Abstract
Macrophages (Mϕ) play a pivotal role in the protection system by recognizing and eliminating invading pathogenic bacteria. Phagocytosis and the killing of invading bacteria are major effector functions of Mϕ. Although the phagocytic and bactericidal activities of Mϕ have been analyzed via several methods using a light microscope, a fluorescence microscope, or a fluorescence-activated cell sorter, expensive materials and equipment are usually required, and the methods are rather complicated. Moreover, it is impossible to determine both the phagocytic and bactericidal activities of Mϕ simultaneously using these methods. In this review, we describe a simple, reproducible, inexpensive, yet old-fashioned method (antibiotic protection assay) for determining the phagocytic and bactericidal activities of Mϕ.
Keywords: Anti-bacterial agent, gentamicin, macrophage, phagocytosis
INTRODUCTION
Mammalian cells usually uptake macromolecules from the extracellular microenvironment through their receptor(s) or by penetration.1 Professional phagocytes such as macrophages (Mϕ) are not exceptional. However, Mϕ have another unique uptake process called phagocytosis, in which Mϕ actively uptake not only macromolecules but also large particles such as bacterial pathogens.1
Mϕ recognize and engulf invading bacteria, and specific vacuoles called phagosomes are then formatted therein.2 The phagosomes mature into phagolysosomes, in which bacterial pathogens encounter various antimicrobial agents such as lysozymes by which bacterial pathogens are digested.3 Reactive oxygen intermediates and nitrogen oxide synthesized in the cytosol participate in the killing of bacterial pathogens by penetrating into phagolysosomes.4
Since Mϕ are particularly important for protection against bacterial infection, analysis of the phagocytic and bactericidal activities of Mϕ is essential for the determination of their functional activities. Bacterial pathogens are categorized into at least two groups on the basis of their kinetics in Mϕ:5 extracellular bacteria and intracellular bacteria. Extracellular bacteria are easily killed by Mϕ, whereas intracellular bacteria show resistance to digestion by Mϕ.5 However, the majority of intracellular bacteria are also killed by Mϕ, although this depends on the activation status of Mϕ.6,7,8,9,10
Several methods are employed to determine the phagocytic and bactericidal activities of Mϕ. Yet, in most cases, expensive materials and equipment are usually required, and the methods are rather complicated. Therefore, this review focuses on a simple, reproducible, inexpensive, yet old-fashioned method for determining the phagocytic and bactericidal activities of Mϕ.
WHAT IS AN ANTIBIOTIC PROTECTION ASSAY?
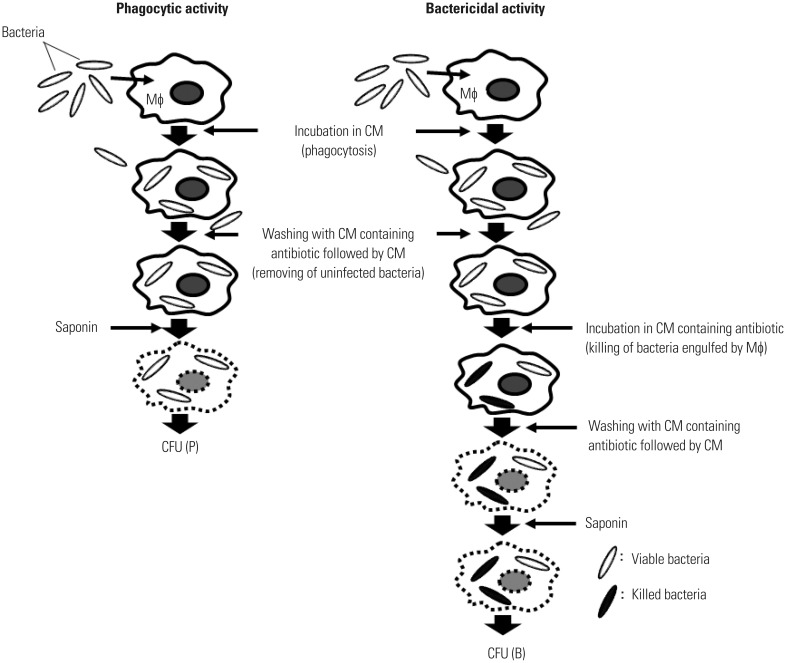
An antibiotic protection assay is traditionally employed to determine the phagocytic and bactericidal activities of Mϕ.6,7,8,9,10,11,12,13 This assay system is based on counting colony-forming units (CFU) in Mϕ after phagocytosis (Fig. 1). After incubating Mϕ with bacteria for a short period of time, the bacteria are engulfed by Mϕ. The phagocytic activity of Mϕ can be determined by counting the CFU in Mϕ at this time point [CFU (P)]. After further incubation for a short period of time, the engulfed bacteria are killed by Mϕ. The number of viable bacteria in Mϕ can be counted at this time point [CFU (B)]. The bactericidal activity of Mϕ can thus be calculated by comparing CFU (P) with CFU (B). Thus, both the phagocytic and bactericidal activities of Mϕ can easily be determined. It is important that bacteria not engulfed by Mϕ must be killed to avoid bacterial growth outside the Mϕ. As antibiotics are essential for killing bacteria that are not engulfed by Mϕ, this assay is known as an antibiotic protection assay.
Fig. 1. An antibiotic protection assay. Two sets of Mϕ (one for the determination of phagocytic activity and the other for the determination of bactericidal activity) are incubated for a short period of time with bacteria in CM to be engulfed by Mϕ. To determine phagocytic activity, Mϕ are washed with CM containing antibiotic to kill extracellular bacteria followed by CM. They are then treated with saponin to release bacteria from Mϕ, and CFUs are determined [CFU (P)]. To determine bactericidal activity, Mϕ infected with bacteria are further incubated for a short period of time in CM containing antibiotic to kill intracellular bacteria followed by CM. They are then treated with saponin, and the number of viable bacteria in Mϕ is determined by counting the CFU after washing with CM [CFU (B)]. Bactericidal activity of Mϕ can be calculated by comparing CFU (P) with CFU (B). CM, complete medium; CFU, colony-forming units; Mϕ, macrophages.
ANTIBIOTICS RECOMMENDED FOR AN ANTIBIOTIC PROTECTION ASSAY
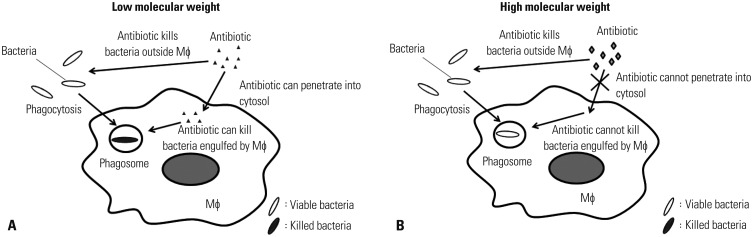
In an antibiotic protection assay, selection of an antibiotic that can effectively kill extracellular (i.e., bacteria not engulfed by Mϕ) yet not intracellular (i.e., bacteria engulfed by Mϕ) bacteria is quite important. As the sensitivity against antibiotics differs for each bacterium, an antibiotic that can effectively kill bacteria must be employed. Antibiotics with low molecular weight pass through plasma membrane of Mϕ. Therefore, the bacteria engulfed by Mϕ are killed by this type of antibiotic (Fig. 2A). In contrast, antibiotics with high molecular weight are unable to penetrate into cytosol. Therefore, the bacteria engulfed by Mϕ are not killed by this type of antibiotic (Fig. 2B). Thus, an antibiotic with a high molecular weight must be used in an antibiotic protection assay.
Fig. 2. Influence of antibiotics with high and low molecular weight on bacteria in Mϕ. Mϕ engulf bacteria by forming specific vacuoles called phagosomes. An antibiotic with a low molecular weight penetrates into the cytosol and kills both extracellular and intracellular bacteria (A). An antibiotic with a high molecular weight is unable to penetrate into the cytosol and thus kills only extracellular bacteria (B). Mϕ, macrophages.
GENTAMICIN (GM) IS WIDELY USED FOR DETERMINING PHAGOCYTIC AND BACTERICIDAL ACTIVITIES OF Mϕ IN AN ANTIBIOTIC PROTECTION ASSAY
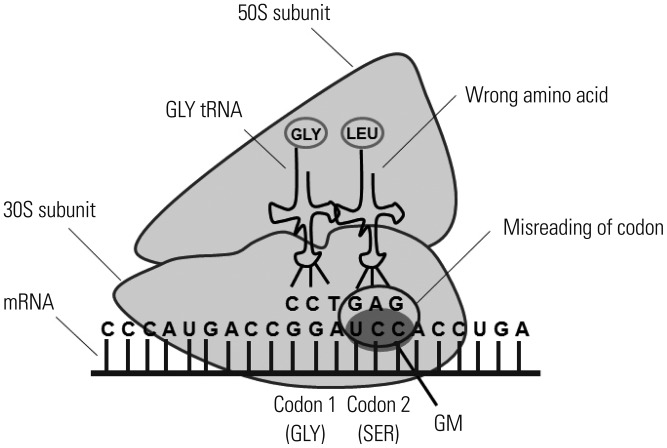
Numerous antibiotics with high molecular weight have been identified previously.14 Among these, gentamicin (GM) is widely employed for an antibiotic protection assay. The reasons are as follows: 1) GM is an aminoglycoside antibiotic that binds to the bacterial ribosome 30S subunit and induces the misreading of a wide range of RNAs (Fig. 3).15 2) GM has a broad-spectrum (Table 1).16,17,18,19,20 3) GM is considered to be unable to penetrate into the cytosol of Mϕ due to its high molecular weight (Table 2).14 It is generally accepted that molecules with a molecular weight of less than 400 g/mol are able to pass through the plasma membrane.21 Therefore, antibiotics with a molecular weight of more than 400 g/mol are recommended for determining the phagocytic and bactericidal activities of Mϕ. There are several antibiotics with a higher molecular weight than that of GM (Table 2). However, these antibiotics are not recommended by several reasons. For example, the molecular weight of erythromycin is markedly higher than that of GM, yet erythromycin expresses bacteriostatic, but not bactericidal, activity.14 Similarly, the molecular weights of kanamycin and streptomycin are higher than those of GM (Table 2).14 However, Pseudomonas spp. show resistance to these antibiotics.22,23 It is needless to say that antibiotics other than GM with high molecular weight can nevertheless be employed for determining the phagocytic and bactericidal activities of Mϕ against particular bacterial pathogens. However, GM is recommended for determining these activities, as this antibiotic has a broad spectrum and kills extracellular, but not intracellular, bacteria. Therefore, we focus on an antibiotic protection assay using GM (GM protection assay) in the following section.
Fig. 3. Killing mechanism of GM. GM inhibits translation of mRNA by binding to the 30S subunit of the ribosome. The irreversible binding of GM to the ribosome causes the misreading of the codons, which in turn causes an error in the proofreading process of translation, leading to incorrect protein expression and bacterial cell death. GM, gentamicin.
Table 1. MIC of GM Against Various Bacteria.
| Organism | MIC (mg/mL) | Reference |
|---|---|---|
| Bacillus cereus | 1.6 | Klein, et al.16 |
| Corynebacterium spp. | 3.0-37.5 | Waitz and Weinstein17 |
| Enterobacter spp. | 0.3-3.0 | Waitz and Weinstein17 |
| Escherichia coli | 0.3-0.75 | Waitz and Weinstein17 |
| Haemophilus influenzae | 7.5 | Waitz and Weinstein17 |
| Lactobacillus spp. | 0.08 | Waitz and Weinstein17 |
| Listeria monocytogenes | 0.03-4.0 | Espaze and Reynaud18 |
| Mycobacterium tuberculosis | 1.0-4.0 | Ho, et al.19 |
| Mycoplasma spp. | 0.75-1.4 | Waitz and Weinstein17 |
| Neisseria gonorrhoeae | 0.8-1.6 | Klein, et al.16 |
| Neisseria meningitidis | 6.3-25 | Klein, et al.16 |
| Pasteurella multocida | 3.0-7.5 | Waitz and Weinstein17 |
| Pseudomonas aeruginosa | 0.3-3.0 | Waitz and Weinstein17 |
| Pseudomonas pseudomallei | 17.5-75.0 | Waitz and Weinstein17 |
| Proteus spp. | 0.75-3.0 | Waitz and Weinstein17 |
| Salmonella spp. | 0.08-0.3 | Waitz and Weinstein17 |
| Shigella spp. | 4.0-16.0 | Wilson, et al.20 |
| Stapylococcus aureus | 0.4-3.1 | Klein, et al.16 |
| Stapylococcus mastitis | 0.01 | Waitz and Weinstein17 |
| Streptococcus agalactiae | 0.3-3.0 | Waitz and Weinstein17 |
| Vibrio spp. | 0.7 | Waitz and Weinstein17 |
MIC, minimal inhibitory concentration; GM, gentamicin.
Table 2. Molecular Weights of Representative Antibiotics.
| Antibiotic | Molecular weight (g/mol) | Reference |
|---|---|---|
| Amoxicillin | 365.4 | Bryskier14 |
| Ampicillin | 349.4 | Bryskier14 |
| Cefaclor | 367.8 | Bryskier14 |
| Cefalexin | 347.4 | Bryskier14 |
| Cephalosporin C | 415.4 | Bryskier14 |
| Chloramphenicol | 323.10 | Bryskier14 |
| Ciclacillin | 341.4 | Bryskier14 |
| Ciprofloxacin | 331.3 | Bryskier14 |
| Erythromycin | 733.9 | Bryskier14 |
| Fosfomycin | 138.1 | Bryskier14 |
| Gentamicin | 477.6 | Bryskier14 |
| Imipenem | 299.3 | Bryskier14 |
| Kanamycin | 484.5 | Bryskier14 |
| Minocycline | 457.5 | Bryskier14 |
| Penicillin G | 334.4 | Bryskier14 |
| Spectinomycin | 332.4 | Bryskier14 |
| Streptomycin | 581.6 | Bryskier14 |
| Tetracycline | 444.4 | Bryskier14 |
EXPERIMENTAL PROCEDURE FOR DETERMINING PHAGOCYTIC ACTIVITY OF Mϕ USING A GM PROTECTION ASSAY
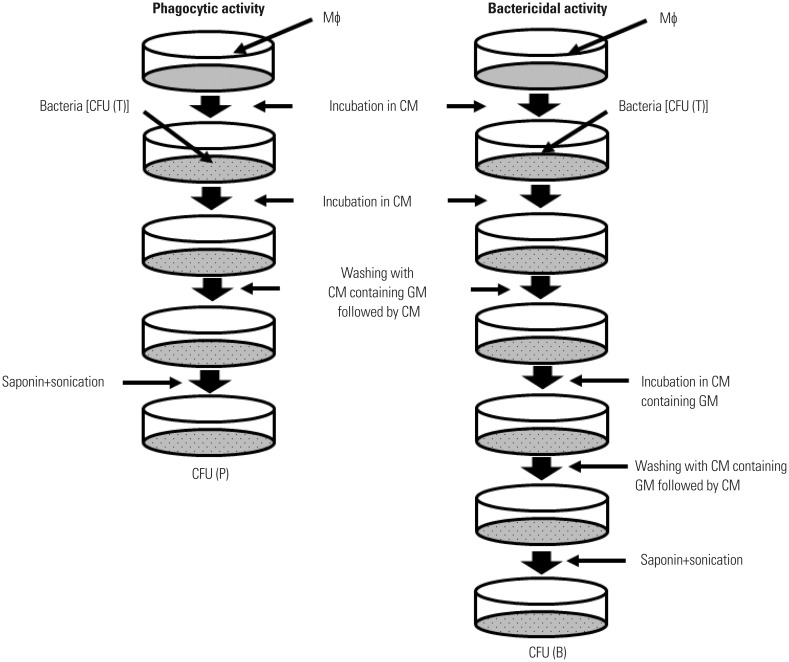
An experimental procedure for determining the phagocytic activity of Mϕ using a GM protection assay is shown in Fig. 4. Mϕ are incubated in RPMI 1640 containing 10% fetal calf serum [designated as complete medium (CM)] for 120 min to adhere to the bottom of tissue culture plates. Cells are incubated with bacteria for a given length of time to ingest bacteria. Subsequently, cells are washed three times with CM containing the optimal concentration of GM to remove non-ingested bacteria. Note that pre-warmed, but not cold, CM should be used throughout the experiment so as not to detach the Mϕ from the bottom of tissue culture plates. After washing with CM, a portion of cells is treated with saponin, which is plated on agar plates after sonication, and the CFUs are determined. Phagocytic activity is calculated as follows: {number of viable bacteria ingested by Mϕ [CFU (P)]/total number of viable bacteria incubated with Mϕ [CFU (T)]}×100 (%). Thus, the percentage of bacteria engulfed by Mϕ (phagocytic activity) can be quantitated.
Fig. 4. Experimental procedure for determining the phagocytic and bactericidal activities of Mϕ using a GM protection assay. Two tissue culture plates are prepared; one to determine phagocytic activity and the other to determine bactericidal activity. Mϕ are incubated in CM for 120 min to adhere to the bottom of tissue culture plates and then incubated with bacteria (Mϕ:bacteria=1:10) for a short period of time in CM to ingest bacteria. To remove non-ingested bacteria, cells are washed three times with CM containing the optimal concentration of GM followed by CM, and CFUs are then determined [CFU (P)]. Infected Mϕ incubated in another plate are further incubated for a short period of time in CM containing the optimal concentration of GM. During this period, engulfed bacteria are killed by Mϕ. Cells are washed three times with CM, and the number of viable bacteria in Mϕ can also be determined by counting CFU after washing with CM [CFU (B)]. Bactericidal activity of Mϕ can be calculated by comparing CFU (P) with CFU (B) after saponin treatment and sonication. GM, gentamicin; CM, complete medium; CFU, colony-forming units; Mϕ, macrophages.
EXPERIMENTAL PROCEDURE FOR DETERMINING BACTERICIDAL ACTIVITY OF Mϕ USING A GM PROTECTION ASSAY
An experimental procedure for determining the bactericidal activity of Mϕ using a GM protection assay is also shown in Fig. 4. Mϕ incubated in CM for 120 min are incubated with bacteria and then washed three times with CM containing the optimal concentration of GM to remove non-ingested bacteria. Subsequently, cells are further incubated in CM containing the optimal concentration of GM for a given length of time to kill non-ingested bacteria followed by CM. Cells are then treated with saponin, plated on agar plates after sonication, and the CFUs are determined. Bactericidal activity is calculated as follows: 100-{number of remaining viable bacteria in Mϕ [CFU (B)]/CFU (P)×100} (%). Thus, the percentage of bacteria killed by Mϕ (bactericidal activity) can be quantitated.
AN ANTIBIOTIC PROTECTION ASSAY FOR BACTERIA SHOWING RESISTANCE TO GM
As described above, GM is widely used to determine the phagocytic and bactericidal activities of Mϕ against various bacteria. However, certain bacteria show resistance to GM (Table 3).15,17 Hence, GM cannot be used to determine the phagocytic and bactericidal activities of Mϕ against these bacteria. In such cases, an antibiotic other than GM with a high molecular weight must be used.
Table 3. Bacteria Showing Resistance to GM.
| Organism | Reference |
|---|---|
| Aeromonas liquefaciens | Waitz and Weinstein17 |
| Bacteroides spp. | Vakulenko and Mobashery15 |
| Burkholderia cepacia | Vakulenko and Mobashery15 |
| Clostridium spp. | Waitz and Weinstein17 |
| Stenotrophomonas maltophilia | Vakulenko and Mobashery15 |
| Streptococcus pneumoniae | Vakulenko and Mobashery15 |
GM, gentamicin.
PRELIMINARY EXPERIMENTS THAT SHOULD BE PERFORMED BEFORE A GM PROTECTION ASSAY
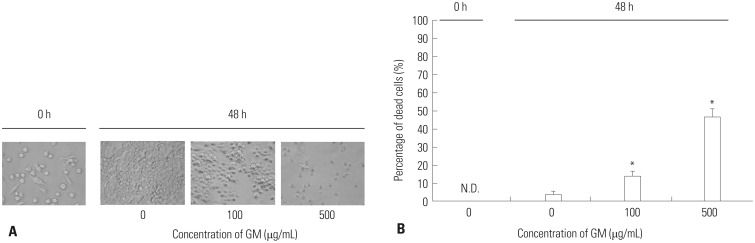
Although the GM protection assay is quite simple, preliminary experiments must be performed. One of the most important points is to determine the optimal conditions (i.e., concentration and length of effectiveness) of GM. Although GM has been considered to be unable to kill bacteria in Mϕ,12,13,24,25,26 several studies have reported that GM, even in high concentrations, kills bacteria in Mϕ.27,28 Therefore, the optimal concentration and length of effectiveness for GM should be determined with care. After determining the minimal inhibitory concentration (MIC), the optimal concentration of GM is then determined. Specifically, Mϕ infected with bacteria are incubated with different concentrations of GM (higher than MIC; e.g., 2.5-fold MIC), and verification that GM does not penetrate into Mϕ should be performed. In addition, further verification as to whether Mϕ are damaged by the concentration of GM should also be performed, as Mϕ are commonly destroyed by high concentrations (e.g., 100 µg/mL) of GM (Fig. 5).
Fig. 5. Influence of GM on Mϕ. (A) RAW264 were incubated with CM containing various concentrations of GM for 48 h, and the morphological changes were observed under phase contrast microscope. Representative data from two independent experiments are shown. (B) RAW264 were incubated with CM containing various concentrations of GM for 48 h, and their viabilities were enumerated via trypan blue exclusion test. Data are presented as mean±SD of two independent experiments. *p<0.05: 0 vs. 100 or 500. N.D., not detectable; GM, gentamicin; CM, complete medium; Mϕ, macrophages.
Saponin facilitates the destruction of plasma and phagosomal membranes of Mϕ by interacting with cholesterols, which are abundant in their plasma membranes (Fig. 6).29 Therefore, before counting CFU in Mϕ, Mϕ must be treated with saponin. We can confirm that Mϕ are completely destroyed by 0.5% saponin.
Fig. 6. Effects of saponin and sonication on plasma and phagosomal membranes of Mϕ. Saponin interacts with cholesterols residing in plasma and phagosomal membranes and forms pores in lipid bilayers. After sonication, internalized bacteria are released. Mϕ, macrophages.
In order to determine the phagocytic and bactericidal activities of Mϕ, the CFUs in Mϕ are counted at different time points after infection. In most cases, the number of viable bacteria engulfed by Mϕ is highest at 45-60 min after incubation with bacteria, and the bacteria are usually killed within 90-120 min after being engulfed by Mϕ. It is needless to say that there are some exceptions; for example, Mycobacterium spp. are not killed within 120 min after being engulfed by Mϕ.30,31
SEVERAL METHODS RECENTLY EMPLOYED FOR DETERMINING PHAGOCYTIC AND BACTERICIDAL ACTIVITIES OF Mϕ
Bacteria engulfed by Mϕ can be detected using a light microscope, a fluorescence microscope, or a flow cytometer.32,33,34,35,36 However, it is difficult to distinguish bacteria engulfed by Mϕ from those merely attached to the plasma membranes of Mϕ by these methods. The problem can be solved by using ethidium bromide.32,33,35,36 Although the phagocytic activity of Mϕ can be determined using these methods, another experiment must be performed to determine the bactericidal activity of Mϕ using MTT and an absorption spectrometer in each case.33,36,37,38,39 Thus, it is possible to determine the phagocytic and bactericidal activities of Mϕ using these methods. However, expensive materials and equipment are required and the methods are rather complicated. Moreover, it is impossible to determine both the phagocytic and bactericidal activities of Mϕ simultaneously using these methods.
CONCLUSION
This review describes a simple, reproducible, inexpensive, yet old-fashioned method for determining the phagocytic and bactericidal activities of Mϕ. Although the phagocytic and bactericidal activities of Mϕ against various bacterial pathogens can be determined by using methods that have been recently employed, it is impossible to determine both activities simultaneously. In addition, these methods require expensive materials and equipment and complicated methods. In contrast, both the phagocytic and bactericidal activities of Mϕ can be determined simultaneously by using an antibiotic protection assay for which expensive materials and equipment are not required. We therefore recommend investigators to reevaluate the antibiotic protection assay.
ACKNOWLEDGEMENTS
This work was supported by a Grant-in-Aid for Scientific Research (22300261 to M.E. and 22590388 to Y.E.) from the Japan Society for the Promotion of Science.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 2.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012;12:492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, et al. The phagosome proteome: insight into phagosome functions. J Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 5.Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol. 2009;7:333–340. doi: 10.1038/nrmicro2112. [DOI] [PubMed] [Google Scholar]
- 6.Buchmeier NA, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skerrett SJ, Martin TR. Recombinant murine interferon-gamma reversibly activates rat alveolar macrophages to kill Legionella pneumophila. J Infect Dis. 1992;166:1354–1361. doi: 10.1093/infdis/166.6.1354. [DOI] [PubMed] [Google Scholar]
- 8.Inoue S, Itagaki S, Amano F. Intracellular killing of Listeria monocytogenes in the J774.1 macrophage-like cell line and the lipopolysaccharide (LPS)-resistant mutant LPS1916 cell line defective in the generation of reactive oxygen intermediates after LPS treatment. Infect Immun. 1995;63:1876–1886. doi: 10.1128/iai.63.5.1876-1886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevanin TM, Moir JW, Read RC. Nitric oxide detoxification systems enhance survival of Neisseria meningitidis in human macrophages and in nasopharyngeal mucosa. Infect Immun. 2005;73:3322–3329. doi: 10.1128/IAI.73.6.3322-3329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emoto M, Yoshida T, Fukuda T, Kawamura I, Mitsuyama M, Kita E, et al. Alpha-galactosylceramide promotes killing of Listeria monocytogenes within the macrophage phagosome through invariant NKT-cell activation. Infect Immun. 2010;78:2667–2676. doi: 10.1128/IAI.01441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandell GL. Interaction of intraleukocytic bacteria and antibiotics. J Clin Invest. 1973;52:1673–1679. doi: 10.1172/JCI107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobo MC, Mandell GL. The effect of antibiotics on Escherichia coli ingested by macrophages. Proc Soc Exp Biol Med. 1973;142:1048–1050. doi: 10.3181/00379727-142-37173. [DOI] [PubMed] [Google Scholar]
- 13.Vaudaux P, Waldvogel FA. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1979;16:743–749. doi: 10.1128/aac.16.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryskier A. Antimicrobial agents. 1st ed. Washington, DC: ASM Press; 2005. [Google Scholar]
- 15.Vakulenko SB, Mobashery S. Versatility of aminoglycosides and prospects for their future. Clin Microbiol Rev. 2003;16:430–450. doi: 10.1128/CMR.16.3.430-450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein JO, Eickhoff TC, Finland M. Gentamicin: activity in vitro and observations in 26 patients. Am J Med Sci. 1964;248:528–544. [PubMed] [Google Scholar]
- 17.Waitz JA, Weinstein MJ. Recent microbiological studies with gentamicin. J Infect Dis. 1969;119:355–360. doi: 10.1093/infdis/119.4-5.355. [DOI] [PubMed] [Google Scholar]
- 18.Espaze EP, Reynaud AE. Antibiotic susceptibilities of Listeria: in vitro studies. Infection. 1988;16(Suppl 2):S160–S164. doi: 10.1007/BF01639741. [DOI] [PubMed] [Google Scholar]
- 19.Ho YI, Chan CY, Cheng AF. In-vitro activities of aminoglycoside-aminocyclitols against mycobacteria. J Antimicrob Chemother. 1997;40:27–32. doi: 10.1093/jac/40.1.27. [DOI] [PubMed] [Google Scholar]
- 20.Wilson G, Easow JM, Mukhopadhyay C, Shivananda PG. Isolation & antimicrobial susceptibility of Shigella from patients with acute gastroenteritis in Western Nepal. Indian J Med Res. 2006;123:145–150. [PubMed] [Google Scholar]
- 21.Schmid S, Knoblauch K. Digestive Organs. In: Bühlmann AA, Froesch ER, editors. Pathophysiology. 1st ed. New York: Springer-Verlag; 1979. pp. 261–315. [Google Scholar]
- 22.Tseng JT, Bryan LE, Van den Elzen HM. Mechanisms and spectrum of streptomycin resistance in a natural population of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1972;2:136–141. doi: 10.1128/aac.2.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ximenes J, Bassoi ON, de Menezes JP, Fry W. Activity of amikacin, gentamicin and kanamycin against Pseudomonas aeruginosa. J Int Med Res. 1976;4:165–175. doi: 10.1177/030006057600400304. [DOI] [PubMed] [Google Scholar]
- 24.de Melo MA, Pechère JC. Effect of mucin on Campylobacter jejuni association and invasion on HEp-2 cells. Microb Pathog. 1988;5:71–76. doi: 10.1016/0882-4010(88)90083-6. [DOI] [PubMed] [Google Scholar]
- 25.Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw JH, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drevets DA, Canono BP, Leenen PJ, Campbell PA. Gentamicin kills intracellular Listeria monocytogenes. Infect Immun. 1994;62:2222–2228. doi: 10.1128/iai.62.6.2222-2228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohya S, Xiong H, Tanabe Y, Arakawa M, Mitsuyama M. Killing mechanism of Listeria monocytogenes in activated macrophages as determined by an improved assay system. J Med Microbiol. 1998;47:211–215. doi: 10.1099/00222615-47-3-211. [DOI] [PubMed] [Google Scholar]
- 29.Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbst S, Schaible UE, Schneider BE. Interferon gamma activated macrophages kill mycobacteria by nitric oxide induced apoptosis. PLoS One. 2011;6:e19105. doi: 10.1371/journal.pone.0019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drevets DA, Campbell PA. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect Immun. 1991;59:2645–2652. doi: 10.1128/iai.59.8.2645-2652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drevets DA, Canono BP, Campbell PA. Listericidal and nonlistericidal mouse macrophages differ in complement receptor type 3-mediated phagocytosis of L. monocytogenes and in preventing escape of the bacteria into the cytoplasm. J Leukoc Biol. 1992;52:70–79. doi: 10.1002/jlb.52.1.70. [DOI] [PubMed] [Google Scholar]
- 34.Utermöhlen O, Karow U, Löhler J, Krönke M. Severe impairment in early host defense against Listeria monocytogenes in mice deficient in acid sphingomyelinase. J Immunol. 2003;170:2621–2628. doi: 10.4049/jimmunol.170.5.2621. [DOI] [PubMed] [Google Scholar]
- 35.Sharma L, Wu W, Dholakiya SL, Gorasiya S, Wu J, Sitapara R, et al. Assessment of phagocytic activity of cultured macrophages using fluorescence microscopy and flow cytometry. Methods Mol Biol. 2014;1172:137–145. doi: 10.1007/978-1-4939-0928-5_12. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko M, Kanayama Y, Emoto Y, Emoto M. Several methods for determination of phagocytic and killing activities of macrophages against Listeria monocytogenes. In: Vicario T, editor. Listeria monocytogenes: incidence, growth behavior and control. 1st ed. New York: Nova Science Publishers; 2015. pp. 15363–163. [Google Scholar]
- 37.Peck R. A one-plate assay for macrophage bactericidal activity. J Immunol Methods. 1985;82:131–140. doi: 10.1016/0022-1759(85)90232-7. [DOI] [PubMed] [Google Scholar]
- 38.Mancuso P, Peters-Golden M, Goel D, Goldberg J, Brock TG, Greenwald-Yarnell M, et al. Disruption of leptin receptor-STAT3 signaling enhances leukotriene production and pulmonary host defense against pneumococcal pneumonia. J Immunol. 2011;186:1081–1090. doi: 10.4049/jimmunol.1001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domingo-Gonzalez R, Katz S, Serezani CH, Moore TA, Levine AM, Moore BB. Prostaglandin E2-induced changes in alveolar macrophage scavenger receptor profiles differentially alter phagocytosis of Pseudomonas aeruginosa and Staphylococcus aureus post-bone marrow transplant. J Immunol. 2013;190:5809–5817. doi: 10.4049/jimmunol.1203274. [DOI] [PMC free article] [PubMed] [Google Scholar]