Abstract
Purpose
The neonatal mortality rate in Japan has currently been at the lowest level in the world. However, it is unclear whether there are still some potentially preventable neonatal deaths. We, therefore, aimed to examine the backgrounds of neonatal death and the possibilities of prevention in a region of Japan.
Materials and Methods
This is a population-based study of neonatal death in Shiga Prefecture of Japan.
Results
The 103 neonatal deaths in our prefecture between 2007 and 2011 were included. After reviewing by a peer-review team, we classified the backgrounds of these neonatal deaths and analyzed end-of-life care approaches associated with prenatal diagnosis. Furthermore, we evaluated the possibilities of preventable neonatal death, suggesting specific recommendations for its prevention. We analyzed 102 (99%) of the neonatal deaths. Congenital malformations and extreme prematurity were the first and the second most common causes of death, respectively. More than half of the congenital abnormalities (59%) including malformations and chromosome abnormality had been diagnosed before births. We had 22 neonates with non-intensive care including eighteen cases with congenital abnormality and four with extreme prematurity. Twenty three cases were judged to have had some possibility of prevention with one having had a strong possibility of prevention. Among specific recommendations of preventable neonatal death, more than half of them were for obstetricians.
Conclusion
There is room to reduce neonatal deaths in Japan. Prevention of neonatal death requires grater prenatal care by obstetricians before birth rather than improved neonatal care by neonatologists after birth.
Keywords: Neonatal death, cause, end of life care, prevention, population-based study
INTRODUCTION
Development of technology and systems in perinatal care has been lowering the perinatal mortality rate for several decades. The neonatal mortality rate in Japan has currently been at the lowest level in the world1,2 and it is still decreasing. Shiga, located in the middle of Japan, is a prefecture with one of the highest birth rates. The neonatal mortality rate of Shiga, however, has been continuously higher than the Japanese average.3 To uncover the reason for this, we conducted a population based study to find ways of reducing neonatal mortality in our region. Our analysis of neonatal death cases in our region was based upon data from the death certificates. From this, we first investigated the backgrounds of neonatal death cases, and we then assessed the possibilities of preventing neonatal death, exploring specific recommendations for how this could be achieved.
MATERIALS AND METHODS
We have 13000 births on average annually in Shiga prefecture, two-thirds of them in 30 primary obstetric clinics and the remaining one-third in eleven hospitals, including four perinatal centers. All clinics and hospitals were enrolled in this study. Since 2012, we have organized a peer-review team consisting of six obstetricians and six neonatologists to analyze the perinatal death cases in our region during the period of 2007 to 2010.
The 103 neonatal deaths of 66682 births in our prefecture during this period were registered in the National Vital Statistics of Japan.3 We inspected all of the neonatal death certificates with permission of the Japanese Ministry of Health, Labor and Welfare. Based on the evaluating of the information, we designed an original questionnaire and sent it to each medical staff who submits a death certificate. The peer-review team retrospectively reviewed the questionnaires returned from the institutions.
Analyzing the questionnaires, primary causes and circumstances of neonatal death were identified. Focusing on end-oflife care approaches, we abstracted primary nonintervention cases. In addition, we assessed the possibilities of preventable neonatal death, formulating recommendations for preventions.
This study was approved by the Institutional Review Board, Shiga University of Medical Science.
RESULTS
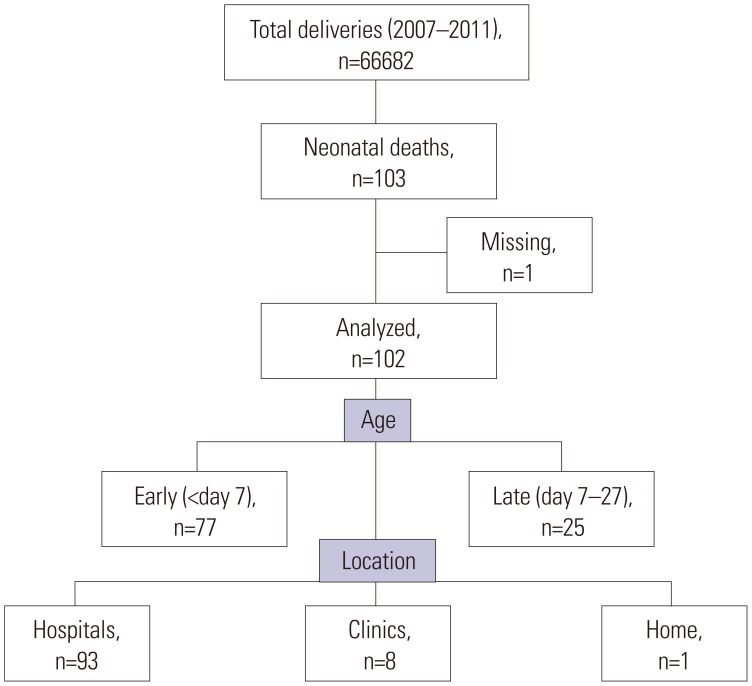
The 103 neonatal deaths among 66682 deliveries in our prefecture during 2007–2011 were registered by the National Vital Statics in Japan. We analyzed 102 (99%) of neonatal deaths. Among the 102 neonatal deaths, 52 (51%) died within 24 hours and 77 (75%) died within day six of life. The numbers of neonatal deaths by locations were 93 (91%), eight (8%), and one (1%), respectively, in hospitals, primary obstetric clinics and home, respectively (Fig. 1).
Fig. 1. Overview of neonatal death in our region.
Cause of death
The primary causes of 102 deaths listed in Table 1, were classified as described previously.4 We were able to classify the most explainable cause of 100 (98%) but were unable to identify two (2%) of them due to lack of sufficient information. Most common causes were congenital malformations including renal (24%), lung (21%), brain (17%), tracheal (14%), skeletal (14%), and cardiac (10%) ones. Extreme prematurity (born at 22–23 weeks of gestation) was the second most common cause. In thirteen perinatal asphyxia cases, three cases of placental abruption were identified. Respiratory complications included four cases of pneumothorax and three of pulmonary hypoplasia. Of eight chromosome abnormality cases, 18 trisomy constituted six (75%) of them. Autopsy of the neonatal deaths was performed in 21 cases (21%).
Table 1. Primary Causes of Neonatal Deaths.
| n | |
|---|---|
| Congenital malformation | 30 |
| Prematurity (<24 wk) | 18 |
| Perinatal asphyxia | 13 |
| Respiratory complications | 10 |
| Chromosome abnormality | 8 |
| Sepsis | 8 |
| Hydrops fetalis | 7 |
| Severe IVH | 4 |
| Metabolic disease | 1 |
| NEC | 1 |
| Unknown | 2 |
| Total | 102 |
IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis.
Prenatal diagnosis and end-of-life care
Congenital abnormalities, including malformations and chromosome abnormality were classified in terms of prenatal diagnosis and non-invasive care (Table 2). More than half of congenital abnormalities (59%) were diagnosed before birth. Although abnormalities of brain, skeleton and kidney were frequently diagnosed (87–100%), only 13% of chromosome abnormality were prenatally diagnosed.
Table 2. Congenital Abnormality in Relation to Prenatal Diagnosis and Non-Invasive Care.
| Congenital abnormality | n | Prenatal diagnosis | Non-invasive care |
|---|---|---|---|
| Chromosome anomaly | 8 | 1 (13%) | 3 (38%) |
| 18 trisomy | 6 | 1 | 3 |
| 21 trisomy | 1 | 0 | 0 |
| Atypical | 1 | 0 | 0 |
| Potter sequence | 7 | 6 (87%) | 6 (87%) |
| Congenital diaphragmatic hernia | 6 | 4 (67%) | 0 |
| Brain anomaly | 5 | 5 (100%) | 5 (100%) |
| Congenital heart anomaly (isolated) | 3 | 2 (67%) | 0 |
| Skeletal dysplasia | 4 | 4 (100%) | 4 (100%) |
| Other | 5 | 0 | 0 |
| Total | 38 | 22 (58%) | 18 (47%) |
Excluding 4 cases of non-invasive care with extreme prematurity. Non-invasive care means neonatal death occurred after primary nonintervention.
We had 22 neonates with non-intensive care, including eighteen cases with congenital abnormality and four with extreme prematurity. The neonates with non-invasive medical care were 49% of congenital abnormalities. All neonates of brain anomaly or skeletal dysplasia with prenatal diagnosis received non-intensive care. Neither neonates with congenital diaphragmatic hernia (CDH) nor congenital heart disease (CHD) received non-intensive care, even though 67% of them were prenatally diagnosed. On the other hand, two cases of 18 trisomy without prenatal diagnosis received non-intensive care because they were diagnosed as such only after birth.
Possibility and recommendation of preventable neonatal death
The audit conference concluded that 75% (77/102) of neonatal deaths could not have been avoided. On the other hand, 23% (23/102) of them were judged to have had some possibility of prevention with one case (1%) having had a strong possibility. The remaining one case could not be judged due to lack of sufficient information. Among the 23 cases with some possibility of prevention, the most common cause of death was perinatal asphyxia (5 cases) with extreme prematurity (4 cases) and sepsis (3 cases) as the second and third most common, respectively. In addition, there was no case of congenital abnormality with non-invasive care among these 23 cases. With nine cases delivered at clinics or home, three cases were determined to have been somewhat preventable and one case easily preventable.
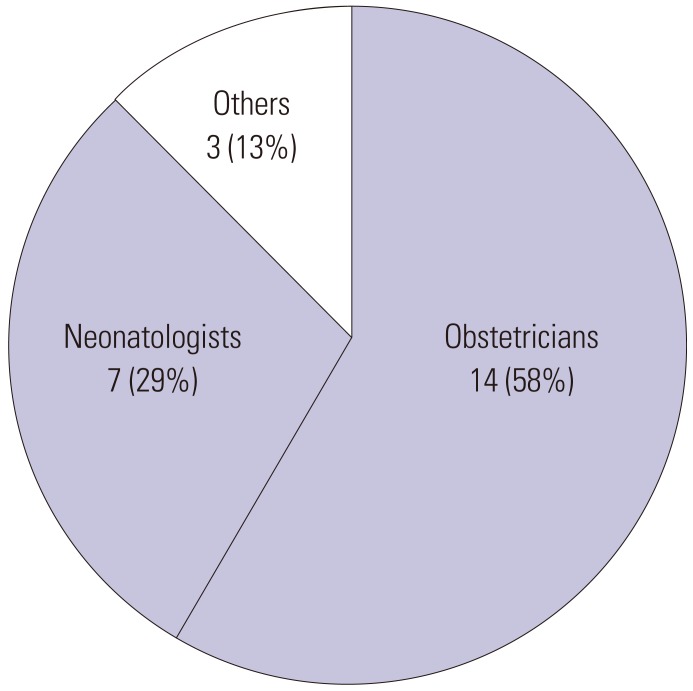
We made 24 recommendations of possible procedures to prevent neonatal deaths and classified them for obstetricians, neonatologists and others (Fig. 2). We had fourteen (58%) recommendations targeting obstetricians for improvement of medical management including managing threatened premature labor, multiple pregnancy, and heart rate monitoring of the fetus. Those recommendations also suggested earlier maternal referral from clinics to higher perinatal centers for neonatal intensive care, including fetal growth restriction, threatened premature labor, and nonreassuring fetal status. There were seven (29%) recommendations, targeting neonatologists with some advice of improving medical management of very premature infants including those with respiratory complications as well as emphasizing that infectious controls should carefully be maintained.
Fig. 2. Target of recommendations in possibly preventable cases.
DISCUSSION
In this study, almost all of the neonatal death cases in our region for five years were reviewed and we ascertained two points. We investigated the background of neonatal death including age, location, causes, prenatal diagnosis, and end-of-life care and concluded that some neonatal deaths could have potentially been prevented, and some recommendations were made to prevent such cases.
Based on Japanese vital statistics, we concluded that congenital malformation and extreme prematurity were, respectively, the first and the second most common cause of death. Our finding is consistent with the data from studies5,6,7,8 of domestic4 and other developed countries', in which neonatal death is mostly attributed to prematurity, congenital anomalies, and perinatal asphyxia. We also showed that few of chromosome anomalies were prenatally diagnosed whereas most of the congenital abnormalities were. Fetal aneuploidy such as 18 and 21 trisomy were determined conventionally in our region during the period. As cell-free DNA screening of fetal aneuploidy has greater specificity than the conventional one,9,10 it's wide use would make it possible to increase accurate prenatal diagnosis of chromosomal anomaly in the future.
In addition, by analyzing the relation to perinatal diagnosis and end-of-life care in congenital abnormality, we found that none of prenatally diagnosed CDH or CHD received primary non-invasive care although the majority of prenatally diagnosed congenital abnormalities did. Neonatal survival in CDH and CHD with prenatal diagnosis was 38–70%11 and 71–90%,12,13 respectively. Based on the relatively high survival rate of these two maladies, intensive care for them with prenatal diagnosis is generally acceptable in our society.
The next important point in our study is prevention of neonatal deaths. We concluded that 23% of neonatal deaths were somewhat preventable with 1% much more so. Our estimate of possible neonatal death prevention in our population-based study differs from that of another study in Japan which estimated only 6% of them were preventable.4 Two factors might explain for this difference. First, all neonatal death cases were judged to be either preventable or not in our study whereas only near term asphyxia cases were judged to be preventable in the other study. Next, we classified preventable neonatal deaths into three grades according to their relative possibility, therefore, it is difficult to compare the proportion of prevention between these two studies.
In terms of preventing neonatal deaths, we could suggest some recommendations on neonatal deaths prevention. The fact that more than half of them were for obstetricians indicates that prevention of neonatal death requires grater prenatal care by obstetricians before birth, compared with improved neonatal care by neonatologists after birth. We concluded with recommendations for obstetricians, which included earlier referral from clinics to perinatal centers upon recognition of high risk pregnancy such as multiple pregnancy, fetal growth restriction, and fetal distress. Improvement of obstetrical care in primary clinics would reduce neonatal deaths.
In the present study, there is a limitation in the lack of identification of the primary cause of the two neonatal deaths occurred after birth: one in obstetrical clinic and another at home. It was impossible to determine the primary cause of death in both cases by autopsy.
In conclusion, we have shown in our regional study the backgrounds of neonatal death and the possibility of prevention with recommendations. We conclude that improving obstetric care before birth is required much more than focusing upon improved neonatal care after birth to reduce neonatal death. This study might contribute to the reduction of neonatal death not only in our region, but also in other areas as well.
ACKNOWLEDGEMENTS
This study was supported by a grant No. 23590594 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
We would like to express our appreciation to the peer-review team members: Masafumi Koshiyama, MD, Yukiyasu Sato, MD, Kenji Nakamura, MD, Daisuke Fujisawa, MD, Takahide Yanagi, MD, Shoji Kaku, MD, Kashirou Nishizawa, MD, Masahito Yamamoto, MD, Yoshihiko Hayashi, MD, Asuka Higuchi, MD, Tsutomu Asano, MD, and Tetsuya Nomura, MD.
Footnotes
This study was presented in part at 59th Medical Meeting of Japan Society for premature and newborn medicine in Matsuyama, Ehime, Japan on November 10th 2014.
The authors have no financial conflicts of interest.
References
- 1.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010-2011. Pediatrics. 2013;131:548–558. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajaratnam JK, Marcus JR, Flaxman AD, Wang H, Levin-Rector A, Dwyer L, et al. Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970-2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet. 2010;375:1988–2008. doi: 10.1016/S0140-6736(10)60703-9. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Health, Labour and Welfare. Prompt Vital Statistics Report, October 2012. Tokyo, Japan: Ministry of Health, Labour and Welfare; 2012. [Google Scholar]
- 4.Sameshima H, Ikenoue T Miyazaki Perinatal Data Group. Risk factors for perinatal deaths in Southern Japan: population-based analysis from 1998 to 2005. Early Hum Dev. 2008;84:319–323. doi: 10.1016/j.earlhumdev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Eventov-Friedman S, Kanevsky H, Bar-Oz B. Neonatal end-of-life care: a single-center NICU experience in Israel over a decade. Pediatrics. 2013;131:e1889–e1896. doi: 10.1542/peds.2012-0981. [DOI] [PubMed] [Google Scholar]
- 6.Berger TM, Hofer A. Causes and circumstances of neonatal deaths in 108 consecutive cases over a 10-year period at the Children's Hospital of Lucerne, Switzerland. Neonatology. 2009;95:157–163. doi: 10.1159/000153100. [DOI] [PubMed] [Google Scholar]
- 7.Simpson CD, Ye XY, Hellmann J, Tomlinson C. Trends in cause-specific mortality at a Canadian outborn NICU. Pediatrics. 2010;126:e1538–e1544. doi: 10.1542/peds.2010-1167. [DOI] [PubMed] [Google Scholar]
- 8.Mathews TJ, Miniño AM, Osterman MJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2008. Pediatrics. 2011;127:146–157. doi: 10.1542/peds.2010-3175. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi DW, Parker RL, Wentworth J, Madankumar R, Saffer C, Das AF, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799–808. doi: 10.1056/NEJMoa1311037. [DOI] [PubMed] [Google Scholar]
- 10.Greene MF, Phimister EG. Screening for trisomies in circulating DNA. N Engl J Med. 2014;370:874–875. doi: 10.1056/NEJMe1401129. [DOI] [PubMed] [Google Scholar]
- 11.Tudorache S, Chiuţu LC, Iliescu DG, Georgescu R, Stoica GA, Simionescu CE, et al. Prenatal diagnosis and perinatal outcome in congenital diaphragmatic hernia. Single tertiary center report. Rom J Morphol Embryol. 2014;55:823–833. [PubMed] [Google Scholar]
- 12.Anagnostou K, Messenger L, Yates R, Kelsall W. Outcome of infants with prenatally diagnosed congenital heart disease delivered outside specialist paediatric cardiac centres. Arch Dis Child Fetal Neonatal Ed. 2013;98:F218–F221. doi: 10.1136/archdischild-2011-300488. [DOI] [PubMed] [Google Scholar]
- 13.Oster ME, Kim CH, Kusano AS, Cragan JD, Dressler P, Hales AR, et al. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol. 2014;113:1036–1040. doi: 10.1016/j.amjcard.2013.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]