Abstract
Purpose
The development of bronchiolitis obliterans syndrome (BOS) after allogeneic hematopoietic stem cell transplantation (HSCT) deteriorates patients' quality of life. This study aimed to analyze the prevalence, clinical features, risk factors and prognostic factors of BOS.
Materials and Methods
This retrospective study included patients who underwent allogeneic HSCT from January 2002 to December 2008 and survived for ≥100 days after transplantation.
Results
Of 860 patients who survived for ≥100 days, 36 (4.2%) met the diagnostic criteria. The duration of BOS development after transplantation was 466.00 (284.00–642.75) [median (interquartile range)] days. The risk factor for the development of BOS was peripheral blood as the stem cell source with a hazard ratio (HR) of 2.550 [95% confidence interval (CI): 1.274–5.104, p=0.008]. In multivariate analysis, pretransplant FEV1/FVC (HR: 0.956, 95% CI: 0.921–0.993, p=0.020) and time from HSCT to diagnosis of BOS (HR: 0.997, 95% CI: 0.994–0.999, p=0.009) were independent prognostic factors associated with mortality.
Conclusion
Peripheral blood as a stem cell source is a risk factor for the development of BOS. A decreased pretransplant FEV1/FVC and shorter duration of time from transplantation to diagnosis of BOS are poor prognostic factors for BOS.
Keywords: Bronchiolitis obliterans syndrome, hematopoietic stem cell transplantation, risk factor, prognosis
INTRODUCTION
Bronchiolitis obliterans syndrome (BOS) is a chronic graft-versus-host disease (cGVHD) of the lung that develops after allogeneic hematopoietic stem cell transplantation (allo-HSCT). BOS is diagnosed either histologically or clinically by demonstrating a new-onset airflow obstruction on a pulmonary function test (PFT) or mosaic patterns that forecast air trapping on high resolution computed tomographic images.1,2,3
BOS was historically thought to be a rare disease. However, various recent reports have shown that the prevalence of BOS is actually higher than previously reported and that BOS causes a deterioration of affected patients' quality of life and is associated with a higher mortality rate.2 Thus, the achievement of an early stage diagnosis by making meticulous observations of patients with a risk factor for BOS and identification of an effective treatment modality are becoming increasingly imperative. Allo-HSCT and cGVHD are well-known risk factors for BOS.4 These conditions are included in the diagnostic criteria for BOS and it may not be appropriate to consider them again as other risk factors.1,2,3,5 Other risk factors are peripheral blood-derived stem cells, busulfan-based conditioning regimens, a >14-month interval from diagnosis of leukemia to transplantation, female donor to male recipient transplantation, prior interstitial pneumonitis and an episode of moderate-to-severe acute GVHD (aGVHD).5 However, previous studies have shown different results regarding these risk factors. Furthermore, studies reported before 2005 did not use consistent diagnostic criteria, therefore, the acceptance of those results is limited.6,7,8,9,10,11,12,13,14
The goal of the present study included analysis of the prevalence, clinical features, risk factors and prognostic factors of BOS using modified National Institutes of Health (NIH) criteria1 in patients who had undergone allo-HSCT at Yeouido St. Mary's Hospital, Seoul, Republic of Korea.
MATERIALS AND METHODS
Subjects
This investigation involved a retrospective review of the medical records of patients who survived for ≥100 days after their first transplantation among those who had undergone allo-HSCT at Yeouido St. Mary's Hospital, Seoul, Republic of Korea from January 2002 to December 2008. Clinical data associated with allo-HSCT of all enrolled patients were collected. Approval was obtained from the Institutional Review Board of Seoul St. Mary's Hospital. The requirement for informed consent was waived by the ethical review board.
The diagnostic criteria for BOS were as follows: 1) In patients who underwent lung biopsy, exhibition of fibrogenic deposition in the small airways or the bronchioles satisfied the diagnostic criteria for BOS, 2) In patients who did not undergo lung biopsy, presence of cGVHD in other organs and the following PFT diagnostic criteria of the modified NIH criteria1 were to be satisfied for a diagnosis of BOS: (1) forced expiratory volume in 1 s (FEV1) of <75% of predicted or decreased of the FEV1 by 10% in comparison to the pretransplant value, (2) FEV1/forced vital capacity (FVC) of <70%, (3) residual volume (RV) or RV/total lung capacity (TLC) >120% of predicted, and (4) evidence of air trapping on high-resolution computed tomography (HRCT). Patients who satisfied the diagnostic criteria but had not developed an active infectious disease were categorized as having BOS.
Pulmonary function testing
The results of the PFTs from Yeouido St. Mary's Hospital were obtained. Pretransplantation PFTs were routinely performed for all patients before performing allo-HSCT as a part of the protocol. Post-transplant PFT results were evaluated for patients with unexplained respiratory symptoms such as significant dyspnea on exertion, decreased exercise tolerance, and a persistent nonproductive cough for ≥100 days after undergoing HSCT.
Radiologic examination
HRCT scans during deep inspiration were obtained throughout the entire thorax with 1.5–2 mm thick axial sections at 1 cm intervals; they were reconstructed using a high spatial frequency algorithm. The tube current was 240 mA at a voltage of 120 kV. Expiratory scans were obtained at the levels of the aortic arch, midway between the aortic arch and the trachea carina, in the tracheal carina, midway between the tracheal carina and the right hemidiaphragm, and 1 cm above the right hemidiaphragm. The reading of HRCT was performed by one radiology specialist (Jung JI15,16,17,18,19,20). During the analysis of each CT examination, the inspiratory images were reviewed before the expiratory images. The expiratory HRCT images were assessed for the presence and lobar distribution of decreased lung attenuation. Mosaic patterns on HRCT indicative of air trapping satisfied the diagnostic criteria for BOS that concur with the modified NIH criteria for BOS. This study used the results of tests performed ≥100 days after transplantation.
Clinical variables
The stem cell source was classified into bone marrow, peripheral blood, or bone marrow plus peripheral blood. Three patients whose stem cell source was cord blood were excluded from analysis. Disease risk at the time of transplantation was classified into two groups. The criteria for high risk included 1) acute leukemia beyond the first remission, 2) chronic myelogenous leukemia beyond the first chronic phase, 3) high-risk myelodysplastic syndrome (International Prognostic Scoring System ≥intermediate-2), 4) multiple myeloma and lymphoma with chemoresistance, or 5) aplastic anemia with a history of immunosuppressive treatment and/or a longer duration of disease infliction (≥3 years) before the transplantation.21 The donor match status was determined by the donor-recipient's human leukocyte antigen (HLA)-A, -B, -C, and -DR status.
With respect to aGVHD, this study included patients in whom aGVHD had been demonstrated histologically or radiologically within 100 days after transplantation and those with aGVHD that was clinically distinctive and underwent treatment appropriate for the diagnosis.
The date of disease diagnosis was considered to be the date of diagnostic confirmation by bone marrow biopsy. The date of BOS diagnosis was considered to be the date of completion of all tests needed to fulfill the modified NIH criteria.
BOS stage
The stage of BOS was classified by FEV1 and maximal mid expiratory flow rate (MMEFR, FEF25–75).22 The BOS stages are classified as follows: BOS 0=FEV1 >90% of baseline and MMEFR >75% of baseline; BOS 0-p=FEV1 81% to 90% of baseline and/or MMEFR ≤75% of baseline; BOS 1=FEV1 66% to 80% of baseline; BOS 2=FEV1 51% to 65% of baseline; and BOS 3=FEV1 50% or less of baseline.
Statistical methods
All statistical analyses were performed using PASW Statistics, version 17 (SPSS Inc., Chicago, IL, USA). Differences between groups were assessed using the chi-squared test or Fisher's exact test for categorical variables and Student's t-test or the Mann-Whitney U test for continuous variables, as appropriate. Binary logistic regression was used to analyze risk factors for BOS. Paired t-tests were performed to compare changes in PFT results after allo-HSCT in patients with BOS. Correlation between days from transplantation to diagnosis of BOS and lung function at diagnosis was analyzed by Pearson's correlation test.
Analysis of overall survival in patients with BOS was based on the Kaplan-Meier method. Differences between survival curves were estimated using the log-rank test. Cox regression was used to identify variables with independent prognostic significance. p values of <0.05 were considered to indicate statistical significance.
RESULTS
Patients' characteristics
Of 976 patients who underwent their first allo-HSCT from January 2002 to December 2008, 113 died within 100 days after transplantation and were excluded from this study. Three patients whose stem cell source was cord blood were also excluded. The characteristics of the remaining 860 patients are listed in Table 1. The mean patient age was 35 years. Of all 860 patients, 560 (65%) received transplants from sibling donors and 738 (86%) received transplants from HLA full-matched donors.
Table 1. Clinical Characteristics of Patients with and without BOS.
| Clinical variable | BOS (n=36) | Non-BOS (n=824) | p value |
|---|---|---|---|
| Recipient age (yrs) | 37.00 (23.75–44.50) | 35.00 (26.00–43.00) | 0.779 |
| Donor age (yrs) | 36.00 (25.25–41.00) | 33.00 (26.00–42.00) | 0.769 |
| Recipient sex, male | 20 (56) | 455 (56) | 0.968 |
| Donor sex, male | 26 (72) | 512 (62) | 0.221 |
| Underlying disease | |||
| AML | 14 (39) | 338 (41) | 0.357 |
| ALL | 8 (22) | 173 (21) | |
| ABL | 1 (3) | 18 (2) | |
| CML | 4 (11) | 71 (9) | |
| MDS | 4 (11) | 62 (8) | |
| AA | 2 (6) | 118 (14) | |
| MM | 2 (6) | 26 (3) | |
| Lymphoma | 1 (3) | 4 (1) | |
| Other | 0 (0) | 14 (2) | |
| Donor type | |||
| Sibling/unrelated | 23 (64)/13 (36) | 537 (65)/287 (35) | 0.875 |
| ABO | |||
| Compatibility/incompatibility | 16 (44)/20 (56) | 422 (51)/402 (49) | 0.426 |
| HLA | |||
| Full match/mismatch | 35 (97)/1 (3) | 703 (85)/121 (15) | 0.045 |
| Stem cell source | |||
| BM only/PB only/BM+PB | 17 (47)/17 (47)/2 (6) | 528 (64)/229 (28)/67 (8) | 0.041 |
| High risk at transplant | 11 (31) | 213 (26) | 0.532 |
| Conditioning regimens | |||
| Myeloablative/reduced intensity | 22 (61)/14 (39) | 599 (73)/225 (27) | 0.129 |
| TBI/non-TBI | 23 (64)/13 (36) | 570 (69)/254 (31) | 0.502 |
| Busulfan-based/other | 10 (28)/26 (72) | 207 (25)/617 (75) | 0.719 |
| GVHD prophylaxis | |||
| CS-based/tacrolimus-based | 24 (67)/12 (33) | 492 (60)/325 (40) | 0.439 |
| MTX-based/other | 34 (94)/2 (6) | 776 (94)/48 (6) | 1.000 |
| Acute GVHD | 14 (39) | 280 (34) | 0.561 |
| Time from diagnosis to transplant, days | 189.50 (140.25–399.25) | 178.00 (137.00–297.00) | 0.748 |
BOS, bronchiolitis obliterans syndrome; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; ABL, acute biphenotypic leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; AA, aplastic anemia; MM, multiple myeloma; ABO, ABO blood group; HLA, human leukocyte antigen; BM, bone marrow; PB, peripheral blood; TBI, total body irradiation; CS, cyclosporine; MTX, methotrexate; GVHD, graft-versus-host disease.
Data are expressed as median (interquartile range) or numbers (%).
The overall prevalence of BOS among patients who survived for ≥100 days after transplantation was 4.2% (36/860). Two of thirty-six patients were diagnosed by lung biopsy. Patients were largely diagnosed from 300 to 700 days after transplantation.
The duration of time from transplantation to BOS diagnosis was 466.00 (284.00–642.75) [median (interquartile range)] days.
Risk factors for BOS
Table 1 and 2 show the results of the univariate analysis of risk factors for BOS. Many previously known risk factors were not significantly different between patients with and without BOS, including increasing recipient age (p=0.779), increasing donor age (p=0.769), female donor to male recipient (p=0.771), lower FEV1/FVC (p=0.406), aGVHD (p=0.561), myeloablative conditioning regimens (p=0.129), total body irradiation (p=0.502), busulfan-based conditioning regimens (p=0.719), and longer duration from diagnosis to transplantation (p=0.748). Only two factors were significantly different: HLA matching (p=0.045) and stem cell source (p=0.041). In the multivariate analysis, peripheral blood as the stem cell source was a significant risk factor for BOS with a hazard ratio (HR) of 2.550 [95% confidence interval (CI): 1.274–5.104, p=0.008] (Table 3).
Table 2. Pretransplant Pulmonary Function Test Results in Patients with and without BOS.
| Clinical variable | BOS (n=36) | Non-BOS (n=824) | p value |
|---|---|---|---|
| FVC, % predicted | 91.86±14.39 | 93.86±12.72 | 0.344 |
| FEV1, % predicted | 91.81±14.71 | 94.70±12.51 | 0.178 |
| FEV1/FVC (%) | 83.81±9.81 | 84.78±6.74 | 0.406 |
| TLC, % predicted | 97.81±12.12 | 98.63±11.23 | 0.668 |
| RV, % predicted | 119.97±33.22 | 117.18±29.46 | 0.580 |
| RV/TLC (%) | 33.36±11.86 | 30.76±7.69 | 0.053 |
| DLCO, % predicted | 77.00±16.78 | 78.52±16.21 | 0.583 |
BOS, bronchiolitis obliterans syndrome; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; TLC, total lung capacity; RV; residual volume; DLCO, carbon monoxide diffusion in the lung.
Data are expressed as means±SD.
Table 3. Logistic Regression for Risk Factors of BOS.
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| HLA | ||
| Mismatch | Reference | |
| Match | 7.044 (0.950–52.208) | 0.056 |
| SC source | ||
| BM only | Reference | |
| PB only | 2.550 (1.274–5.104) | 0.008 |
| BM+PB | 0.972 (0.219–4.313) | 0.970 |
BOS, bronchiolitis obliterans syndrome; CI, confidence interval; HLA, human leukocyte antigen; SC, stem cell; BM, bone marrow; PB, peripheral blood.
Goodness of fit (Hosmer-Lemeshow) chi-squared p value=0.516.
Clinical characteristics of BOS
The changes between the pretransplant PFT results and PFT results at the time of BOS diagnosis are summarized in Table 4. PFT variables such as FVC, FEV1, FEV1/FVC, and carbon dioxide diffusion in the lung (DLCO) decreased significantly (p<0.001). RV and the ratio of RV to TLC (RV/TLC), which are markers of air trapping, increased significantly (p<0.001). Most patients were diagnosed with BOS in an advanced state. Among 36 BOS patients, 2 (5.6%) patients were stage 0, 4 (11.1%) patients were stage 1, 4 patients (11.1%) were stage 2, and 26 (72.2%) patients were stage 3. There was no significant correlation between days from transplantation to diagnosis of BOS and FEV1 (%) at diagnosis of BOS.
Table 4. Changes in Pulmonary Function at Diagnosis of BOS.
| Variable | Pretransplant | At diagnosis | p value |
|---|---|---|---|
| FVC, % predicted | 91.86±14.39 | 61.58±16.09 | <0.001 |
| FEV1, % predicted | 91.81±14.71 | 40.25±21.61 | <0.001 |
| FEV1/FVC, % | 83.81±9.81 | 53.17±22.63 | <0.001 |
| TLC, % predicted | 97.81±12.12 | 93.45±16.16 | 0.060 |
| RV, % predicted | 119.97±33.22 | 179.33±55.02 | <0.001 |
| RV/TLC, % | 33.36±11.86 | 52.36±10.49 | <0.001 |
| DLCO, % predicted | 77.00±16.78 | 53.35±20.59 | <0.001 |
BOS, bronchiolitis obliterans syndrome; DLCO, carbon monoxide diffusion in the lung; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; RV, residual volume; TLC, total lung capacity.
Survival analysis of BOS
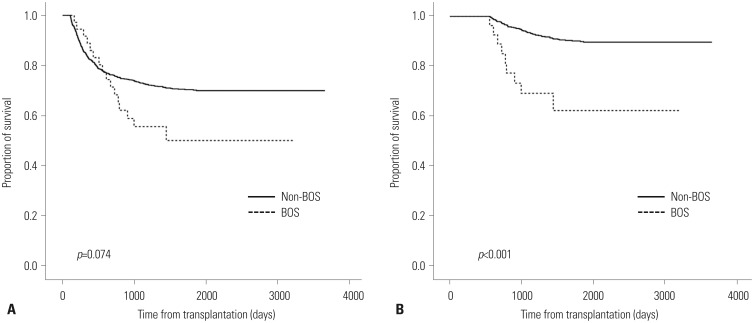
Patients with BOS were treated mainly by immunosuppressive agent (systemic corticosteroids, tacrolimus, cyclosporine, and mycophenolate mofetil). For patients with severe dyspnea symptom, inhaled corticosteroid plus long acting bronchodilator was added. Sixteen of the thirty-six patients who developed BOS died. Eleven of these sixteen patients died of BOS-associated causes. There was no significant difference in the survival rate between patients with and without BOS (p=0.074) (Fig. 1A). However, the survival curve was crossed on day 554 after transplantation. A significant difference in survival was observed in the subgroup analysis of patients who survived for >554 days (p<0.001) (Fig. 1B). Patients with BOS showed a continuous occurrence of death compared with patients without BOS. Nevertheless, a plateau was observed in both groups. Interestingly, the plateau occurred earlier in patients with than without BOS (day 1440 vs. day 1862, BOS vs. non-BOS, respectively).
Fig. 1. Overall survival (OS) curves of patients with and without bronchiolitis obliterans syndrome (BOS). OS of patients who survived for (A) >100 days and (B) >554 days after HSCT. HSCT, hematopoietic stem cell transplantation.
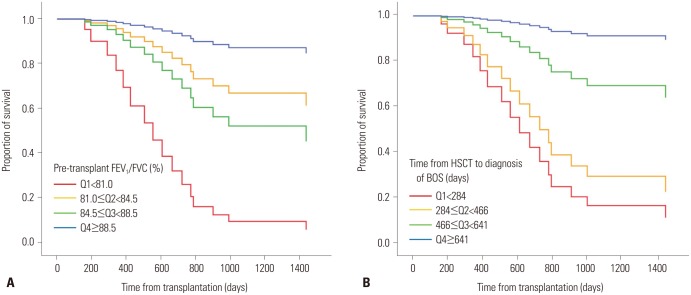
Univariate and multivariate analyses were performed to identify prognostic factors associated with the survival of the 36 patients with BOS. In univariate analysis, neither BOS severity nor FEV1/FVC at diagnosis of BOS were not significant factors associated with survival. The significant risk factors associated with mortality in the univariate analysis were the decreased pretransplant FEV1/FVC and shorter duration of time from transplantation to diagnosis of BOS. In multivariate analysis, decreased pretransplant FEV1/FVC (HR: 0.956, 95% CI: 0.921–0.993, p=0.020) and shorter duration of time from HSCT to diagnosis of BOS (HR: 0.997, 95% CI: 0.994–0.999, p=0.009) were also independent prognostic factors associated with mortality. Fig. 2 shows the survival curve according to the factors. When adjusted for the time from transplantation to diagnosis of BOS, the 3-year survival rate according to the pretransplant FEV1/FVC was 87.4% (fourth quartile; ≥88.5%), 52.2% (third quartile; 84.5–88.5%), 67.1% (second quartile; 81.0–84.5%), and 9.8% (first quartile; <81.0%). Compared with the highest quartile of the pretransplant FEV1/FVC group, the lowest quartile group had an HR of 17.346 (95% CI: 2.593–116.019; p=0.003). When adjusted for the pretransplant FEV1/FVC, the 3-year survival rate according to the time from transplantation to diagnosis of BOS was 91.5% (fourth quartile; ≥641 days), 69.8% (third quartile; 466–641 days), 29.9% (second quartile, 284–466 days), and 16.8% (first quartile; <284 days). Compared with the highest quartile of the time from transplantation to diagnosis of BOS, the lowest quartile had an HR of 19.658 (95% CI: 2.068–186.828; p=0.010) (Table 5).
Fig. 2. Survival curve for patients with bronchiolitis obliterans syndrome (BOS) according to prognostic factors (A: according to pretransplant FEV1/FVC adjusted for duration of time from HSCT to diagnosis of BOS, B: according to time from HSCT to diagnosis of BOS adjusted for pretransplant FEV1/FVC). Q, quartile; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HSCT, hematopoietic stem cell transplantation.
Table 5. Cox Regression for Mortality in Patients with BOS.
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| Continuous variables | ||
| Pre-transplant FEV1/FVC (%) | 0.956 (0.921–0.993) | 0.020 |
| Time from HSCT to diagnosis of BOS (days) | 0.997 (0.994–0.999) | 0.009 |
| Categorical variables | ||
| Pretransplant FEV1/FVC (%) | ||
| Q4 (≥88.5) | Reference | |
| Q3 (84.5–88.5) | 4.750 (0.761–29.659) | 0.095 |
| Q2 (81.0–84.5) | 2.933 (0.533–16.143) | 0.216 |
| Q1 (<81.0) | 17.346 (2.593–116.019) | 0.003 |
| Time from HSCT to diagnosis of BOS (days) | ||
| Q4 (≥641) | Reference | |
| Q3 (466–641) | 3.991 (0.444–35.851) | 0.217 |
| Q2 (284–466) | 13.342 (1.408–126.417) | 0.024 |
| Q1 (<284) | 19.658 (2.068–186.828) | 0.010 |
BOS, bronchiolitis obliterans syndrome; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HSCT, hematopoietic stem cell transplantation; Q, quartile.
DISCUSSION
In this study, we analyzed the prevalence, risk factors, clinical features, and survival-associated factors of BOS among patients who satisfied the modified NIH criteria1 proposed in 2009.
The prevalence of BOS among patients who survived for ≥100 days after allo-HSCT (n=860) was 4.2% in this study. This result is compatible with that in a previous report by Au, et al.,23 which showed that the overall prevalence of BOS for all patients undergoing allo-HSCT (n=1145) was 5.5%. Both studies used modified NIH criteria1 to define BOS. The small difference in prevalence may have been caused by the use of different PFT strategies. In their study, PFTs were performed regardless of the presence of symptoms 1 year after transplantation. In our center, however, PFTs were performed only in patients with clinical symptoms which led to the exclusion of clinically asymptomatic patients with BOS. Thus, the prevalence rate of BOS in the present investigation should represent that of relatively advanced, symptomatic post-transplantation BOS. Actually, most patients were diagnosed in the advanced stage of BOS (stage 3) in this study. Compared with previous study,23 lung function of patients in this study is lower. Mean FEV1 (%) in this study was 40.25%, while median FEV1 (%) was 59% in previous study. Interestingly in this study, however, there was no significant correlation between days from transplantation to diagnosis of BOS and FEV1 (%) at diagnosis of BOS.
We compared the results of PFTs (spirometry, lung volume, and DLCO) before and after diagnosis of BOS. This comparison was possible because we routinely perform PFTs, including lung volume and DLCO, before HSCT. The FVC, FEV1, and FEV1/FVC were significantly lower after than before HSCT. Increases in the RV and RV/TLC were also observed. These results correspond to the development of obstructive lung disease. The PFT results at the time of diagnosis of BOS in this study showed a pattern similar to that in the above-mentioned study by Au, et al.23 However, much higher degree of air trapping was observed in this study. This may have been due to the fact that we did not routinely perform PFTs after transplantation. Thus, patients with early mild symptomatic BOS may have been missed or their diagnosis may have been delayed until the advanced stage.
Many previously reported risk factors for BOS4,5 were not found to be statistically significant in this study. Only peripheral blood as the stem cell source was a significant risk factor for the development of BOS in this study. The reason for this discrepancy remains unclear.
Two previous studies reported that peripheral blood as the stem cell source is a risk factor for BOS.5,24 Although the reason for this has not been elucidated, cGVHD is more common in patients who have undergone peripheral blood stem cell transplantation than in patients who have undergone bone marrow transplantation. BOS, which is one type of cGVHD, is thus believed to be more common in patients who have undergone peripheral blood stem cell transplantation.
In previous reports, cGVHD was analyzed as a potential risk factor for BOS. However, the present study applied stringent diagnostic standards for BOS. Patients without cGVHD in other organs were excluded. Thus, we did not analyze cGVHD as a risk factor for BOS because it was already included in the diagnostic criteria.
A low FEV1/FVC, which denotes pretransplant airflow obstruction, has been reported as a risk factor for airway obstruction after transplantation.11,13 However, Au, et al.23 reported that a lower baseline FEV1/FVC is not a significant risk factor for the development of BOS. In the present study, we also found no significant relationship between the pretransplant PFT findings and development of BOS; rather, the pretransplant FEV1/FVC was a significant prognostic factor for BOS. Further studies regarding the effect of pretransplant PFT findings on the development and prognosis of BOS are mandatory.
No difference was observed in the overall survival rate between patients with and without BOS (p=0.074). However, the overall survival rate of patients with BOS 554 days after transplantation was significantly lower than that in patients without BOS (p<0.001). This suggests that, ultimately the prognosis after 554 days was poorer in patients with than without BOS. A poorer prognosis among patients with than without BOS has been reported.23,24 Additionally, cGVHD is generally the leading cause of long-term mortality following allogenic HSCT.25,26,27 The implication of these findings is that BOS, which is a type of cGVHD, may increase the long-term mortality rate.
In this study, a decreased pretransplant FEV1/FVC and shorter duration from transplantation to diagnosis of BOS were poor prognostic factors in patients with BOS. A poor prognosis of early onset BOS was also reported in lung transplant recipients.28,29 Our result is compatible with those of previous studies. Unfortunately, a few studies reported shorter duration from transplantation to diagnosis of BOS as a poor prognostic factor in HSCT recipients. The reason for the poor prognosis of early onset BOS has not been elucidated, and further studies are necessary. A poor prognosis of early onset BOS implies the need for early diagnosis through continuous PFTs as well as early and intensive intervention. To our knowledge, this is the first report to show that a low pretransplant FEV1/FVC is a poor prognostic factor for BOS. BOS is a cGVHD with airflow obstruction as the principle mechanism. Thus, FEV1/FVC, which is marker of airway obstruction, may be related to a poor prognosis. Further studies are needed to explain our result.
This study had two limitations. First, it was a retrospective study of medical records. Thus, possible selection bias may have occurred and some data may have been missing. However, we analyzed all consecutive transplantation patients without selection. Thus, there was little selection bias. Moreover, HSCT is performed according to a standardized protocol in our center. All information regarding transplantation was prospectively collected and routinely stored. Thus, there was little chance of missing data despite that fact that this was a retrospective review. Second, evaluations for BOS were performed only in symptomatic patients. Thus, unrecognized and undiagnosed cases of BOS may have been missed. Patients with BOS characterized by a mild degree of airflow obstruction may not have been identified. Further studies using routine PFT follow-up after transplantation regardless of symptoms are needed to overcome this limitation.
In conclusion, peripheral blood as a stem cell source is a risk factor for the development of BOS. A decreased pretransplant FEV1/FVC and shorter duration from transplantation to diagnosis of BOS are poor prognostic factors for BOS.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S106–S114. doi: 10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien JW. Preventing and managing bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplantation. Expert Rev Respir Med. 2011;5:127–135. doi: 10.1586/ers.10.79. [DOI] [PubMed] [Google Scholar]
- 4.Pandya CM, Soubani AO. Bronchiolitis obliterans following hematopoietic stem cell transplantation: a clinical update. Clin Transplant. 2010;24:291–306. doi: 10.1111/j.1399-0012.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- 5.Santo Tomas LH, Loberiza FR, Jr, Klein JP, Layde PM, Lipchik RJ, Rizzo JD, et al. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005;128:153–161. doi: 10.1378/chest.128.1.153. [DOI] [PubMed] [Google Scholar]
- 6.Clark JG, Schwartz DA, Flournoy N, Sullivan KM, Crawford SW, Thomas ED. Risk factors for airflow obstruction in recipients of bone marrow transplants. Ann Intern Med. 1987;107:648–656. doi: 10.7326/0003-4819-107-5-648. [DOI] [PubMed] [Google Scholar]
- 7.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood. 1988;72:621–627. [PubMed] [Google Scholar]
- 8.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation. Clinical presentation and course. Ann Intern Med. 1989;111:368–376. doi: 10.7326/0003-4819-111-5-368. [DOI] [PubMed] [Google Scholar]
- 9.Yokoi T, Hirabayashi N, Ito M, Uno Y, Tsuzuki T, Yatabe Y, et al. Broncho-bronchiolitis obliterans as a complication of bone marrow transplantation: a clinicopathological study of eight autopsy cases. Nagoya BMT Group. Virchows Arch. 1997;431:275–282. doi: 10.1007/s004280050099. [DOI] [PubMed] [Google Scholar]
- 10.Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28:425–434. doi: 10.1038/sj.bmt.1703142. [DOI] [PubMed] [Google Scholar]
- 11.Chien JW, Martin PJ, Gooley TA, Flowers ME, Heckbert SR, Nichols WG, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 12.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 13.Chien JW, Martin PJ, Flowers ME, Nichols WG, Clark JG. Implications of early airflow decline after myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;33:759–764. doi: 10.1038/sj.bmt.1704422. [DOI] [PubMed] [Google Scholar]
- 14.Yoshihara S, Tateishi U, Ando T, Kunitoh H, Suyama H, Onishi Y, et al. Lower incidence of Bronchiolitis obliterans in allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning compared with myeloablative conditioning. Bone Marrow Transplant. 2005;35:1195–1200. doi: 10.1038/sj.bmt.1704985. [DOI] [PubMed] [Google Scholar]
- 15.Choi MH, Jung JI, Chung WD, Kim YJ, Lee SE, Han DH, et al. Acute pulmonary complications in patients with hematologic malignancies. Radiographics. 2014;34:1755–1768. doi: 10.1148/rg.346130107. [DOI] [PubMed] [Google Scholar]
- 16.Jung JI, Jung WS, Hahn ST, Min CK, Kim CC, Park SH. Bronchiolitis obliterans after allogenic bone marrow transplantation: HRCT findings. Korean J Radiol. 2004;5:107–113. doi: 10.3348/kjr.2004.5.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh JK, Jung JI, Han DH, Ahn MI, Park SH, Cho BS, et al. Multidetector row computed tomography quantification of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation: a pilot study. J Thorac Imaging. 2013;28:114–120. doi: 10.1097/RTI.0b013e3182690b42. [DOI] [PubMed] [Google Scholar]
- 18.Kim TY, Jung JI, Kim YJ, Kim HW, Lee HG. CT and MRI evaluation of cardiac complications in patients with hematologic diseases: a pictorial review. Int J Cardiovasc Imaging. 2015;31(Suppl 2):159–167. doi: 10.1007/s10554-015-0610-5. [DOI] [PubMed] [Google Scholar]
- 19.Jung JI, Lee DG, Kim YJ, Yoon HK, Kim CC, Park SH. Pulmonary tuberculosis after hematopoietic stem cell transplantation: radiologic findings. J Thorac Imaging. 2009;24:10–16. doi: 10.1097/RTI.0b013e31818c6b97. [DOI] [PubMed] [Google Scholar]
- 20.Hwang SS, Kim HH, Park SH, Jung JI, Jang HS. The value of CTguided percutaneous needle aspiration in immunocompromised patients with suspected pulmonary infection. AJR Am J Roentgenol. 2000;175:235–238. doi: 10.2214/ajr.175.1.1750235. [DOI] [PubMed] [Google Scholar]
- 21.Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009;23:78–84. doi: 10.1038/leu.2008.276. [DOI] [PubMed] [Google Scholar]
- 22.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 23.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakaseko C, Ozawa S, Sakaida E, Sakai M, Kanda Y, Oshima K, et al. Incidence, risk factors and outcomes of bronchiolitis obliterans after allogeneic stem cell transplantation. Int J Hematol. 2011;93:375–382. doi: 10.1007/s12185-011-0809-8. [DOI] [PubMed] [Google Scholar]
- 25.Socié G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 26.Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin PJ, Counts GW, Jr, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato M, Ohmori-Matsuda K, Saito T, Matsuda Y, Hwang DM, Waddell TK, et al. Time-dependent changes in the risk of death in pure bronchiolitis obliterans syndrome (BOS) J Heart Lung Transplant. 2013;32:484–491. doi: 10.1016/j.healun.2013.01.1054. [DOI] [PubMed] [Google Scholar]
- 29.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med. 2010;182:784–789. doi: 10.1164/rccm.201002-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]