Abstract
Purpose
An epidemiological study of myasthenia gravis (MG) has not been performed in Korea. The purpose of this study was to estimate the prevalence and incidence of MG in Korea.
Materials and Methods
Health Insurance Review and Assessment (HIRA) data from 2010 to 2014 were searched for MG codes as defined by the International Classification of Diseases, 10th revision. After identifying MG cases, we estimated the prevalence and annual incidence of MG based on the HIRA database and Korean population data.
Results
During the study period, 10138 MG cases were identified. The prevalence of MG was 10.42 cases per 100000 people in 2010 and this increased every year to 12.99 cases per 100000 people in 2014. The average incidence of MG between 2011 and 2014 was 0.69 cases per 100000 person-years. The prevalence and incidence were higher in the older (≥50 years) age group than in the younger (<50 years) age group [prevalence: 9.26 vs. 19.24 per 100000, relative risk 2.077, 95% confidence interval (CI) 1.976-2.183, p<0.001; incidence: 0.47 vs. 1.18 per 100000, relative risk 2.490, 95% CI 2.006-3.091, p<0.001].
Conclusion
This study was the first nationwide population-based epidemiological study of MG in Korea. The prevalence and incidence of MG were consistent with those of previous studies. We found an increase in the prevalence of MG and a predominance of elderly MG patients.
Keywords: Myasthenia gravis, prevalence, incidence, epidemiology, nationwide population-based, Korea
INTRODUCTION
Myasthenia gravis (MG) is an autoimmune disorder affecting neuromuscular transmission.1,2 MG is the most common neuromuscular junction disorder, although estimates of its frequency vary. A large number of population-based epidemiological studies of MG have been performed worldwide since the 1950s. A meta-analysis of 55 epidemiological studies estimated a prevalence of 77.7 per million people and an incidence of 5.3 per million person-years.3 However, the prevalence and incidence of MG varied markedly between the populations investigated. The prevalence ranged from 15 to 179 per million people, and the incidence ranged from 1.7 to 21.3 cases per million person-years.3 Therefore, in unstudied populations, the prevalence and incidence rates are very hard to extrapolate from previous studies. In Korea, no population-based epidemiological studies were known to have been performed.
The Republic of Korea has a public medical insurance system, National Health Insurance (NHI), which covers almost all of the Korean population.4 Under NHI, the Health Insurance Review and Assessment Service (HIRA) evaluates the medical fees, quality of care, and adequacy of medical services. To perform such evaluations on these public services, HIRA collects data for each patient, including diagnoses, treatments, procedures, and prescription drugs. The HIRA database has been used for a number of epidemiological studies of medical disorders, for example, multiple sclerosis, Huntington's disease, and postherpetic neuralgia.5,6,7
The purpose of this study was to estimate the prevalence and incidence of MG in Korea by utilizing the HIRA database.
MATERIALS AND METHODS
Sources of data and data selection
Under the Korean NHI system, health care institutions (i.e., hospitals and clinics) ask the NHI Service to pay for their medical expenses. After HIRA reviews the NHI claims, the NHI Service reimburses the health care institutions for their medical expenses. HIRA data are obtained from the payment claims form that is generated from each inpatient or outpatient visit to a health care institution. HIRA provides detailed data including patient demographics, diagnoses, procedures, and any prescription drugs taken. For research analyses, personal identifiers are replaced by surrogate identification numbers to protect personal information. Researchers can combine data from multiple claims into a single entry for each patient.
We filtered the HIRA data to determine the total MG population. Among all payment claims between 2010 and 2014, we extracted only claims in which the main or secondary cause of the visit was MG (G70 or G70.0), as defined by the International Classification of Diseases, 10th revision. To increase the diagnostic accuracy, we selected only exact cases by using the operational definition of MG. Only the data of individuals who visited a neurologist, pediatrician, or ophthalmologist were included in this study. An individual was defined as having MG if he or she visited a health care institution for MG at least twice during the study period. To estimate the annual incidence, a new MG case was defined as an individual who met both of the following criteria: 1) payment claims had never been made before each year in the study period; and 2) the prescription drug code for pyridostigmine bromide or oral corticosteroids, such as prednisolone, was present. The number of new MG cases in 2010 could not be calculated due to the lack of data for the previous year.
Estimation of prevalence and incidence
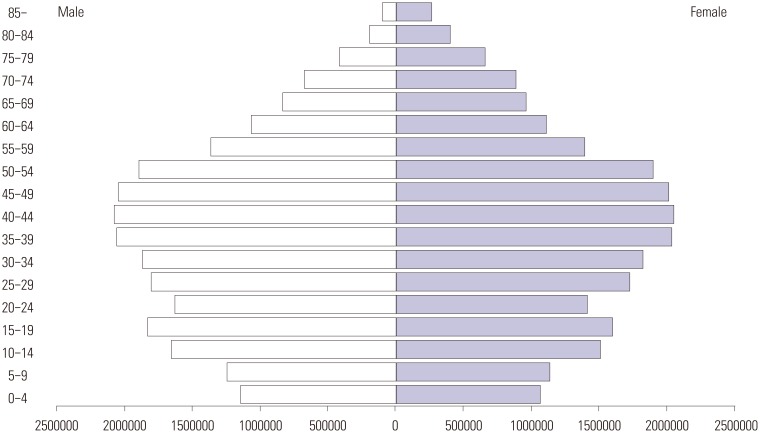
The population data for the Republic of Korea between 2010 and 2014 were obtained from the Korean Statistical Information Service (KOSIS; http://kosis.kr/). The total population ranged from 47.99 to 50.42 million during the study period. The population stratified by sex and 5-year age intervals was also provided by KOSIS (Fig. 1). The annual prevalence and incidence were calculated by dividing the number of total MG and new MG cases by the total population of each year, respectively. As the incidence and prevalence of MG varied among sex and age groups, averaged sex- and age-specific prevalence and incidence were calculated based on the numbers of total MG and new MG cases in each sex and 5-year age interval and the stratified population data.
Fig. 1. The population pyramid in 2010, South Korea.
This study was approved by the Institutional Review Board (IRB) of Severance Hospital, Yonsei University Health System (IRB No.: 4-2015-0330). HIRA data access was approved and offered by HIRA.
Statistics
A chi-square test was performed to compare prevalence and incidence according to sex and age groups, and 95% confidence intervals (CIs) that did not contain 1 and p<0.05 were considered statistically significant for all analyses. All statistical analyses were performed using SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
The incidence and prevalence of MG are presented in Table 1.
Table 1. Incidence and Prevalence of MG in Korea.
| Year | Total population | Total cases | F/M ratio of cases* | Prevalence rate per 100000 | New cases | Incidence rate per 100000 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | M | F | T | M | F | T | M | F | T | M | F | T | M | F | ||
| 2010 | 47990761 | 23840896 | 24149865 | 5001 | 1920 | 3081 | 1.6 (1.1–3.0) | 10.42 | 8.05 | 12.76 | ||||||
| 2011 | 49779440 | 24942339 | 24837101 | 5493 | 2134 | 3359 | 1.5 (1.2–2.8) | 11.03 | 8.56 | 13.52 | 292 | 116 | 176 | 0.59 | 0.47 | 0.71 |
| 2012 | 50004441 | 25039557 | 24964884 | 6201 | 2411 | 3790 | 1.6 (1.2–2.8) | 12.40 | 9.63 | 15.18 | 390 | 174 | 216 | 0.78 | 0.69 | 0.87 |
| 2013 | 50219669 | 25132612 | 25087057 | 6513 | 2557 | 3956 | 1.5 (1.2–2.4) | 12.97 | 10.17 | 15.77 | 355 | 165 | 190 | 0.71 | 0.66 | 0.76 |
| 2014 | 50423955 | 25219810 | 25204145 | 6551 | 2601 | 3950 | 1.5 (1.3–2.2) | 12.99 | 10.31 | 15.67 | 279 | 109 | 170 | 0.55 | 0.43 | 0.67 |
F, female; M, male; T, total; MG, myasthenia gravis.
*(bracket), range of F/M ratio according to age group.
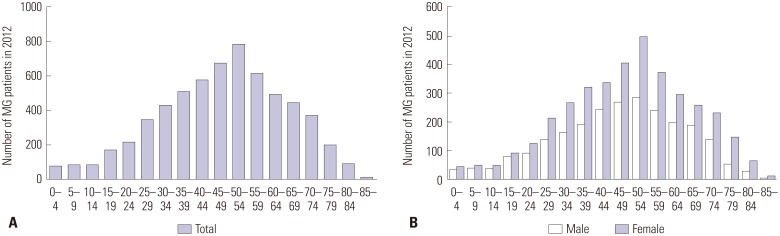
A total of 10138 MG cases (male: 4133; female: 6005) were identified during the study period. Fig. 2 demonstrates the number of MG cases according to age in 2012. The number of MG cases increased steadily from people aged 10-14 years to those aged 50-54 years. After peaking at 50-54 years of age, the number of cases decreased continuously as age increased. This pattern was similar in both males and females. The number of MG cases was approximately 1.5 times higher in females than in males. The ratio of females to males ranged from 1.1 to 3.0 according to age.
Fig. 2. The number of MG cases in 2012 (A: by age group, B: by sex and age group). MG, myasthenia gravis.
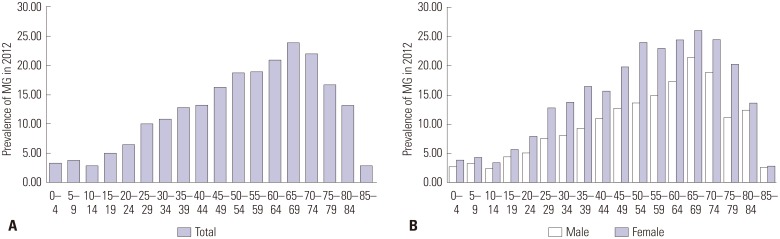
The annual prevalence of MG increased steadily from 10.42 cases per 100000 people in 2010 to 12.99 cases per 100000 people in 2014. This rising tendency was observed in both males and females. The prevalence ratio of females to males was approximately 1.6. Fig. 3 shows the age-specific prevalence in 2012. In females, the prevalence of MG tended to increase from people aged 10-14 years to those aged 50-54 years; then, the prevalence reached a plateau up to those aged 75-79 years. After the age of 80, the prevalence decreased sharply as age increased. In males, the prevalence tended to increase with age from 10-14 years to 65-69 years. After peaking at 65-69 years, the prevalence tended to decrease as age increased. The prevalence was higher in the older (≥50) age groups than in the younger (<50) age groups [prevalence: 9.26 vs. 19.24 per 100000; relative risk (RR): 2.077; 95% CI: 1.976-2.183; p<0.001] (Table 2).
Fig. 3. The prevalence in 2012 (A: by age group, B: by sex and age group). MG, myasthenia gravis.
Table 2. Comparison of Prevalence and Average Incidence between Younger-Age and Older-Age MG Cases.
| Young age (<50) | Old age (≥50) | Relative risk (95% CI) | p value | |
|---|---|---|---|---|
| Prevalence (per 100000) | 9.26 | 19.24 | 2.077 (1.976–2.183) | <0.001 |
| Incidence (per 100000) | 0.47 | 1.18 | 2.490 (2.006–3.091) | <0.001 |
CI, confidence interval; MG, myasthenia gravis.
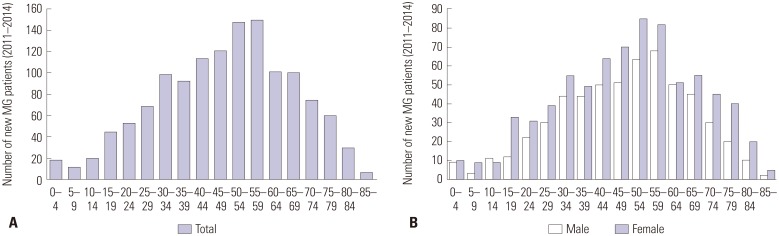
There were 1316 new MG cases (male: 564; female: 752) during the study period. The number of new MG cases according to age is illustrated in Fig. 4. The number of new MG cases tended to increase with age from 5-9 years. The number of new MG cases peaked at 55-59 years. After the age of 60, the number of new MG cases decreased as age increased. Both males and females showed similar tendencies. In those aged 15-19 years, however, a difference in new MG cases between males and females was noticeable.
Fig. 4. The new MG cases during the study period (A: by age group, B: by sex and age group). MG, myasthenia gravis.
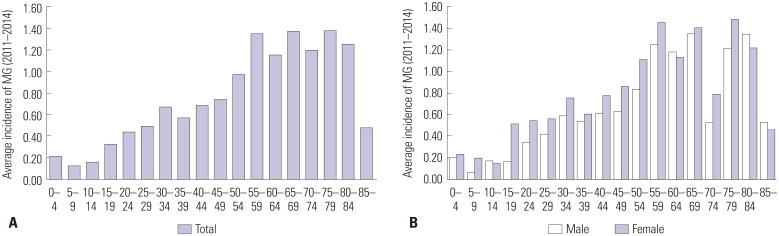
The average incidence of MG between 2011 and 2014 was 0.69 cases per 100000 person-years. The annual incidence ranged from 0.55 to 0.71 cases per 100000 person-years. Fig. 5 presents the age-specific incidence during the study period. The incidence tended to increase from 10-14 years to 55-59 years; then, the incidence reached a plateau up to 80-84 years. After the age of 85, a steep drop-off in the incidence was observed. The incidence was higher in the older (≥50) age groups than in the younger (<50) age groups (incidence: 0.47 vs. 1.18 per 100000; RR: 2.490; 95% CI: 2.006-3.091; p<0.001) (Table 2). Although the pattern of age-specific incidence was similar in males and females, the incidence in those aged 15-19 years was significantly higher in females than in males (0.51 vs. 0.16 per 100000; RR: 3.115; 95% CI: 1.609-6.031; p<0.001) (Table 3).
Fig. 5. Average incidence from 2011 to 2014 (A: by age group, B: by sex and age group). MG, myasthenia gravis.
Table 3. Average Age- and Sex-Specific Incidence of MG, 2010-2014.
| Age group (yr) | Cases | Average incidence per 100000 | Relative risk (95% CI) | F/M incidence rate p value | ||||
|---|---|---|---|---|---|---|---|---|
| T | M | F | T | M | F | |||
| 0–4 | 19 | 9 | 10 | 0.21 | 0.20 | 0.23 | 1.179 (0.479–2.900) | 0.720 |
| 5–9 | 12 | 3 | 9 | 0.13 | 0.06 | 0.20 | 3.240 (0.877–11.966) | 0.062 |
| 10–14 | 20 | 11 | 9 | 0.16 | 0.17 | 0.15 | 0.892 (0.370–2.152) | 0.799 |
| 15–19 | 45 | 12 | 33 | 0.33 | 0.16 | 0.51 | 3.115 (1.609–6.031) | <0.001 |
| 20–24 | 53 | 22 | 31 | 0.43 | 0.34 | 0.54 | 1.602 (0.927–2.766) | 0.088 |
| 25–29 | 69 | 30 | 39 | 0.49 | 0.42 | 0.56 | 1.350 (0.839–2.173) | 0.215 |
| 30–34 | 99 | 44 | 55 | 0.67 | 0.59 | 0.75 | 1.276 (0.858–1.896) | 0.228 |
| 35–39 | 93 | 44 | 49 | 0.57 | 0.53 | 0.60 | 1.125 (0.749–1.691) | 0.570 |
| 40–44 | 114 | 50 | 64 | 0.69 | 0.60 | 0.78 | 1.287 (0.889–1.863) | 0.180 |
| 45–49 | 121 | 51 | 70 | 0.74 | 0.62 | 0.86 | 1.383 (0.964–1.984) | 0.077 |
| 50–54 | 148 | 63 | 85 | 0.97 | 0.83 | 1.11 | 1.334 (0.963–1.847) | 0.082 |
| 55–59 | 150 | 68 | 82 | 1.36 | 1.25 | 1.46 | 1.167 (0.846–1.610) | 0.346 |
| 60–64 | 101 | 50 | 51 | 1.16 | 1.18 | 1.13 | 0.958 (0.649–1.415) | 0.830 |
| 65–69 | 100 | 45 | 55 | 1.38 | 1.35 | 1.40 | 1.040 (0.702–1.543) | 0.844 |
| 70–74 | 75 | 30 | 45 | 1.20 | 1.11 | 1.26 | 1.130 (0.712–1.794) | 0.604 |
| 75–79 | 60 | 20 | 40 | 1.38 | 1.22 | 1.48 | 1.219 (0.713–2.086) | 0.468 |
| 80–84 | 30 | 10 | 20 | 1.26 | 1.34 | 1.22 | 0.908 (0.425–1.941) | 0.804 |
| ≥85 | 7 | 2 | 5 | 0.48 | 0.53 | 0.46 | 0.871 (0.169–4.490) | 0.869 |
F, female; M, male; T, total; CI, confidence interval; MG, myasthenia gravis.
DISCUSSION
The present study is the first population-based epidemiological study on MG in the Republic of Korea, which has a population of about 50 million. In addition, this study used data representing the entire national population. The prevalence of MG was estimated as 10.42-12.99 per 100000 people, and the incidence was 0.69 cases per 100000 person-years. These results were consistent with those of previous studies in which the prevalence ranged from 1.5 to 17.9 per 100000 people and the incidence ranged from 0.17 to 2.13 cases per 100000 person-years.3,8,9
Several issues of the present study must be considered. First, we used operational definitions of MG cases and new MG cases for diagnostic accuracy. In this study, case identification was based on diagnostic coding by medical doctors. Neurologists, pediatricians, and ophthalmologists are considered to be MG specialists in Korea. In addition, most patients have easy access to medical specialists throughout the country. Therefore, almost all suspected patients of MG visit at least one of these three kinds of medical specialists in Korea. This is one reason why MG cases could not be determined according to medical specialty. Considering that MG is a chronic disease, individuals with only one visit for MG were also excluded. To define new MG cases, we used prescription drug codes for pyridostigmine bromide or oral corticosteroids. HIRA provided only 5 years of data. If the drug codes were not applied, new MG cases would have included certain amounts of well-controlled MG patients without medication who made visits to a doctor only at long intervals. For example, MG patients classified as minimal manifestation-0 (Myasthenia Gravis Foundation of America post-intervention status) who visited a doctor in 2009 and 2011 would have been regarded as new MG cases in 2011 in this study. Second, the age of age-specific incidence represents that of the first visit for MG, not the age of symptom onset in this study. The age of symptom onset was not available in the HIRA database. According to a previous study, the median periods from onset of MG to the first visit to a doctor and from onset of MG to diagnosis were 1.0 and 6.1 months, respectively.10 Although the age of the first visit for MG was greater than that of MG onset, the age difference may have had only a minor influence on age-specific incidence based on 5-year age intervals.
The most noticeable observation of this study was the high prevalence and incidence of MG in the older age group. A Taiwanese population-based epidemiological study also showed the predominance of elderly MG patients.11 The incidence of elderly-onset MG has been increasing across the world.12 In Japan, the incidence of MG patients with an onset age of 65 years or more has doubled in the past two decades.13 A number of epidemiological studies from the United States and Europe also revealed a similar tendency. Distinct immunological characteristics of elderly patients, the occurrence of thymoma, and environmental factors have been suggested as causes of the increasing incidence of elderly MG patients. However, the reason for this growing incidence remains unclear.
In the most elderly age group of this study, the incidence of MG dropped off very steeply. This pattern was also observed in a previous study, which demonstrated the high prevalence of unrecognized positive anti-acetylcholine receptor antibodies in the most elderly age group.14 In addition, ocular myasthenia is more common in older people than in younger people.15 The diagnosis of ocular myasthenia can be difficult. These findings suggest that MG may be underdiagnosed in a substantial number of very elderly people. The prevalence of this study also decreased after the age of 70. Although the underdiagnosis of MG may be one of the reasons, this finding remains difficult to explain. Another possible explanation is the low accessibility of health care institutions for very elderly patients with MG. In this study, only MG patients who visited health care institutions were identified as MG cases. If they did not visit a doctor, it was not possible for them to be included as subjects in this study.
Korea is one of the fastest aging countries in the world. It is anticipated that the proportion of elderly people will be more than 20% by 2026.16 Considering the high prevalence and incidence of MG in the older age group, the number of elderly patients with MG will increase very rapidly in Korea. As elderly patients are prone to additional diseases, physicians will be faced more frequently with complicated MG patients, for example, MG patients with diabetes mellitus or osteoporosis. The management of MG patients will be more complex, and the medical costs associated with MG will likely increase in the coming years. Further studies on the comorbidities and medical costs of MG are necessary to prepare for the increasing number of elderly patients with MG.
Although the characteristic bimodal pattern of age- and sex-specific incidence was not demonstrated in this study, the incidence at 15-19 years of age was significantly higher in females than in males. This observation may reflect female predominance, particularly in early-onset MG. As in other autoimmune diseases, female predominance has been thought to represent certain effects of sex hormones.17 A previous animal model study showed that estrogen enhances susceptibility to experimental autoimmune MG through the action on acetylcholine receptor-specific T and B cell responses.18 Fluctuations in disease activity during pregnancy or menstrual periods also suggest the possible effects of sex hormones on MG.19,20
There were several limitations in this study. First, we identified MG cases based on diagnostic coding. Clinical information including medical history, anti-acetylcholine receptor antibody titer, and results of electrodiagnostic and pharmacological tests were not available in the HIRA database. Accordingly, there was concern regarding the diagnostic accuracy. Second, we used operational definitions for MG cases and new MG cases to increase the diagnostic accuracy. Therefore, we could have missed actual cases of MG that did not meet the inclusion criteria. Diagnosis of MG depends mainly on the physician's decision. In a substantial number of patients with MG, particularly ocular type, laboratory tests for MG diagnosis did not support the clinical diagnosis. For this reason, the issue of diagnostic accuracy is present in all epidemiological studies of MG. Third, the age data of this study was based on the time at which patients visited a doctor for MG. Consequently, the age-specific incidence results must be interpreted with caution. In this study, the bimodal pattern of the ageand sex-specific distributions, which was an early peak in the twenties for females and a late peak in those over 50 for males, was not obvious, although the incidence for those aged 15-19 years was significantly higher in females than in males. In addition, the high frequency of childhood-onset MG, which has been reported in Asian countries, was not demonstrated. The issues related to the age data may partially account for these discrepancies between the previous studies and the present study. Further studies combining the HIRA database with a national registry or survey for MG are necessary to improve the accuracy of diagnosis and clinical information, including the age of symptom onset.
In conclusion, this first Korean epidemiological study on MG estimated the prevalence as 10.42-12.99 per 100000 people and the incidence as 0.69 cases per 100000 person-years in the early 2010s. The prevalence and incidence were higher in the older age group. It is recommended to develop a strategy for the growing number of elderly patients with MG.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Lewis RA, Selwa JF, Lisak RP. Myasthenia gravis: immunological mechanisms and immunotherapy. Ann Neurol. 1995;37(Suppl 1):S51–S62. doi: 10.1002/ana.410370707. [DOI] [PubMed] [Google Scholar]
- 2.Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8:475–490. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr AS, Cardwell CR, McCarron PO, McConville J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010;10:46. doi: 10.1186/1471-2377-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health. 2014;36:e2014008. doi: 10.4178/epih/e2014008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim NH, Kim HJ, Cheong HK, Kim BJ, Lee KH, Kim EH, et al. Prevalence of multiple sclerosis in Korea. Neurology. 2010;75:1432–1438. doi: 10.1212/WNL.0b013e3181f88191. [DOI] [PubMed] [Google Scholar]
- 6.Cheong C, Lee TJ. Prevalence and healthcare utilization of herpes zoster and postherpetic neuralgia in South Korea: disparity among patients with different immune statuses. Epidemiol Health. 2014;36:e2014012. doi: 10.4178/epih/e2014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HS, Lyoo CH, Lee PH, Kim SJ, Park MY, Ma HI, et al. Current Status of Huntington's Disease in Korea: A Nationwide Survey and National Registry Analysis. J Mov Disord. 2015;8:14–20. doi: 10.14802/jmd.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32:150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 9.Phillips LH. The epidemiology of myasthenia gravis. Semin Neurol. 2004;24:17–20. doi: 10.1055/s-2004-829593. [DOI] [PubMed] [Google Scholar]
- 10.Oöpik M, Kaasik AE, Jakobsen J. A population based epidemiological study on myasthenia gravis in Estonia. J Neurol Neurosurg Psychiatry. 2003;74:1638–1643. doi: 10.1136/jnnp.74.12.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai CH, Tseng HF. Nationwide population-based epidemiological study of myasthenia gravis in taiwan. Neuroepidemiology. 2010;35:66–71. doi: 10.1159/000311012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aarli JA. Late-onset myasthenia gravis: a changing scene. Arch Neurol. 1999;56:25–27. doi: 10.1001/archneur.56.1.25. [DOI] [PubMed] [Google Scholar]
- 13.Murai H, Yamashita N, Watanabe M, Nomura Y, Motomura M, Yoshikawa H, et al. Characteristics of myasthenia gravis according to onset-age: Japanese nationwide survey. J Neurol Sci. 2011;305:97–102. doi: 10.1016/j.jns.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Vincent A, Clover L, Buckley C, Grimley Evans J, Rothwell PM; UK Myasthenia Gravis Survey. Evidence of underdiagnosis of myasthenia gravis in older people. J Neurol Neurosurg Psychiatry. 2003;74:1105–1108. doi: 10.1136/jnnp.74.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zivkoviń SA, Clemens PR, Lacomis D. Characteristics of late-onset myasthenia gravis. J Neurol. 2012;259:2167–2171. doi: 10.1007/s00415-012-6478-6. [DOI] [PubMed] [Google Scholar]
- 16.Lowe-Lee F. Is Korea ready for the demographic revolution? The World's Most Rapidly Aging Society with the Most Rapidly Declining Fertility Rate. [accessed on 2015 September 3]. Available at: http://www.keia.org/sites/default/files/publications/04Exchange09.pdf.
- 17.Ansar Ahmed S, Penhale WJ, Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol. 1985;121:531–551. [PMC free article] [PubMed] [Google Scholar]
- 18.Delpy L, Douin-Echinard V, Garidou L, Bruand C, Saoudi A, Guéry JC. Estrogen enhances susceptibility to experimental autoimmune myasthenia gravis by promoting type 1-polarized immune responses. J Immunol. 2005;175:5050–5057. doi: 10.4049/jimmunol.175.8.5050. [DOI] [PubMed] [Google Scholar]
- 19.Leker RR, Karni A, Abramsky O. Exacerbation of myasthenia gravis during the menstrual period. J Neurol Sci. 1998;156:107–111. doi: 10.1016/s0022-510x(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 20.Djelmis J, Sostarko M, Mayer D, Ivanisevic M. Myasthenia gravis in pregnancy: report on 69 cases. Eur J Obstet Gynecol Reprod Biol. 2002;104:21–25. doi: 10.1016/s0301-2115(02)00051-9. [DOI] [PubMed] [Google Scholar]