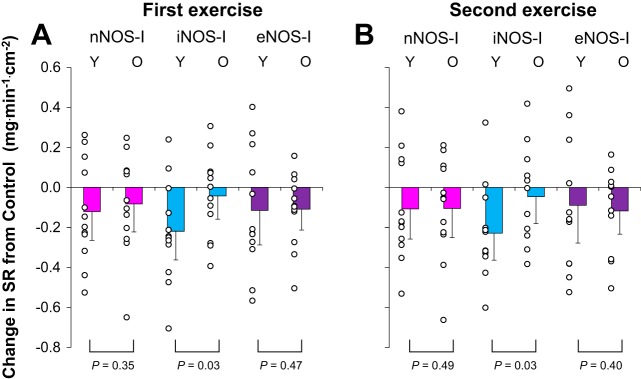
Fig. 2.
The change (Δ) in sweat rate (SR) from lactated Ringer site (control) and each treatment site in younger (Y, n = 12) and older (O, n = 12) adults. Drugs employed are 1) lactated Ringer (control), or each NOS inhibitor, including 2) nNOS inhibitor (nNOS-I), 3) iNOS inhibitor (iNOS-I), or 4) eNOS inhibitor (eNOS-I). Values are means ± 95% confidence interval. First (A) and second (B) exercise indicate last 5 min of each exercise.
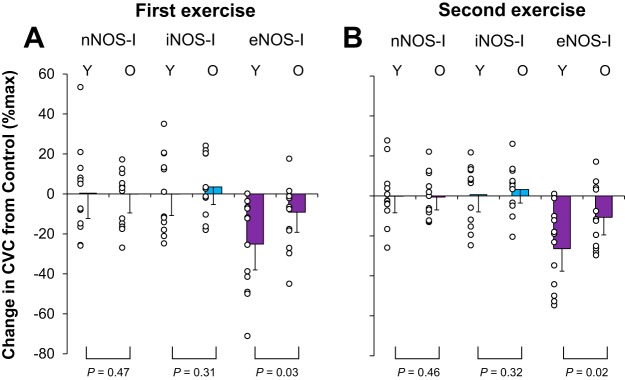
Fig. 4.
The change (Δ) in cutaneous vascular conductance (CVC) from lactated Ringer site (control) and each treatment site in younger (Y, n = 12) and older (O, n = 12) adults. Drugs employed are 1) lactated Ringer (control), or each NOS inhibitor, including 2) nNOS inhibitor (nNOS-I), 3) iNOS inhibitor (iNOS-I), or 4) eNOS inhibitor (eNOS-I). Values are means ± 95% confidence interval. First (A) and second (B) exercise indicate last 5 min of each exercise.
Table 2.
Esophageal and mean skin temperatures, heart rate, and mean arterial pressure at rest and during the two exercise and recovery periods
|
Recovery 2 |
||||||
|---|---|---|---|---|---|---|
| Rest | Exercise 1 (30 min) | Recovery 1 (20 min) | Exercise 2 (30 min) | 20 min | 40 min | |
| Esophageal temperature, °C | ||||||
| Younger (n = 10) | 37.22 ± 0.17 | 37.72 ± 0.14* | 37.43 ± 0.13* | 37.85 ± 0.15* | 37.52 ± 0.11* | 37.50 ± 0.10* |
| Older (n = 10) | 37.17 ± 0.15 | 37.78 ± 0.14* | 37.51 ± 0.16* | 37.94 ± 0.14* | 37.61 ± 0.16* | 37.51 ± 0.15* |
| Mean skin temperature, °C | ||||||
| Younger (n = 12) | 35.12 ± 0.18 | 35.70 ± 0.15* | 35.47 ± 0.13* | 35.78 ± 0.15* | 35.53 ± 0.16* | 35.35 ± 0.14* |
| Older (n = 12) | 34.92 ± 0.17 | 35.66 ± 0.14* | 35.34 ± 0.19* | 35.79 ± 0.17* | 35.35 ± 0.22* | 35.18 ± 0.15* |
| Heart rate, beats/min | ||||||
| Younger (n = 11) | 76 ± 6 | 128 ± 8* | 86 ± 5* | 134 ± 9* | 91 ± 5* | 87 ± 6 |
| Older (n = 12) | 68 ± 5 | 111 ± 9*† | 77 ± 8*† | 116 ± 10*† | 79 ± 8*† | 78 ± 8*† |
| Heart rate reserve, %Reserve | ||||||
| Younger (n = 11) | 7 ± 3 | 48 ± 8* | 15 ± 6* | 52 ± 9* | 19 ± 5* | 14 ± 6* |
| Older (n = 12) | 6 ± 4 | 54 ± 7* | 19 ± 7* | 58 ± 8* | 23 ± 10* | 18 ± 7* |
| Mean arterial pressure, mmHg | ||||||
| Younger (n = 12) | 88 ± 4 | 96 ± 3* | 88 ± 4 | 93 ± 4* | 85 ± 4* | 85 ± 4* |
| Older (n = 12) | 92 ± 4 | 103 ± 5*† | 94 ± 5 | 101 ± 5*† | 91 ± 5 | 92 ± 4† |
Values are means ± 95% confidence interval. Values indicate an average of the final 10 min of the corresponding period.
P < 0.05 vs. Rest.
P < 0.05 vs. Younger.
RESULTS
Local forearm sweat rate.
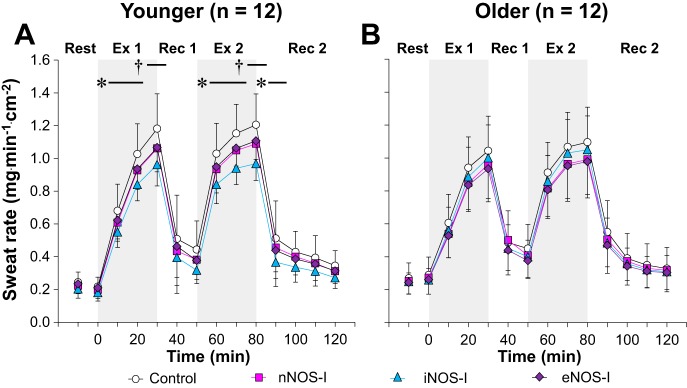
Figure 1 depicts the local sweating response at each treatment site in both younger (Fig. 1A) and older (Fig. 1B) adults. Sweat rate at the control site was similar between age groups at each time point during the intermittent exercise protocol (P > 0.64 for main effect of age and interaction between age and time). In the younger adults, no differences in local sweat rate were observed between treatment sites at baseline rest or at the onset of exercise (time 0) (all P > 0.81). However, inhibition of each NOS isoform (i.e., nNOS, iNOS, and eNOS) attenuated local sweat rate relative to control during the first and second exercise bouts (all P < 0.05). Furthermore, sweat rate was reduced at the iNOS-I site compared with the nNOS-I and eNOS-I sites during the last 5 min of the first and second exercise (all P < 0.05). Conversely, no differences were observed between treatment sites at any point during either recovery period (all P > 0.10) except a lower sweat rate at the iNOS-I site relative to control during the first 10 min of second recovery. In the older adults, no differences in local forearm sweat rate were observed between treatment sites throughout baseline rest and at time 0 as well as during both exercise bouts and recovery periods (P ≥ 0.11 for main effect of treatment site and interaction between treatment site and time).
Fig. 1.
Time-course changes in sweat rate during exercise at a fixed rate of metabolic heat production (400 W) in younger (A, n = 12) and older (B, n = 12) adults. Drugs employed are 1) lactated Ringer (control, open circles), or each nitric oxide synthase (NOS) inhibitor, including 2) neuronal NOS (nNOS) inhibitor (nNOS-I, squares), 3) inducible NOS (iNOS) inhibitor (iNOS-I, triangles), or 4) endothelial NOS (eNOS) inhibitor (eNOS-I, diamonds). Values are means ± 95% confidence interval. Each value during exercise and recovery represents the average of the last 5 min of each 10-min interval. Start of intermittent exercise (time 0) indicates resting values 5 min before exercise. Ex 1, first exercise; Rec 1, first recovery; Ex 2, second exercise; Rec 2, second recovery; †All NOS inhibitor sites different from control, and iNOS-I site different from nNOS-I and eNOS-I sites (all P < 0.05); *iNOS-I site different from control (P < 0.05).
Figure 2 depicts a comparison between younger and older adults for nNOS-, iNOS-, and eNOS-dependent sweating at the end of each exercise bout. During both exercise bouts, iNOS-dependent sweating was greater in the younger relative to older adults (both P = 0.03). Conversely, nNOS- and eNOS-dependent sweating during each exercise were similar between age groups (all P ≥ 0.35). The magnitudes of iNOS-dependent sweating were positively correlated with those of nNOS- and eNOS-dependent sweating in younger (all P < 0.05, R > 0.73) but not older (all P > 0.05, 0.13 > R > 0.01) adults. Age did not correlate with the magnitude of nNOS-, iNOS-, and eNOS-dependent sweating in older adults (all P > 0.05, 0.18 > R > −0.33).
Local forearm cutaneous vascular response.
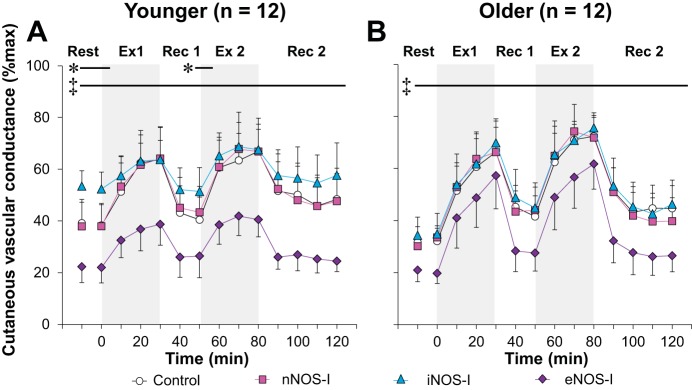
Local forearm CVC at each treatment site in the younger (Fig. 3A) and older (Fig. 3B) adults is illustrated in Fig. 3. CVC measured at the control site was similar between groups throughout the intermittent exercise protocol (P > 0.41 for main effect of age and interaction between age and time). In the younger adults, CVC at the eNOS-I site was reduced compared with the control site at all time points throughout baseline rest and time 0 as well as both exercise and recovery periods (all P ≤ 0.05). In contrast, CVC at the iNOS-I site was higher relative to the control site at baseline rest, time 0, and the last 10 min of first recovery (all P ≤ 0.05), whereas there was no difference in CVC between nNOS-I and control sites throughout the protocol (P > 0.52) in younger adults. In the older adults, CVC at the eNOS-I site was lower than that at the control site throughout the entire intermittent exercise protocol (all P ≤ 0.05); however, CVC at the nNOS-I or iNOS-I site was not different from that at the control site (all P ≥ 0.09).
Fig. 3.
Time-course changes in cutaneous vascular conductance during exercise at a fixed rate of metabolic heat production (400 W) in younger (A, n = 12) and older (B, n = 12) adults. Drugs employed are 1) lactated Ringer (control, open circles), or each NOS inhibitor including 2) nNOS inhibitor (nNOS-I, squares), 3) iNOS inhibitor (iNOS-I, triangles), or 4) eNOS inhibitor (eNOS-I, diamonds). Values are means ± 95% confidence interval. Each value during exercise and recovery represents the average of the last 5 min of each 10-min interval. Start of intermittent exercise (time 0) indicates resting values 5 min before exercise. Ex 1, first exercise; Rec 1, first recovery; Ex 2, second exercise; Rec 2, second recovery; *iNOS-I site different from control (P < 0.05); ‡eNOS-I site different from control (P < 0.05).
The magnitude of nNOS-, iNOS-, and eNOS-dependent cutaneous vasodilation are compared between the younger and older adults in Fig. 4. eNOS-dependent cutaneous vasodilation was reduced in the older relative to younger adults (both P ≤ 0.03), whereas nNOS- and iNOS-dependent cutaneous vasodilation were not different between age groups (all P ≥ 0.31). Age did not correlate with the magnitude of nNOS-, iNOS-, and eNOS-dependent cutaneous vasodilation in older adults (all P > 0.05, 0.35 > R > −0.28).
Local forearm absolute maximal CVC (in perfusion units/mmHg) did not differ between the younger and older adults at the control site (younger 1.77 ± 0.25 vs. older 1.56 ± 0.28) or the nNOS-I (younger 1.53 ± 0.24 vs. older 1.69 ± 0.32), iNOS-I (younger 1.84 ± 0.25 vs. older 1.79 ± 0.40), and eNOS-I (younger 1.80 ± 0.21 vs. older 1.58 ± 0.28) skin sites as well as between treatment sites within each group (all P > 0.25 for main effects of age and treatment site, and interaction between age and treatment site).
Body temperature, cardiovascular, and hydration status variables.
The body (i.e., esophageal and skin) temperature and cardiovascular (i.e., mean arterial pressure and heart rate) responses are presented in Table 2. Neither esophageal nor mean skin temperatures differed between the younger and older adults throughout the protocol (all P ≥ 0.13 for main effect of age and interaction between age and time). During both exercise bouts, as well as the first and second recovery periods, heart rate was lower in the older relative to younger adults (all P ≤ 0.05). However, when expressed as a percentage of heart rate reserve, no age-related differences were observed (P > 0.40 for main effect of age and interaction between age and time). Furthermore, the older adults exhibited greater mean arterial pressure responses compared with the younger adults during both exercise bouts and at the 40-min time point of the second recovery (all P ≤ 0.05). Finally, body mass was similarly (P = 0.56) reduced from baseline levels by 1.8 ± 0.2% in younger adults and by 1.8 ± 0.2% in older adults following the experiment (both P < 0.01).
DISCUSSION
The primary finding of the current study is that nNOS, iNOS, and eNOS are all involved in the sweating response in younger adults during exercise in the heat, albeit iNOS is the major contributor. Furthermore, we demonstrate that iNOS inhibition does not alter sweating and cutaneous vasodilation in exercising older adults. We also observed that eNOS inhibition attenuated cutaneous vasodilation in both age groups throughout baseline rest as well as both exercise bouts and recovery periods, but the magnitude of eNOS-dependent cutaneous vasodilation during exercise was comparatively smaller in the older adults.
Sweating.
Previous studies have shown that NOS contributes to the sweating response in young adults during exercise in the heat (9, 28, 45, 50). Our data indicate that this response is primarily mediated by iNOS (Fig. 1A). It is generally thought that, in younger healthy adults, basal iNOS expression is minimal (5, 8, 49). However, a functional role for iNOS in younger adults under normal physiological circumstances was previously suggested by Bruning et al. (3), who demonstrated that iNOS partially contributes to the initial cutaneous vasodilatory response to local heating of the skin. Our results extend upon this finding by demonstrating that iNOS functionally contributes to the sweating response in younger adults performing moderate intensity exercise in the heat.
The precise mechanism(s) by which iNOS contributes to sweating during exercise is unclear. Given that heat stress can increase the expression of iNOS (2), and iNOS expression can occur as early as 60 min after stimulation of transcription (37), the prolonged exposure (>60 min) to a warm environment (35°C) prior to the start of exercise in the current study may have been sufficient to increase functional iNOS activity in the skin. Indeed, we confirmed an effect of iNOS on cutaneous blood flow during baseline rest before exercise (see below). Alternatively, Bruning et al. (3) showed that iNOS intrinsically exists in healthy, younger human skin and thus the activation of this iNOS may contribute to iNOS-dependent sweating during exercise. However, it is important to note that the presence of iNOS has not been directly observed in human eccrine sweat glands (40). Therefore, NO produced by iNOS located in the skin elsewhere than the sweat glands (e.g., vessels, melanocytes, keratinocytes) likely contributes to NOS-dependent sweating. As a potential mechanism, exercise-induced hyperthermia may activate heat shock protein 90 in the skin (keratinocytes) (52), which in turn activates iNOS. Indeed, heat shock protein 90-mediated activation of iNOS has been previously observed in vitro (54).
The iNOS-dependent sweating during exercise that was observed in younger adults was not seen in the older adults (Fig. 1B), which is an extension of our previous studies demonstrating that age-related impairments in NOS-dependent sweating during exercise (11, 45). The mechanism(s) underlying the impaired iNOS-mediated sweating are unclear. It is known however, that aging can increase the levels of reactive oxygen species including superoxide in the skin (17). Superoxide reacts readily with NO to form peroxynitrite, consequently decreasing NO bioavailability, and, by extension, may impair NOS-dependent sweating.
In addition to iNOS, both eNOS and nNOS contribute to NOS-dependent sweating in exercising younger adults, although their respective contributions are smaller relative to that of iNOS (Fig. 1A). eNOS- and nNOS-dependent sweating during exercise likely results from activations of eNOS and nNOS (40, 55) secondary to elevated Ca2+ concentration in the sweat glands. Indeed, it is generally accepted that both eNOS and nNOS can be activated by Ca2+. Moreover, acetylcholine released from sympathetic cholinergic nerves during heat stress activates muscarinic receptor located on sweat glands, which can elicit increases in intracellular Ca2+ levels in sweat glands (39).
We found large interindividual differences in the magnitude of each NOS isoforms contribution to sweating in younger adults (Fig. 2). In addition, we observed that greater iNOS-dependent sweating was associated with greater eNOS- and nNOS-dependent sweating. This may indicate that the NOS isoforms work sequentially to augment sweating during exercise in the heat. In line with this, it has been previously demonstrated that eNOS activity can be regulated by nNOS (19). A nonadditive NOS isoform contribution to sweating is also suggested by the fact that the sum of reduction in sweat rate induced by each NOS isoform inhibitor explains ∼40% of total sweat rate, which is much greater than the ∼20% reduction seen in sweat rate using a nonselective NOS inhibitor (9). However, future studies are warranted to elucidate the interaction(s), if any, between NOS isoforms and their influence on the sweating response.
Cutaneous vasodilation.
An unexpected finding of the current study was that iNOS inhibition increased CVC relative to control in the younger adults during baseline rest (Fig. 3A). Since iNOS inhibition does not affect normothermic CVC (3, 43), our result suggests that passive exposure to a hot environment (35°C) activates iNOS, which in turn attenuates cutaneous vasodilation. This may in part be explained by sensory nerve-dependent iNOS activation which is induced by increases in local skin temperature (3). Given that eNOS contributes to cutaneous vascular regulation during rest in a hot environment (Fig. 3A), iNOS may reduce NO produced from eNOS, which attenuates cutaneous vasodilation. For instance, iNOS can upregulate arginase (38), which can convert NO precursor l-arginine into l-ornithine and urea, consequently reducing NO bioavailability. Furthermore, older adults did not exhibit this iNOS-dependent reduction in CVC during baseline rest. As a potential mechanism, aging may have already upregulated arginase in the skin as reported previously (18); thus a heat-induced increase in iNOS activity may not result in further upregulation of arginase in older adults.
NOS contributes to cutaneous vasodilation during exercise in the heat (9, 28, 30, 50). In line with this, we observed a clear eNOS component for cutaneous vasodilation in younger adults during moderate-intensity exercise (at ∼49% peak oxygen uptake) in the heat (35°C) (Fig. 3A). This is consistent with the recent study by McNamara et al. (29) who employed a slightly higher intensity exercise (at ∼60% peak oxygen uptake) in non-heat-stressed conditions (∼23–24°C). However, we also observed eNOS-dependent cutaneous vasodilation during a prolonged ambient heat exposure (>60 min) at rest prior to the start of exercise as well as during the postexercise recovery periods of the intermittent exercise protocol (Fig. 3A). Our findings differ from the observations by McNamara et al. (29) who reported no eNOS-dependent cutaneous vasodilation during resting prior to exercise. This discrepancy may be due to differences in mean skin temperature. In the present study, subjects were exposed to an ambient heat stress prior to exercise, resulting in mean skin temperatures of ∼35°C. On the other hand, the non-heat stress (∼23–24°C) conditions employed by McNamara et al. (29) would have likely resulted in lower mean skin temperature (skin temperature not reported in their study) compared with the current study. It is also noteworthy that previous studies have shown that nNOS, but not eNOS, mediates cutaneous vasodilation during passive whole body increases in body temperature achieved with a water-perfused suit (20, 21, 29). Since a water-perfused suit was used to clamp mean skin temperature at ∼39°C (20, 21), it is plausible that the comparatively higher mean skin temperature may have activated nNOS rather than eNOS. The influence of mean skin temperature on NOS-dependent cutaneous vasodilation requires further scrutiny.
As with younger adults, older adults exhibited eNOS-dependent cutaneous vasodilation throughout baseline rest as well as during both exercise bouts and recovery periods (Fig. 3B). This is an extension of our previous work in which it was demonstrated that local administration of a nonselective NOS inhibitor decreased cutaneous vasodilation in older adults during exercise in the heat (11). However, it is important to emphasize that the contribution of eNOS to cutaneous vasodilation is reduced in older relative to younger adults (Fig. 4). This impairment in eNOS-dependent cutaneous vasodilation in older adults may be the result of age-related increases in superoxide and/or arginase (18, 48), which can reduce NO bioavailability.
Despite attenuations in the contribution of eNOS to cutaneous vasodilation, older adults were able to maintain CVC comparable to younger adults throughout the experimental protocol (see results). We previously found that cyclooxygenase (COX) does not contribute to cutaneous vasodilation in older adults exercising in the heat (11). Therefore it is likely that upregulated NOS- and COX-independent mechanisms compensate for reduced NOS-dependent cutaneous vasodilation in older adults. For example, endothelium-derived hyperpolarizing factors can contribute to regulation of cutaneous vasodilation (4, 6, 26), which may underlie the NOS- and COX-independent contributions to cutaneous vasodilation during exercise in older adults.
Age-related decrement in heat loss responses.
Local forearm sweating (Fig. 1) and CVC (Fig. 3) at the control site, as well as core body temperature (Table 2), did not differ between the younger and older adults in the current study. At first glance, this seems to reflect that whole body heat dissipation was not different between groups. However, age-related impairments in whole body heat loss during exercise have recently been reported despite no differences in local heat loss responses and core body temperature (24, 46). It may be that more heat is stored in areas within the body (e.g., muscle and or splanchnic organs) that are not measured/detected by the core body temperature sensor (22). In addition, given that the magnitude of age-related decrement of local heat loss responses can vary across skin sites (42), we may have seen a clearer aging effect if different skin site were recruited, especially in areas of high sweat output (e.g, chest, back) (22); or, the lack of differences in the heat loss responses between age groups in the present study may be due to the fact that we recruited active older adults. Specifically, regular exercise can improve local (33) and whole body (46) heat loss responses, which would counteract the effect of aging. Further, the lack of age-related differences in heat loss responses may have been related to the fact that our older subjects were 60 ± 6 yr of age. Had we sedentary adults older than the participants of the current study (e.g., >60 yr), we may have seen age-related reductions in local sweat rate and CVC with greater elevations in core body temperature during exercise, as well as greater decrement in the contribution of each NOS isoform to the heat loss responses. However, the fact remains that our data indicate that aging modulates the mechanisms underlying cutaneous vasodilation and sweating.
Considerations.
In the present study, we were unable to confirm the extent to which each NOS isoform inhibitor affected the other NOS isoform(s); however, both the present and previous studies support the specificity of each NOS isoform inhibitor. For instance, we demonstrated that eNOS inhibition markedly reduced CVC during exercise in both groups (Fig. 3). If nNOS and iNOS inhibitors attenuate eNOS activity, we should have observed a reduction in CVC during exercise, which was not the case. Additionally, Kellogg et al. (21) showed that eNOS inhibition did not influence nNOS-dependent cutaneous vasodilation during passive heating at rest. Furthermore, they also demonstrated that nNOS inhibition did not influence the eNOS-dependent cutaneous vasodilation induced by locally heating the skin (21). Finally, Smith et al. (43) showed that nNOS inhibition did not replicate the effect of iNOS inhibition (i.e., augmented cutaneous vasodilation during local heating). Therefore, while the specificity of each NOS blockade cannot be directly determined, it is unlikely that each agent employed greatly influenced any isoform other than the intended target.
Our results cannot be applied to different exercise protocols such as longer-duration exercise where there would be greater increases in core body temperature. Also, we observed NOS-dependent mechanisms under a moderate exercise intensity in this study (i.e., ∼50% peak oxygen uptake). We previously found that higher-intensity exercise in the heat (∼70% peak oxygen uptake) diminishes NOS-dependent cutaneous vasodilation and sweating (9, 30). Therefore, had we employed a higher-intensity exercise, we may have not seen the contribution of each NOS isoform to sweating and cutaneous vasodilation.
Perspectives.
Increases in iNOS expression and/or activation can be harmful as described by cutaneous vascular dysfunction in essential hypertensive adults (43). However, iNOS can be functionally important as it protects cells and tissues from infections (35) and stress-related injuries (2). In line with the latter concept, our results demonstrate that iNOS functionally contributes to the sweating response in physically active younger adults during exercise in the heat. We also show that this iNOS contribution is attenuated in physically active older adults. Therefore, restoring iNOS function may augment the sweating response during exercise in older adults. It has been shown that reductions in oxidative stress (17) and tetrahydrobiopterin (BH4) supplementation (44) increase NO bioavailability and thus restore NOS-dependent cutaneous vasodilation in resting older adults during whole body passive heating. Therefore, it is plausible that targeting the aforementioned physiological pathways may restore iNOS-dependent sweating in older adults, thereby augmenting heat loss.
Conclusions.
We show that iNOS is the main contributor to NOS-dependent sweating in physically active younger adults during exercise in the heat, albeit comparatively minor roles for eNOS and nNOS were also observed. Further, we demonstrate that NOS-dependent cutaneous vasodilation in both physically active younger and older adults is mediated by eNOS but the magnitude of its contribution was comparatively reduced in older adults. Finally, we demonstrate that iNOS does not functionally contribute to sweating and cutaneous vasodilation in exercising physically active older adults.
GRANTS
This study was supported by the Natural Sciences and Engineering Research Council Discovery Grant of Canada (RGPIN-06313-2014), Discovery Grants Program-Accelerator Supplements (RGPAS-462252-2014), and by a Canada Foundation for Innovation Leaders Opportunity Fund (22529) (funds held by G. P. Kenny). G. P. Kenny is supported by a University of Ottawa Research Chair Award. N. Fujii is supported by the Human and Environmental Physiology Research Unit. R. D. Meade is supported by a Queen Elizabeth II Graduate Scholarship in Science and Technology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.F. and G.P.K. conception and design of research; N.F., R.D.M., P.A., I.F.-b., J.C.L., and P.B. performed experiments; N.F. and I.F.-b. analyzed data; N.F., R.D.M., L.M.A., and G.P.K. interpreted results of experiments; N.F. prepared figures; N.F. drafted manuscript; N.F., R.D.M., L.M.A., P.A., I.F.-b., J.C.L., P.B., and G.P.K. edited and revised manuscript; N.F., R.D.M., L.M.A., P.A., I.F.-b., J.C.L., P.B., and G.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We greatly appreciate all of the volunteers for taking time to participate in this study and M. Poirier for technical assistance. We also thank M. Sabino of Can-Trol Environmental Systems Limited (Markham, ON, Canada) for support.
All experiments took place at the Human and Environmental Physiology Research Unit located at the University of Ottawa.
REFERENCES
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaud C, Godin-Ribuot D, Bottari S, Peinnequin A, Joyeux M, Demenge P, Ribuot C. iNOS is a mediator of the heat stress-induced preconditioning against myocardial infarction in vivo in the rat. Cardiovasc Res 58: 118–125, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol 112: 2019–2026, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res 83: 279–286, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Cracowski JL, Gaillard-Bigot F, Cracowski C, Sors C, Roustit M, Millet C. Involvement of cytochrome epoxygenase metabolites in cutaneous postocclusive hyperemia in humans. J Appl Physiol 114: 245–251, 2013. [DOI] [PubMed] [Google Scholar]
- 7.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17: 863–871, 1916. [Google Scholar]
- 8.Ferrini M, Magee TR, Vernet D, Rajfer J, Gonzalez-Cadavid NF. Aging-related expression of inducible nitric oxide synthase and markers of tissue damage in the rat penis. Biol Reprod 64: 974–982, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Fujii N, McGinn R, Stapleton JM, Paull G, Meade RD, Kenny GP. Evidence for cyclooxygenase-dependent sweating in young males during intermittent exercise in the heat. J Physiol 592: 5327–5339, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii N, Meade RD, Paull G, McGinn R, Foudil-Bey I, Akbari P, Kenny GP. Can intradermal administration of angiotensin II influence human heat loss responses during whole-body heat stress? J Appl Physiol (1985) 118: 1145–1153, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii N, Paull G, Meade RD, McGinn R, Stapleton JM, Akbari P, Kenny GP. Do nitric oxide synthase and cyclooxygenase contribute to the heat loss responses in older males exercising in the heat? J Physiol 593: 3169–3180, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagnon D, Jay O, Kenny GP. The evaporative requirement for heat balance determines whole-body sweat rate during exercise under conditions permitting full evaporation. J Physiol 591: 2925–2935, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJR, Knowles RG. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem 272: 4959–4963, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Giudicelli JF, Richer C, Mattei A. Pharmacokinetics and biological effects of captopril and hydrochlorothiazide after acute and chronic administration either alone or in combination in hypertensive patients. Br J Clin Pharmacol 23: S51–S61, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy JD, Dubois EF. The technic of measuring radiation and convection. J Nutr 15: 461–475, 1938. [Google Scholar]
- 16.Hodges GJ, Sparks PA. Noradrenaline and neuropeptide Y contribute to initial, but not sustained, vasodilatation to local skin warming in humans. Exp Physiol 99: 381–392, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol 291: H2965–H2970, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Holowatz LA, Thompson CS, Kenney WL. l-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol 574: 573–581, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Idigo WO, Reilly S, Zhang MH, Zhang YH, Jayaram R, Carnicer R, Crabtree MJ, Balligand JL, Casadei B. Regulation of endothelial nitric-oxide synthase (NOS) S-glutathionylation by neuronal NOS: evidence of a functional interaction between myocardial constitutive NOS isoforms. J Biol Chem 287: 43665–43673, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellogg DL Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellogg DL Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol (1985) 107: 1438–1444, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny GP, Jay O. Thermometry, calorimetry, and mean body temperature during heat stress. Compr Physiol 3: 1689–1719, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Larose J, Boulay P, Sigal RJ, Wright HE, Kenny GP. Age-related decrements in heat dissipation during physical activity occur as early as the age of 40. PLos One 8: e83148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larose J, Boulay P, Wright-Beatty HE, Sigal RJ, Hardcastle S, Kenny GP. Age-related differences in heat loss capacity occur under both dry and humid heat stress conditions. J Appl Physiol (1985) 117: 69–79, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larose J, Wright HE, Stapleton J, Sigal RJ, Boulay P, Hardcastle S, Kenny GP. Whole body heat loss is reduced in older males during short bouts of intermittent exercise. Am J Physiol Regul Integr Comp Physiol 305: R619–R629, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585: 295–303, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGinn R, Fujii N, Swift B, Lamarche DT, Kenny GP. Adenosine receptor inhibition attenuates the suppression of postexercise cutaneous blood flow. J Physiol 592: 2667–2678, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGinn R, Paull G, Meade RD, Fujii N, Kenny GP. Mechanisms underlying the postexercise baroreceptor-mediated suppression of heat loss. Physiol Rep 2: e12168, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNamara TC, Keen JT, Simmons GH, Alexander LM, Wong BJ. Endothelial nitric oxide synthase mediates the nitric oxide component of reflex cutaneous vasodilatation during dynamic exercise in humans. J Physiol 592: 5317–5326, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meade RD, Fujii N, Alexander LM, Paull G, Louie JC, Flouris AD, Kenny GP. Local infusion of ascorbate augments NO-dependent cutaneous vasodilatation during intense exercise in the heat. J Physiol 593: 4055–4065, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol 109: 1239–1246, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishi Y. Measurement of thermal balance in man. In: Bioengineering, Thermal Physiology and Comfort, edited by Cena K, Clark JA. New York: Elsevier, 1981, p. 29–39. [Google Scholar]
- 33.Okazaki K, Kamijo Y, Takeno Y, Okumoto T, Masuki S, Nose H. Effects of exercise training on thermoregulatory responses and blood volume in older men. J Appl Physiol 93: 1630–1637, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira PR, Brum L, Fronza M, Bernardi LS, Masiero SMK, Dalmora SL. Development and validation of a liquid chromatography-tandem mass spectrometry method for the determination of ezetimibe in human plasma and pharmaceutical formulations. Chromatographia 63: 315–320, 2006. [Google Scholar]
- 35.Ramsey KH, Sigar IM, Rana SV, Gupta J, Holland SM, Byrne GI. Role for inducible nitric oxide synthase in protection from chronic Chlamydia trachomatis urogenital disease in mice and its regulation by oxygen free radicals. Infect Immun 69: 7374–7379, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Manas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 8: 226–238, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Sade K, Schwartz D, Wolman Y, Schwartz I, Chernichovski T, Blum M, Brazowski E, Keynan S, Raz I, Blantz RC, Iaina A. Time course of lipopolysaccharide-induced nitric oxide synthase mRNA expression in rat glomeruli. J Lab Clin Med 134: 471–477, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Santhanam L, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible NO synthase dependent S-nitrosylation and activation of arginase 1 contribute to age-related endothelial dysfunction. Circ Res 101: 692–702, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Sato K, Sato F. Relationship between quin2-determined cytosolic [Ca2+] and sweat secretion. Am J Physiol Cell Physiol 254: C310–C317, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu Y, Sakai M, Umemura Y, Ueda H. Expression of endothelial nitric oxide synthase in human eccrine clear cells. Br J Dermatol 136: 572–574, 1997. [PubMed] [Google Scholar]
- 41.Siri WE. The gross composition of the body. Adv Biol Med Phys 4: 239–280, 1956. [DOI] [PubMed] [Google Scholar]
- 42.Smith CJ, Alexander LM, Kenney WL. Nonuniform, age-related decrements in regional sweating and skin blood flow. Am J Physiol Regul Integr Comp Physiol 305: R877–R885, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J Appl Physiol (1985) 112: 791–797, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stapleton JM, Fujii N, Carter M, Kenny GP. Diminished nitric oxide-dependent sweating in older males during intermittent exercise in the heat. Exp Physiol 99: 921–932, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Stapleton JM, Poirier MP, Flouris AD, Boulay P, Sigal RJ, Malcolm J, Kenny GP. Aging impairs heat loss, but when does it matter? J Appl Physiol (1985) 118: 299–309, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szucs T. Cilazapril. A review. Drugs 41, Suppl 1: 18–24, 1991. [DOI] [PubMed] [Google Scholar]
- 48.Treiber N, Maity P, Singh K, Ferchiu F, Wlaschek M, Scharffetter-Kochanek K. The role of manganese superoxide dismutase in skin aging. Dermatoendocrinology 4: 232–235, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaziri ND, Ni Z, Oveisi F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension 31: 1248–1254, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Welch G, Foote KM, Hansen C, Mack GW. Nonselective NOS inhibition blunts the sweat response to exercise in a warm environment. J Appl Physiol 106: 796–803, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilde MI, McTavish D. Tamsulosin: a review of its pharmacological properties and therapeutic potential in the management of symptomatic benign prostatic hyperplasia. Drugs 52: 883–898, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Wilson N, McArdle A, Guerin D, Tasker H, Wareing P, Foster CS, Jackson MJ, Rhodes LE. Hyperthermia to normal human skin in vivo upregulates heat shock proteins 27, 60, 72i and 90. J Cutan Pathol 27: 176–182, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Wolff DJ, Lubeskie A. Inactivation of nitric oxide synthase isoforms by diaminoguanidine and NG-amino-l-arginine. Arch Biochem Biophys 325: 227–234, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida M, Xia Y. Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. J Biol Chem 278: 36953–36958, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Zancanaro C, Merigo F, Crescimanno C, Orlandini S, Osculati A. Immunohistochemical evidence suggests intrinsic regulatory activity of human eccrine sweat glands. J Anat 194: 433–444, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by Nω-propyl-l-arginine. J Med Chem 40: 3869–3870, 1997. [DOI] [PubMed] [Google Scholar]