Abstract
Purpose
Japanese hop (Humulus spp.) and mugwort (Artemisia spp.) are notable causes of autumn pollinosis in East Asia. However, Japanese hop and mugwort pollen extracts, which are widely used for the diagnosis, have not been standardized. This study was performed to standardize Japanese hop and mugwort pollen extracts.
Materials and Methods
Allergen extracts were prepared in a standardized way using locally collected Humulus japonicus and purchased Artemisia vulgaris pollens. The immunoglobulin E (IgE) reactivities of prepared extracts were compared with commercial extracts via IgE immunoblotting and inhibition analyses. Intradermal skin tests were performed to determine the bioequivalent allergy unit (BAU).
Results
The IgE reactive components of the extracts via IgE immunoblotting were similar to those of commercial extracts. A 11-kDa allergen showed the strongest IgE reactivity in Japanese hop, as did a 28-kDa allergen in mugwort pollen extracts. Allergenic potencies of the investigatory Japanese hop and mugwort extracts were essentially indistinguishable from the commercial ones. Sums of erythema of 50 mm by the intradermal skin test (ΣED50) were calculated to be 14.4th and 13.6th three-fold dilutions for Japanese hop and mugwort extracts, respectively. Therefore, the allergenic activity of the prepared extracts was 90827.4 BAU/mg for Japanese hop and 34412 BAU/mg for mugwort.
Conclusion
We produced Japanese hop and mugwort pollen extracts using a standardized method. Standardized Japanese hop and mugwort pollen extracts will facilitate the production of improved diagnostic and immunotherapeutic reagents.
Keywords: Allergen standardization, mugwort, Japanese hop
INTRODUCTION
Japanese hop, Humulus japonicus, is a major cause of autumn pollinosis in East Asia, including China, Japan, and Korea.1 This highly allergenic plant is a fast-growing, prickly, invasive twining bine that smothers whatever it can climb. It is primarily a weed of pastures, hayfields, and other non-crop areas. This invasive species was added to the European and Mediterranean Plant Protection Organization (EPPO) Alert list in 2007 (www.eppo.int) and was also listed as an invasive alien plant in 2012 (www.invasive.org), as it began to invade European countries, Greenland, Canada, and the United States (Virginia, Tennessee, North Carolina, and West Virginia). This wind-pollinated plant produces a large amount of pollen,2 which is abundant in the atmosphere during the autumn.3,4 Thus, Japanese hop could be a major cause of pollinosis not only in East Asia but also in European and American countries in the near future unless its invasion is adequately controlled. In a study conducted on 812 patients visiting a specialized clinic in Seoul between 1984 and 1987, 15.9% were found to be sensitized to Japanese hop.5 Furthermore, the sensitization rate of Japanese hop in the southern part of Gyeonggi Province in Korea is reported to have increased gradually from 7.1% in 1999 to 8.0% in 2005 and then to 9.6% in 2008.6
Mugwort is another major cause of autumn pollinosis in Asia. In a multicenter study, 9.6% of 2554 Korean respiratory allergy subjects enrolled in 2001 were positive for mugwort allergy based on a skin prick test.7 However, in a recent study, a difference in the sensitivity to mugwort was reported between skin prick (13.6%) and ImmunoCAP (22.9%) tests, implying the need for further standardization for better diagnosis.8
Standardization of allergen extract is essential for the development of diagnostic and immunotherapy reagents for respiratory allergic diseases.9 However, many commercial pollen extracts are not standardized. Non-standardized extracts are labeled on the basis of protein nitrogen unit (PNU) or weight of source material per volume of buffer used for the allergen extraction (w/v). A target maintenance dose of 0.5 mL of 1:100 or 1:200 w/v was suggested for immunotherapy.10
Difference in allergen composition among commercial mugwort extracts have been reported11 despite descriptions of preparation using a reference extract.12 Geographical differences among plant flora may influence the sensitization profiles and allergic responses. Artemisia princeps is known to be the dominant species in Korea.13 However, immunoglobulin E (IgE) reactive components from six different species (A. vulgaris, A. scroparia, A. princeps, A. tridentate, A. anuua, and A. campestris) were found to be very similar and highly cross-reactive,14 a finding that was reproduced by Katial, et al.15 These results suggest that a pollen extract from a single species is sufficient for diagnosis and treatment of mugwort, sagebrush, and wormwood allergies. In Korea, most of the mugwort-sensitized subjects were found to be co-sensitized to chrysanthemum and dandelion, which belong to the family Compositae (Asteraceae), and extensive cross-allergenicity among them was also described.16
Allergen extracts have been standardized not only by determining biological potency of the extract but also by measuring the concentrations of major allergens.17 However, the major allergen of Japanese hop has not been characterized. Profilin was reported to be a major allergen of Japanese hop from China.18 However, this was not the case in Korea.19 In this study, we prepared the Japanese hop and mugwort pollen extracts using a standardized method and estimated the potency for use as a reference standard. Importantly, this was the first report on the bioequivalent allergy unit (BAU) of Japanese hop and mugwort pollen extract estimated via intradermal skin test.20
MATERIALS AND METHODS
Allergen extracts
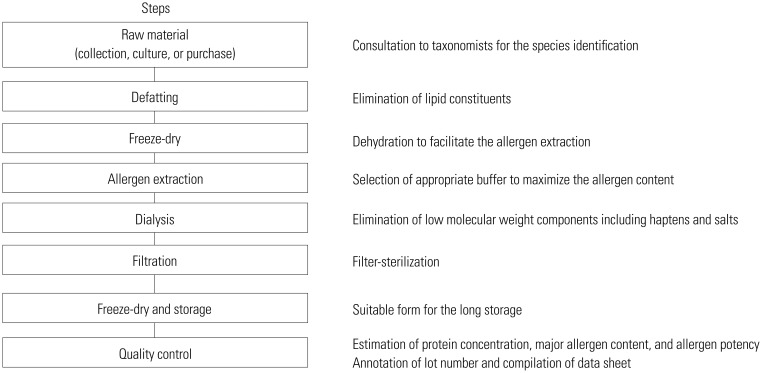
A. vulgaris pollen was purchased from Allergon (Angelholm, Sweden). Japanese hop pollen was collected from fields in Seoul in September 2011. Pollens were defatted three times with ethyl ether. For allergen extraction, 1:4 (w/v) phosphate buffered saline (pH 7.4) was added and stirred for 48 hours at 4℃. The extract was dialyzed (cutoff 3.5-kDa; Spectrum, Houston, TX, USA) extensively against distilled water. The sample was centrifuged, and the supernatant was then filtered (0.22 µm pore; Millipore, Bedford, MA, USA), lyophilized, and kept at -70℃ before use. Key steps for the preparation of pollen allergen extracts are summarized in Fig. 1. Commercial skin test reagent of H. japonicus was obtained from Allergopharma (Reinbeck, Germany). A commercial mugwort pollen extract (1:20 w/v; Hollister-Stier Laboratories, Spokane, WA, USA) was also purchased for in vitro comparison.
Fig. 1. Pollen allergen extraction procedure. Key steps and key points are summarized.
Subjects
The study was approved by the Institutional Review Board (4-2009-0717). Informed consent was obtained before skin testing and blood drawing. Twenty two Japanese hop-allergic subjects (age range, 19 to 62 years; mean 39 years) and 20 mugwort-allergic patients who visited the Allergy-Asthma Center at Severance Hospital in Seoul, Korea were enrolled for the in vivo standardization of Japanese hop and mugwort pollen extracts (Table 1 and 2). Inclusion criteria for the in vivo standardization were 1) apparent symptoms of rhinitis, such as rhinorrhea, sneezing, coughing, and itching of the eyes and nose during the pollen season and 2) a more than twofold increase in the wheal size of pollen extracts compared to histamine controls in the skin prick test. Intradermal skin tests were performed on the enrolled subjects.
Table 1. Clinical Features of the Enrolled Japanese Hop-Allergic Subjects.
| Subject | Gender/age | D50 | BAU | Symptom/diagnosis | Sensitization profile (ImmunoCAP or skin prick test) |
sIgE to w22 (class) |
Total IgE |
|---|---|---|---|---|---|---|---|
| HJ-001 | F/57 | 13.60 | 64439.4 | AR, AS | d2, w22 | >100 (6) | ND |
| HJ-002 | F/54 | ND | ND | AR, AS, CC, UT | d2, e5, w22 | >100 (6) | ND |
| HJ-003 | F/44 | ND | ND | AR, AS | d2, e1, w7, w22 | 95.5 (5) | 474 |
| HJ-004 | F/57 | 15.00 | 300000 | AR | d2, e5, w22, t3, t7 | 35.8 (5) | ND |
| HJ-005 | F/51 | 15.00 | 300000 | AC, AR, AS, OAS | d2, i6, e82, m5, t2, t3, t5, t7, w1, w7, w12, w22 | ND | 330 |
| HJ-006 | M/27 | 15.00 | 300000 | AE, OAS, UT | d2, w22, t2, t3, t5, t7, e1, w22 | 66.4 (5) | 335 |
| HJ-007 | F/25 | 13.20 | 41524.36 | AC, AR | w1, w6, w7, w22 | 42.6 (4) | 99.6 |
| HJ-008 | M/29 | 13.90 | 89595.85 | AC, AD, AR | d2, i6, w22 | 20.9 (4) | 192 |
| HJ-009 | M/50 | 12.30 | 15448.77 | AC, AR | w1, w22 | 31.4 (4) | ND |
| HJ-010 | M/59 | 14.35 | 146890.1 | AR | w6, w7, w22, t2, t3, t7 | 16.9 (3) | ND |
| HJ-011 | M/26 | 14.6 | 193318.2 | AR | d2, w6, w22 | 2.06 (2) | ND |
| HJ-012 | F/54 | ND | ND | AR | w9, w22, e6, e82 | ND | ND |
| HJ-013 | F/29 | 17.2 | 3363474 | AC, AD, CD, AR | d2, e1, e5, m3, m81, w1, w22, t3, t7 | 56.3 (5) | 2131 |
| HJ-014 | F/31 | 14.6 | 373719.3 | AC, AR | w1, w6, w8, w9, w10, w22, g2, g3, g4, g5, g6, t2, t3, t7, t8, t10, t11, t12, t13, t15, t19, t213 | ND | ND |
| HJ-015 | M/50 | 16.3 | 1251350 | AS, AR | d2, w22, t3, t7 | 13.9 (3) | ND |
| HJ-016 | M/19 | 14.8 | 240822.5 | AR, AC, OAS | d1, d2, w6, w22, g6, t3, t7 | 4.58 (3) | 968 |
| HJ-017 | M/19 | ND | ND | AR, AC | d1, d2, m1, w1, d5, w9, w10, w19, w22, g2, g3, g5, g6, t2, t3, t7, t8, t10, t11, t12, t15, t17, t19, t70, t213 | ND | ND |
| HJ-018 | M/23 | ND | ND | AR | w5, w8, w19, w22 | ND | ND |
| HJ-019 | M/37 | ND | ND | AR, AC, UT | d1, d2, e2, w22 | 19.2 (4) | ND |
| HJ-020 | F/31 | 14.6 | 193318.2 | AR, AS | d1, d2, w22 | 5.80 (3) | 99.3 |
| HJ-021 | F/66 | ND | ND | AR, AS | d1, d2, w1, w6, w22 | 8.74 (3) | 38.4 |
| HJ-022 | F/29 | 12.4 | 17242.73 | AR, AS, CC, UT | d1, d2, d72, e2, e5, w9, w22, t8, t10, t12, tx | ND | ND |
| Mean | 14.4 | 459409.5 |
AC, allergic conjunctivitis; AD, atopic dermatitis; AE, angioedema; AR, allergic rhinitis; AS, asthma; CC, chronic cough; CD, contact dermatitis; OAS, oral allergy syndrome; UT, urticaria; BAU, bioequivalent allergen unit; IgE, immunoglobulin E.
d1, Dermatophagoides pteronyssinus; d2, D. farinae; d72, Tyrophagus putrescentiae; i6, German cockroach; e2, dog hair; e5, dog dander; e6, Guinea pig epithelium; e82, rabbit epithelium; m3, Aspergilus fumigates; m5, Candida albicans; w1, ragweed; w6, mugwort; w7, Chrysanthemum leucanthemum; w8, dandelion; w9, plantain; w10, goosefoot; w12, goldenrod; w22, Japanese hop; wx, weed mix; g2, Bermuda grass; g3, cocksfoot; g4, meadow fescue; g5, rye-grass; g6, timothy grass; t2, alder; t3, common silver birch; t5, beech; t7, white oak; t8, elm; t10, walnut; t11, platanus; t12, willow; t13, mulberry; t15, white ash; t17, Japanese cedar; t19, acacia; t213, pine; tx, tree mix.
Table 2. Clinical Features of Enrolled Mugwort-Allergic Subjects.
| Subjects | Gender/age | D50 | BAU | Symptom/diagnosis | Sensitization profile (ImmunoCAP or skin prick test) |
sIgE to w6 (class) |
Total IgE |
|---|---|---|---|---|---|---|---|
| AV-001 | F/28 | 11.7 | 30077.65 | AR | t2, t3, t7, t8, t10, t11, t12, t70, t14, t16, g8, w1, w8, w10, w22, w21, d72, d1, d2, e1, e5 | ND | ND |
| AV-002 | M/45 | 10.3 | 7417.058 | AR | t7, t11, t12, t70, w1, w6, w7, w8, w10, w12, m1, d1, i6 | 8.45 (3) | ND |
| AV-003 | F/43 | 15 | 815484.5 | AR, AC | w1, w6, w8 | ND | ND |
| AV-004 | M/45 | 13.6 | 201096 | AR, AS, drug allergy | t5, t7, t8, t10, t11, t19, t16, g8, g5, g12, g6, w1, w6, w7, w8, w10, w12, w9, w22, m1, m2, m5, d72, d1, d2, e1, e5, e3, e84, e73, i206 | 5.65 (3) | ND |
| AV-005 | M/20 | 12 | 40600.58 | AR | t2, t3, t5, t7, w1, w6, w7, w8, w12, m3, d72, d1, d2 | 0.61 (1) | 284 |
| AV-006 | M/52 | ND | ND | AR, OAS | t1, t2, t3, t7, t8, t10, t11, t12, t70, t11, t16, t6, g6, w1, w6, w8, w10, w22, m1, e1, e5, e3 | ND | ND |
| AV-007 | M/46 | 14.9 | 737880.9 | AR | t2, t3, t7, t8, t10, t12, t70, t11, t15, t16, w6, w8, w9, w22, m1 | ND | ND |
| AV-008 | F/58 | ND | ND | AR, AC | t3, w1, w6, w22, d2, e5 | 25.5 (4) | 784 |
| AV-009 | F/46 | 12 | 40600.58 | AR, AS, drug allergy | w1, w6, w8 | ND | ND |
| AV-010 | M/19 | 14 | 300000 | AR, AC, OAS | t1, t2, t3, t7, t8, t10, t70, t15, t19, t16, g8, g2, g3, g6, w6, w8, w22, d72, d1, d2, e1, e5, i6, i206 | 4.81 (3) | 968 |
| AV-011 | M/34 | 14.9 | 737880.9 | AR, AC | t1, t2, t3, t7, t8, t10, t11, t12, t70, t11, t15, t19, t16, t16, t6, g8, g2, g3, g5, g6, w1, w6, w10, w9, w22, e1, e5, f11, d2 | ND | ND |
| AV-012 | M/19 | ND | ND | AR, AC | t1, t2, t3, t7, t8, t10, t11, t12, t70, t11, t15, t19, t14, t16, t6, g8, g2, g3, g5, g6, w1, w6, w10, w9, w22, w21, m1, m2, d1, d2, e3, i206, f11, f4 | ND | ND |
| AV-013 | F/53 | 13.3 | 148975.6 | AR, AS, drug allergy | t10, t16, g6, w6, w8, w22, w21, m1, m2, m3, m5, m6, d72, d1, d2, e1, e5, e3, e4, e6, i6, i206 | 3.66 (3) | ND |
| AV-014 | M/41 | 14.4 | 447547.4 | AR | t1, g6, w6, w8, d1 | ND | ND |
| AV-015 | F/37 | 14.6 | 546635.6 | AR | t10, w1, w6, w8, w10, m2, d1, d2, e5 | ND | ND |
| AV-016 | M/23 | ND | ND | AR | w6, w8, w22, w21, m1 | ND | ND |
| AV-017 | F/20 | ND | ND | AR, chronic urticaria, drug allergy | w6, w8 | ND | ND |
| AV-018 | M/42 | 15.6 | 1485910 | AR | t1, g2, w1, w6, w8, w10, w22, d72, d1, d2, i6 | ND | ND |
| AV-019 | F/37 | 12.6 | 73979.09 | AR | w1, w6, w8, w10, w9, d1, d2, e1, e5, i6 | ND | ND |
| AV-020 | M/38 | 13 | 110363.8 | AR, AS, AD | t6, w6, w8, d1, d2, e1, e5 | ND | ND |
| Mean | 13.55 | 60691.3 |
AR, allergic rhinitis; AS, asthma; AC, allergic conjunctivitis; OAS, oral allergy syndrome; BAU, bioequivalent allergen unit; IgE, immunoglobulin E.
t1, acer; t2, alder; t3, birch; t5, beech; t7, oak; t8, elm; t10, walnut tree; t11, elder; t12, willow; t14, poplar; t16, pine; t19, acacia; t70, mulberry; g2, Bermuda grass; g3, cocksfoot; g5, rye-grass; g6, timothy grass; g8, meadow grass; g12, cultivated rye; w1, ragweed; w6, mugwort; w8, dandelion; w9, plantain; w10, goosefoot; w21, wall pellitory; w22, Japanese hop; m1, Penicillium; m2, Cladosporium; m3, Aspergilus; m5, Candia; m6, Alternaria; d1, Dermatophagodes pteronyssinus; d2, D. farinae; d72, Tyrophagus putrescentiae; e1, cat dander; e3, horse dander; e4, cow dander; e5, dog dander; e6, Guinea pig epithelium; e73, rat epithelium; e84, hamster epithelium; i6, German cockroach; i206, American cockroach; f4, wheat; f11, buckwheat.
SDS-PAGE and IgE immunoblot analysis
The lyophilized extract was reconstituted in distilled water or buffer, and the protein concentration was determined via Bradford assay (Bio-Rad, Hercules, CA, USA). Thereafter, the extract was aliquoted and stored at -70℃ until use. The protein profile and IgE reactive components were examined by performing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. Lyophilized extracts were reconstituted in a solution containing 0.9% NaCl and 0.03% human serum albumin. Extracts (30 µg of protein each) were separated onto 15% gels under reducing conditions. Proteins were stained with Coomassie blue or transferred onto polyvinylidene difluoride (PVDF) membrane (0.45 µm, Millipore). Then, the membrane was incubated with 1:4 diluted sera (pooled serum from eight subjects or healthy controls). IgE antibodies were probed with alkaline phosphate-conjugated goat anti-human IgE (1:1000; Sigma-Aldrich, St. Louis, MO, USA) for 1 hour, and the color was developed using nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (Promega, Madison, WI, USA).
Evaluation of allergen potency via quantitative intradermal skin test
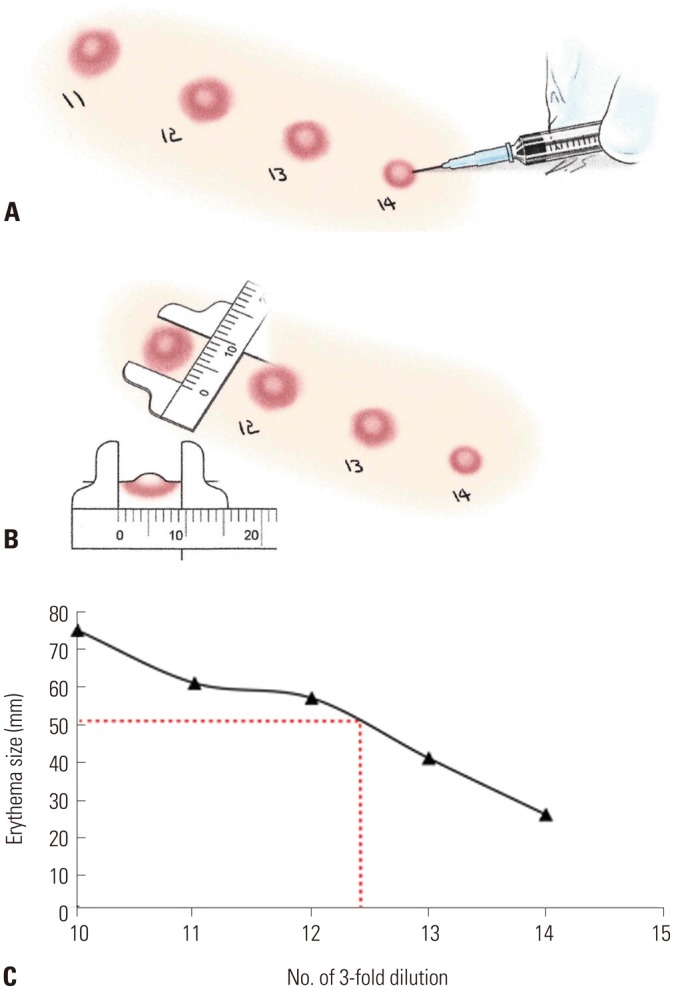
Procedure for the determination of allergen potency via intradermal skin test is illustrated in Fig. 2. Intradermal skin tests were performed to determine the potency BAU of the extract.20 Briefly, injections were made superficially with 50 µL of serial threefold dilutions of the extracts in 0.9% NaCl, 0.4% phenol, and 0.03% human serum albumin solution. At 15 minutes, the sum of the longest and midpoint orthogonal diameters of erythema (ΣE) in millimeters was recorded. The best-fit linear regression lines of the mean of triplicate ΣE vs. log3 dose were calculated for each individual tested in order to analyze the potency of the extract. An extract concentration with an erythema diameter sum of 50 mm of the 14th threefold dilution (ΣED50) was arbitrarily designated as 100000 BAU/mL.
Fig. 2. In vivo standardization. Serially diluted allergen extracts were intradermally injected in subjects (A), and erythema size was measured after 15 to 20 minutes (B). The allergen potency was determined by calculating the dilution that induced an erythema diameter sum of 50 mm (C).
RESULTS
Protein analysis of allergen extracts
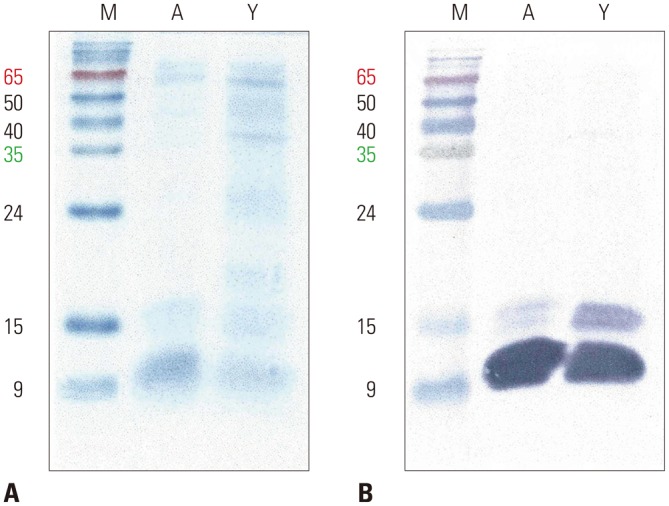
Both commercial and collected Japanese hop pollen extracts showed strong bands of 11- and 15-kDa proteins on SDS-PAGE analysis despite several differences for proteins of higher molecular weight (20–60-kDa) (Fig. 3). An allergen of 11-kDa from both commercial and collected extracts exhibited the strongest IgE reactivity in IgE immunoblot analysis. A protein of 15-kDa from our collected extract showed somewhat stronger IgE reactivity compared to that of the commercial extract.
Fig. 3. SDS-PAGE (A) and IgE immunoblot (B) analyses of Japanese hop pollen extracts. Twenty microlligrams of proteins were separated on 15% polyacrylamide gel under reducing conditions. IgE reactive proteins were probed with a pooled serum of Japanese hop-sensitized patients. M, molecular weight standards; A, commercial (allergopharma) Japanese hop extract; Y, our (Yonsei) collected Japanese hop extract. SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; IgE, immunoglobulin E.
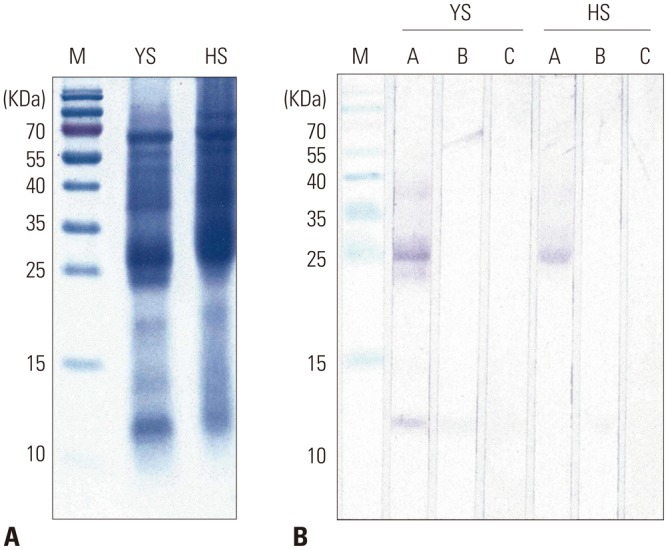
The pattern of protein bands on SDS-PAGE of our mugwort extract was similar to that of Hollister-Stier mugwort extract (Fig. 4A). A stronger band of about 28-kDa was shown from the commercial extract. However, a thicker band of 12-kDa protein was observed from our extract (Fig. 4B). In IgE immunoblotting, strong IgE reactivity was also detected at around 28-kDa from both extracts. However, a stronger IgE reaction to approximately 12- and 20-kDa allergens was shown only from our extract.
Fig. 4. SDS-PAGE (A) and IgE immunoblot (B) analyses of mugwort extracts. M, molecular mass marker; YS, Yonsei extract; HS, Hollister-Stier extract; A, mugwort-sensitized sera; B, non-atopic sera; C, buffer control.
Comparison of allergic activity of standardized Japanese hop pollen extract with commercial skin prick test extract
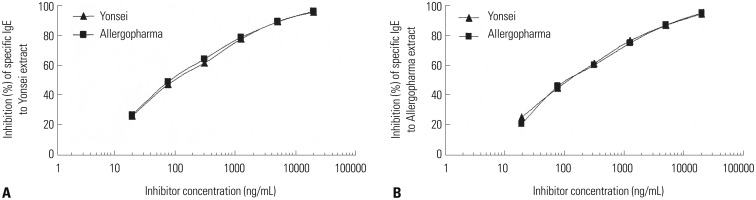
The biologic activity of our prepared extract was 88983.6 BAU/mg. Activity of the prepared skin prick extract was compared with that of commercial skin test reagent via inhibition ELISA (Fig. 5).
Fig. 5. ELISA inhibition analysis of Japanese hop extracts. IgE reactivity to our (Yonsei) collected (A) and commercial (Allergopharma) (B) extracts was inhibited at a various concentrations. ELISA, enzyme-linked immunosorbent assay.
Commercial skin prick test reagent inhibited our extract to a maximum of 96.0%, while our extract inhibited 95.7%. Our extract was able to inhibit the commercial skin prick extract to a maximum of 94.7%, while the commercial extract inhibited 95.2%. The allergic activities of both extracts were essentially indistinguishable.
Allergen potency of the prepared standardized Japanese hop pollen extract measured via quantitative intradermal skin test
Intradermal tests were performed using prepared standardized Japanese hop pollen extract on 15 patients who were highly sensitized to H. japonicus. ΣED50 was calculated to be a 14.4th dilution. Therefore, allergenic activity of the prepared standardized extract was 172572 BAU/mL (90827.4 BAU/mg) (Table 1).
Allergen potency of mugwort pollen extract
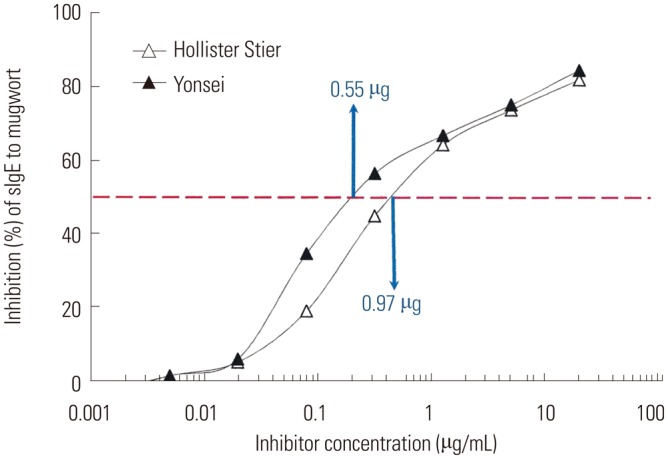
The protein concentration of our mugwort extract was 1.105 mg/mL, whereas that of Hollister-Stier extract was 0.22 mg/mL as determined via Bradford assay. ImmunoCAP inhibition was performed to compare the allergen potencies of the extracts. Hollister-Stier extract was able to inhibit IgE reactivity to a maximum of 82.0%, while our extract inhibited IgE reactivity up to 84.5% (Fig. 6). Inhibition curves from these two extracts were not parallel at the inhibitor concentration from about 0.02 to 1.25 µg/mL. Fifty inhibitory concentrations were determined to 0.97 µg/mL for Hollister-Stier extract and 0.55 µg/mL for our extract.
Fig. 6. In vitro standardization of mugwort extract. Competitive inhibition CAP was performed to estimate the potency of the extract using a pooled serum of eight subjects.
The average ΣED50 of investigated reference material determined from 15 highly allergic subjects was 13.6, and the allergen potency of the mugwort reference material was calculated to be 60691 BAU/mL (Table 2). Therefore, the allergen potency of Hollister-Stier extract should be about 34412 BAU/mg.
DISCUSSION
Standardization of allergen extracts is essential for the development of diagnostic and immunotherapeutic reagents. However, standardization of Japanese hop pollen extract has not been performed despite it being a major cause of autumn pollinosis in East Asia. Therefore, we produced reference material for Japanese hop extract and compared its allergenicity with a commercial extract, which is commonly used for diagnosis. Furthermore, potency of the allergen extract was determined via intradermal skin test for the first time. Commercial Japanese hop extract is provided in a PNU. One PNU is known to contain approximately 0.06 µg of protein.21 In this study, reference material for Japanese hop extract was determined to have 90827.4 BAU/mg (equivalent to 5.45 BAU/PNU if 1 PNU is 0.06 µg of protein) (Table 1), and commercial extract (its allergen potency was indistinguishable from our extract) is provided in a 5000 PNU/mL unit (Fig. 4). Consequently, it could be calculated that 1 PNU is equivalent to 6.58 BAU.
Mugwort is a common cause of pollinosis in autumn. However, non-standardized mugwort pollen extracts are still used for diagnosis and immunotherapy. In this study, allergen potency of the mugwort pollen extract, which was prepared in a standardized way was estimated via quantitative intradermal skin test and compared with a commercial extract.
Six allergens from mugwort have been described (www.allergen.org): Art v 1, a defensin-like protein (28-kDa); Art v 2, pathogenesis-related protein 1 (20-kDa); Art v 3, non-specific lipid transfer protein (12-kDa); Art v 4, profilin (14-kDa); Art v 5, polcalcin (10-kDa); and Art v 6, pectate lyase (44-kDa). In the extract, 28-kDa allergen, a putative Art v 1, exhibited strong IgE reactivity in both extracts on IgE immunoblotting (Fig. 4). Differences in IgE reactivity to 12-kDa (a putative Art v 3) and 20-kDa (a putative Art v 2) proteins may reflect the different concentrations of these allergens. Different allergen compositions also resulted in the unparalleled inhibition curve (Fig. 6). Quantification of major allergen content is helpful for better standardization of the allergen extracts.17 A major allergen was cloned, and monoclonal antibodies were developed for the quantification of Art v 1.22,23 However, two-site ELISA kits for the quantification of mugwort allergen are not commercially available.
In this study, the allergen potency of the standardized reference mugwort pollen extract was determined to be 60691 BAU/mL and to include 54924 BAU/mg of protein, which could be translated as 3.295 BAU/PNU.21 The potency of the commercial extract for immunotherapy (1:20 w/v, Hollister Stier) was calculated to be 34412 BAU/mL based on 50% inhibitory concentrations. The probable effective dose for the non-standardized mugwort pollen extracts was estimated to be 0.5 mL of 1:100 or 1:200 w/v.10 Therefore, approximately 3400 to 6800 BAU could be an effective dose for immunotherapy. Further studies are necessary to determine the optimal dose for effective immunotherapy.
Quantification of major allergen content is important for the standardization of allergen extract. Major allergen concentrations in the extract were found to be strongly correlated with the potency of the extract, though with significant differences in the major allergen concentration at equal allergen potency.17,24 Furthermore, 5–20 µg of major allergen is thought to be administered in a maintenance phase for effective immunotherapy. The concentration of the 15-kDa allergen, a putative profilin, was found to be different from those of the commercial and collected Japanese hop pollen extracts (Fig. 1). However, profilin is not a major allergen, as noted in our previous report.19 Thus, there is an urgent need to characterize the 11-kDa allergen for better standardization of Japanese hop pollen extract.
The allergen extract produced in this study could be used as a reference material for standardization. Its allergen potency (unit) determined via quantitative intradermal skin test could also contribute to better standardization.9,25 The newly acquired data obtained via in vivo standardization of Japanese hop and mugwort pollen extract could be helpful for the development of diagnostics and immunotherapeutics, especially for Japanese hop allergy.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korean Healthcare Technology R&D project, Ministry of Health and Welfare, Republic of Korea (A092076). The authors would like to thank Dong-Su Jang, MFA (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea), for his help with the illustrations.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Park HS, Coi SY, Nahm DH, Kim HY. Revival of Hop Japanese pollinosis in asthmatic subjects in Kyungki area. J Asthma Allergy Clin Immunol. 1998;18:52–60. [Google Scholar]
- 2.Weber RW. On the cover. Hop. Ann Allergy Asthma Immunol. 2008;100:A4. doi: 10.1016/s1081-1206(10)60437-1. [DOI] [PubMed] [Google Scholar]
- 3.Hong CS, Hwang Y, Oh SH, Kim HJ, Huh KB, Lee SY. Survey of the airborne pollens in Seoul, Korea. Yonsei Med J. 1986;27:114–120. doi: 10.3349/ymj.1986.27.2.114. [DOI] [PubMed] [Google Scholar]
- 4.Park HS, Nahm DH, Suh CH, Lee SM, Choi SY, Jung KS, et al. Evidence of Hop Japanese pollinosis in Korea: IgE sensitization and identification of allergenic components. J Allergy Clin Immunol. 1997;100:475–479. doi: 10.1016/s0091-6749(97)70138-6. [DOI] [PubMed] [Google Scholar]
- 5.Yoon YW, Lee MK, Park HS, Park SS, Hong CS. The skin test reactivity and the level of the total IgE in the allergic patients. Allergy. 1989;9:385–398. [Google Scholar]
- 6.Lee JW, Choi GS, Kim JE, Jin HJ, Kim JH, Ye YM, et al. Changes in sensitization rates to pollen allergens in allergic patients in the southern part of Gyeonggi province over the last 10 years. Korean J Asthma Allergy Clin Immunol. 2011;31:33–40. [Google Scholar]
- 7.Kim TB, Kim KM, Kim SH, Kang HR, Chang YS, Kim CW, et al. Sensitization rates for inhalant allergens in Korea; a multi-center study. J Asthma Allergy Clin Immunol. 2003;23:483–493. [Google Scholar]
- 8.Park HJ, Lee JH, Park KH, Ann HW, Jin MN, Choi SY, et al. A nationwide survey of inhalant allergens sensitization and levels of indoor major allergens in Korea. Allergy Asthma Immunol Res. 2014;6:222–227. doi: 10.4168/aair.2014.6.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011;52:393–400. doi: 10.3349/ymj.2011.52.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1–S55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Ingemann L, Formgren H, Løwenstein H, Ipsen H. The use of a reference allergenic extract in the evaluation of allergen products. Allergy. 1985;40:273–281. doi: 10.1111/j.1398-9995.1985.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 12.Ipsen H, Formgren H, Løwenstein H, Ingemann L. Immunochemical and biological characterization of a mugwort (Artemisia vulgaris) pollen extract. Allergy. 1985;40:289–294. doi: 10.1111/j.1398-9995.1985.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 13.Park HS, Hong CS, Choi HJ, Hahm KS. Identification and partial purification of pollen allergens from Artemisia princeps. Yonsei Med J. 1989;30:346–354. doi: 10.3349/ymj.1989.30.4.346. [DOI] [PubMed] [Google Scholar]
- 14.Brandys J, Grimsøen A, Nilsen BM, Paulsen BS, Park HS, Hong CS. Cross-reactivity between pollen extracts from six Artemisia species. Planta Med. 1993;59:221–228. doi: 10.1055/s-2006-959656. [DOI] [PubMed] [Google Scholar]
- 15.Katial RK, Lin FL, Stafford WW, Ledoux RA, Westley CR, Weber RW. Mugwort and sage (Artemisia) pollen cross-reactivity: ELISA inhibition and immunoblot evaluation. Ann Allergy Asthma Immunol. 1997;79:340–346. doi: 10.1016/s1081-1206(10)63025-6. [DOI] [PubMed] [Google Scholar]
- 16.Lee YW, Choi SY, Lee EK, Sohn JH, Park JW, Hong CS. Cross-allergenicity of pollens from the Compositae family: Artemisia vulgaris, Dendranthema grandiflorum, and Taraxacum officinale. Ann Allergy Asthma Immunol. 2007;99:526–533. doi: 10.1016/S1081-1206(10)60382-1. [DOI] [PubMed] [Google Scholar]
- 17.van Ree R. Indoor allergens: relevance of major allergen measurements and standardization. J Allergy Clin Immunol. 2007;119:270–277. doi: 10.1016/j.jaci.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Tao AL, He SH. Cloning, expression, and characterization of pollen allergens from Humulus scandens (Lour) Merr and Ambrosia artemisiifolia L. Acta Pharmacol Sin. 2005;26:1225–1232. doi: 10.1111/j.1745-7254.2005.00194.x. [DOI] [PubMed] [Google Scholar]
- 19.Jeong KY, Han IS, Choi SY, Lee JH, Lee JS, Hong CS, et al. Allergenicity of recombinant profilins from Japanese hop, Humulus japonicus. J Investig Allergol Clin Immunol. 2013;23:345–350. [PubMed] [Google Scholar]
- 20.Turkeltaub PC, Rastogi SC, Baer H, Anderson MC, Norman PS. A standardized quantitative skin-test assay of allergen potency and stability: studies on the allergen dose-response curve and effect of wheal, erythema, and patient selection on assay results. J Allergy Clin Immunol. 1982;70:343–352. doi: 10.1016/0091-6749(82)90023-9. [DOI] [PubMed] [Google Scholar]
- 21.May JC, Sih JT, Miller JR, Seligmann EB., Jr Optimization of parameters in protein nitrogen unit precipitation procedure for allergenic extracts. J Allergy Clin Immunol. 1979;63:87–97. doi: 10.1016/0091-6749(79)90197-0. [DOI] [PubMed] [Google Scholar]
- 22.Himly M, Jahn-Schmid B, Dedic A, Kelemen P, Wopfner N, Altmann F, et al. Art v 1, the major allergen of mugwort pollen, is a modular glycoprotein with a defensin-like and a hydroxyprolinerich domain. FASEB J. 2003;17:106–108. doi: 10.1096/fj.02-0472fje. [DOI] [PubMed] [Google Scholar]
- 23.Jimeno L, Duffort O, Serrano C, Barber D, Polo F. Monoclonal antibody-based ELISA to quantify the major allergen of Artemisia vulgaris pollen, Art v 1. Allergy. 2004;59:995–1001. doi: 10.1111/j.1398-9995.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 24.Dreborg S, Einarsson R. The major allergen content of allergenic preparations reflect their biological activity. Allergy. 1992;47(4 Pt 2):418–423. doi: 10.1111/j.1398-9995.1992.tb02082.x. [DOI] [PubMed] [Google Scholar]
- 25.Jeong KY, Lee JH, Kim EJ, Lee JS, Cho SH, Hong SJ, et al. Current status of standardization of inhalant allergen extracts in Korea. Allergy Asthma Immunol Res. 2014;6:196–200. doi: 10.4168/aair.2014.6.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]