Abstract
Purpose
To develop a model based on non-invasive clinical and ultrasonographic parameters for predicting the likelihood of subsequent histologic chorioamnionitis in women with preterm premature rupture of membranes (PPROM) and to determine whether the inclusion of invasive test results improves the predictive value of the model.
Materials and Methods
This retrospective cohort study included 146 consecutive women presenting with PPROM (20–33 weeks). Transvaginal ultrasonographic assessment of cervical length was performed. Maternal serum C-reactive protein (CRP) levels and white blood cell (WBC) counts were measured after amniocentesis. Amniotic fluid (AF) obtained by amniocentesis was cultured, and interleukin-6 (IL-6) levels and WBC counts were determined. The primary outcome measure was histologic chorioamnionitis.
Results
Risk scores based on serum CRP concentrations and gestational age (model 1) were calculated for each patient. The model was shown to have adequate goodness of fit and an area under the receiver operating characteristic curve (AUC) of 0.742. When including AF test results (e.g., AF IL-6 levels) in model 1, serum CRP concentrations were found to be insignificant, and thus, were excluded from model 2, comprising AF IL-6 levels and gestational age. No significant difference in AUC was found between models 1 and 2.
Conclusion
For women with PPROM, the newly developed model incorporating non-invasive parameters (serum CRP and gestational age) was moderately predictive of histologic chorioamnionitis. The inclusion of invasive test results added no predictive information to the model in this setting.
Keywords: Amniotic fluid, histologic chorioamnionitis; C-reactive protein; gestational age; non-invasive model; preterm premature rupture of membranes
INTRODUCTION
Histologic chorioamnionitis occurs in approximately 50–60% of women with preterm premature rupture of membranes (PPROM).1,2,3 This condition is thought to represent antenatal inflammation/infection, which contributes to impending preterm birth and neonatal sepsis, chronic lung disease, and brain injury.1,2,3,4,5,6 Early and accurate diagnosis of chorioamnionitis is important, although it is limited by the fact that placental pathology cannot be evaluated before delivery. Several studies have attempted to identify rapid yet highly sensitive and specific diagnostic markers for diagnosing chorioamnionitis by analyzing factors in amniotic fluid (AF) obtained during amniocentesis, such as AF interleukin-6 (IL-6): this parameter has been reported as a significant predictor.1,4,5 However, amniocentesis is an invasive procedure with accompanying risks, and a decreased AF volume in cases of PPROM often makes it difficult or impossible to obtain AF. Consequently, alternative approaches for non-invasive and rapid identification of chorioamnionitis are needed for women with PPROM.
Previous studies have shown that the measurement of C-reactive protein (CRP) levels or white blood cell (WBC) counts in maternal blood may be useful non-invasive techniques for diagnosing histologic chorioamnionitis before birth in women with PPROM.7,8 It has also been reported that gestational age at the time of rupture and parity are significant risk factors for histologic chorioamnionitis.1,5 However, none of these factors appear to be sufficiently sensitive or specific on an individual basis. This is perhaps because the development of imminent chorioamnionitis before or after membrane rupture during preterm gestation may potentially be influenced by various pathogenic factors, including the hosts' innate immune function and status, anatomical factors, mucosal immunity, and maternal and/or fetal genotypes.9 It is likely that the only way to better predict the development of histologic chorioamnionitis is to use a combination of different markers. The purposes of the present study was to develop a model based on noninvasive clinical and ultrasonographic parameters for predicting the likelihood of subsequent histologic chorioamnionitis in women with PPROM and to determine whether the additional inclusion of invasive test results improves the predictive value of the model.
MATERIALS AND METHODS
The present study was conducted as a retrospective cohort study, including consecutive women diagnosed with PPROM at Seoul National University Bundang Hospital (Seongnam, Korea) between June 2004 and August 2013. The inclusion criteria were 1) singleton gestation, 2) a live fetus with a gestational age between 20+0 and 33+6 weeks, 3) transabdominal amniocentesis performed to assess the microbiologic and inflammatory status of the amniotic cavity and/or fetal lung maturity, 4) maternal blood drawn to measure WBC counts and CRP levels at the time of amniocentesis, 5) cervical length measured at the time of amniocentesis, 6) absence of active labor, defined by the presence of cervical dilatation >3 cm by sterile speculum examination, 7) no history of prior or subsequent cervical cerclage, and 8) absence of a major congenital anomaly. PPROM was defined as spontaneous rupture of the fetal membranes before the onset of uterine contractions, as diagnosed by a sterile speculum examination to confirm both AF pooling in the vagina and a positive nitrazine test. Digital examinations were not performed until the onset of labor (defined by the presence of regular and painful uterine contractions). Non-invasive clinical data collected at the time of enrollment included demographic variables (maternal age, parity, and number of previous preterm deliveries), gestational age at the time of assessment, CRP level, maternal WBC counts, and cervical length measured by transvaginal ultrasonography. Amniocentesis was immediately offered for the microbiologic assessment of the amniotic cavity to all women who were admitted with a diagnosis of PPROM. Throughout the study period, maternal WBC counts and serum CRP levels were routinely determined at the time of amniocentesis for all women with PPROM. The primary outcome was histologic chorioamnionitis and the secondary outcome was clinical chorioamnionitis. The data from a large portion of our study population were previously reported.10 The study was approved by the Institutional Review Board of the Seoul National University Bundang Hospital (IRB no. B-1105/128-102). Written informed consent was obtained from all study subjects for the amniocentesis procedure and use of AF samples for research purposes.
AF was retrieved by transabdominal amniocentesis using an aseptic technique with ultrasound guidance. Aerobic and anaerobic bacteria, as well as genital mycoplasma (Ureaplasma urealyticum and Mycoplasma hominis), present in the AF samples were cultured according to methods previously described in detail.11 An aliquot of AF was transferred to the hematology laboratory and examined in a hemocytometer chamber for the presence of WBCs. The absolute WBC count was calculated by multiplying the area examined by a factor of 10 and expressed as the number of cells per cubic mL. The remaining AF was centrifuged, and the supernatant was aliquoted and stored at -70℃ until assayed. The samples were not subjected to freeze-thaw cycles before being assayed. IL-6 concentrations were measured with an enzyme-linked immunosorbent assay human IL-6 DuoSet Kit (R&D System, Minneapolis, MN, USA). The range of the IL-6 standard curve was 7.8–600 pg/mL. The assay was carried out by strictly following the instructions provided by the manufacturer, and all samples were measured in duplicate. The calculated intra- and inter-assay coefficients of variation were <10%. Maternal blood WBC counts were determined using an automated hemocytometer (XE-2100; Sysmex, Tokyo, Japan). The CRP concentration was measured with a latex-enhanced turbidimetric immunoassay (Denka Seiken, Tokyo, Japan) using an automated analyzer (Toshiba 200FR; Toshiba, Tokyo, Japan).
Transvaginal ultrasonographic assessment of cervical length was performed by appropriately trained doctors immediately after amniocentesis, using either an Envisor (Philips Medical System, Eindhoven, the Netherlands) or an Accuvix XQ (Medison Co. Ltd., Seoul, Korea) ultrasound machine with a 6.0 MHz transducer covered with a sterile condom. The method used to measure the cervical length has been previously described. 12 Three measurements were taken, and the shortest distance was considered as the cervical length.
Histologic chorioamnionitis was diagnosed in the presence of acute inflammatory changes in any of the tissue samples (amnion, chorion-decidua, umbilical cord, and chorionic plate), using previously published criteria.4 Funisitis was diagnosed by the presence of neutrophil infiltration into the umbilical vessel walls or Wharton's jelly. Clinical chorioamnionitis was defined according to the criteria proposed by Gibbs, et al.13 Composite neonatal morbidity was defined as the presence of one or more of the following, which were diagnosed according to the definitions previously described in detail: respiratory distress syndrome,4 bronchopulmonary dysplasia,14 early-onset neonatal sepsis,15 periventricular leukomalacia,16 and necrotizing enterocolitis.17 Prophylactic antibiotics were given, although the type of antibiotic was left to the discretion of the attending obstetrician. Ampicillin was the primary antibiotic used. Meanwhile, erythromycin or azithromycin was additionally administered in many cases. Treatment with corticosteroids and antibiotics was started after amniocentesis.
Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution of the data. Univariate analysis was conducted with Student's t-test, Mann-Whitney U test, χ2 test, or Fisher's exact test. A multivariable logistic regression analysis was then performed using the forward stepwise technique to develop models for predicting the development of histologic chorioamnionitis. Two separate models were constructed for each non-invasive variable along with the combination of non-invasive and invasive parameters. Model 1 only included non-invasive variables. Model 2 included several variables (AF WBC count, AF IL-6 level, and AF culture results) associated with invasive amniocentesis, in addition to the non-invasive parameters. The linearity assumption was assessed by fitting models with spline transformations and testing the extra parameters against zero. For the logistic regression model, all factors were entered as dichotomous variables, because the assumption of linearity in the logit was not satisfied, especially for gestational age, maternal blood WBC count, AF WBC count, and IL-6 concentration. Receiver operating characteristic (ROC) curves were used to identify the best cut-off values for the dichotomization of variables. The optimal cut-off values were obtained based on the Youden index maximum: [(sensitivity+specificity)-1].18 Risk scores based on each model were calculated according to the coefficients of variables obtained from the forward stepwise modeling. p-values <0.2 from the univariate analysis were required for entry into the stepwise modeling, and a p-value <0.05 was required for final inclusion in the model. Goodness-of-fit of the logistic regression model was assessed by the Hosmer-Leme-show test. Tests for interaction were performed by entering cross-product terms into the logistic models. The discriminatory ability of the model was assessed by the ROC curve analysis. The value that maximized the sum of the sensitivity and specificity was considered the best cut-off point. To determine whether or not additional invasive test results improved the prediction of histologic chorioamnionitis based on the non-invasive model, we estimated the statistical significance of the difference in the area under the ROC curve (AUC) between models 1 and 2 using the method previously described by Hanley and McNeil.19 All reported p-values were two-sided, and p-values <0.05 were considered statistically significant. SPSS for Windows, version 20.0 (IBM SPSS Statistics, Chicago, IL, USA) was used for the statistical analyses.
RESULTS
During the study period, there were 8401 live singleton births at our tertiary referral hospital. Two hundred seventy-three women were diagnosed with PPROM between 20+0 and 33+6 weeks of gestation. Of these 273 women, 181 consecutive women diagnosed with PPROM who fulfilled the inclusion criteria were recruited for this study. Out of the 181 women, AF samples from seven women were not available for IL-6 measurement (four patients with a negative AF culture and three patients with a positive culture); 17 had an incomplete data set [lack of placental pathology (n=6), lack of AF WBC counts (n=2), lack of cervical length measurements (n=7), or lack of maternal serum CRP levels at the time of amniocentesis (n=2)]; three had a history of cervical cerclage placement during the index pregnancy; and eight patients were transferred to another hospital. These women were subsequently excluded from the study, leaving a total of 146 women suitable for evaluating the relationship between histologic chorioamnionitis and the covariates. The mean gestational age (±standard deviation) at the time of amniocentesis was 30.1±3.3 weeks, ranging from 21+1 to 33+6 weeks. The prevalences of histological and clinical chorioamnionitis were 50.7% (74/146) and 8.9% (13/146), respectively. The overall rate of microbial invasion of the amniotic cavity was 34.9% (51/146). Microorganisms isolated from the amniotic cavity included Ureaplasma urealyticum (n=41), Mycoplasma hominis (n=28), Escherichia coli (n=3), Peptostreptococcus species (n=2), Streptococcus agalactiae (n=2), Streptococcus viridans (n=2), Lactobacillus species (n=1), Staphylococcus aureus (n=1), and unidentified Gram-positive cocci (n=1). Polymicrobial invasion was present in 30 cases [59% (30/51)].
Table 1 shows the demographic and clinical characteristics of the women according to the presence or absence of histologic chorioamnionitis. There were no statistically significant differences in the mean maternal age, cervical length, the distribution of parous and non-parous women, or the prevalence of previous spontaneous preterm delivery between the two groups. However, women with histologic chorioamnionitis had a significantly lower mean gestational age at the time of amniocentesis and delivery, higher mean maternal serum CRP levels and WBC counts, and a higher rate of clinical chorioamnionitis than did those without histologic chorioamnionitis. Moreover, the median AF WBC counts and AF IL-6 levels, and the proportion of positive AF cultures were significantly higher for women with histologic chorioamnionitis than subjects without the disorder.
Table 1. Demographic and Clinical Characteristics of the Study Population According to the Presence or Absence of Histologic Chorioamnionitis.
| Histologic chorioamnionitis | p value | ||
|---|---|---|---|
| Presence (n=74) | Absence (n=72) | ||
| Maternal age (yrs) | 32.2±3.6 | 31.4±4.6 | 0.221 |
| Nulliparity | 37% (27/74) | 49% (35/72) | 0.138 |
| Previous spontaneous preterm birth (<37 wks) | 5% (4/74) | 10% (7/72) | 0.323 |
| Gestational age at assessment (wks) | 29.1±3.3 | 31.2±2.9 | <0.001 |
| Gestational age at delivery (wks) | 30.3±3.3 | 32.6±2.4 | <0.001 |
| Cervical length by ultrasound (mm) | 22.2±12.4 | 23.9±11.1 | 0.372 |
| Sampling-to-delivery interval [hrs, median (95% Cl)] | 123 (70–176) | 253 (105–401) | 0.257* |
| Maternal blood WBC count (cells/mm3) | 12037±3623 | 10434±3516 | <0.01 |
| Serum C-reactive protein (mg/L) | 12.7±1.68 | 4.8±5.2 | <0.001 |
| Amniotic fluid IL-6 (ng/mL) | 15.0±23.2 | 2.8±6.7 | <0.001 |
| Amniotic fluid WBC count (cells/mm3) | 1790±8426 | 311±1429 | <0.001 |
| Positive amniotic fluid cultures | 46% (34/74) | 24% (17/72) | <0.01 |
| Funisitis | 47% (35/74) | 0% (0/72) | <0.001 |
| Clinical chorioamnionitis | 14% (10/74) | 4% (3/72) | 0.047 |
| Use of antibiotics | 96% (71/74) | 94% (68/72) | 0.671 |
| Use of antenatal corticosteroids | 81% (60/74) | 86% (62/72) | 0.412 |
| Composite neonatal morbidity† | 49% (33/68) | 28% (20/71) | <0.05 |
| Early-onset neonatal sepsis | 7% (5/68) | 10% (7/71) | 0.599 |
| Respiratory distress syndrome | 32% (22/68) | 18% (13/71) | 0.057 |
| Bronchopulmonary dysplasia | 25% (17/68) | 7% (5/71) | <0.005 |
| Necrotizing enterocolitis | 4% (3/68) | 0% (0/71) | 0.114 |
| Periventricular leukomalacia | 9% (6/68) | 11% (8/71) | 0.632 |
| Neonatal mortality† | 1% (1/68) | 1% (1/71) | 1.000 |
| Birth weight (g) | 1584±518 | 1991±510 | <0.001 |
CI, confidence interval; WBC, white blood cell; IL-6, interleukin-6.
Values are presented as the mean±standard deviation or % (n) unless otherwise indicated.
*Log-rank test, †Seven infants who were not actively resuscitated at birth due to extremely low gestational age (less than 23.4 weeks) were excluded.
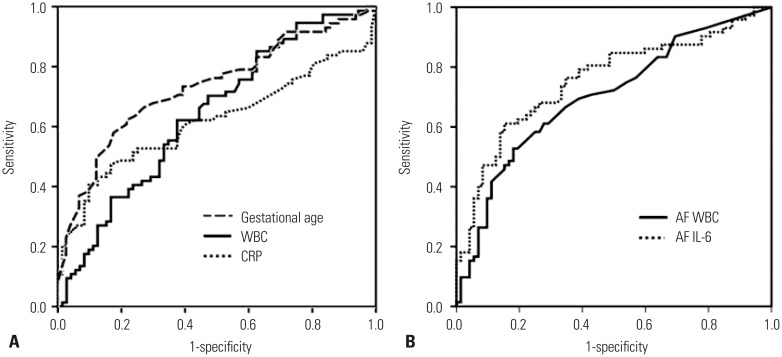
The ROC curves of non-invasive and invasive parameters for predicting histologic chorioamnionitis are presented in Fig. 1. All the curves were above the 45° line, indicating a significant relationship between these factors and histologic chorioamnionitis. The best cut-off values for predicting histologic chorioamnionitis were 32.0 weeks for gestational age (sensitivity of 61% and specificity of 78%), 10600 cells/mm3 for maternal blood WBC count (sensitivity of 62% and specificity of 61%), 5.1 mg/L for serum CRP concentration (sensitivity of 53% and specificity of 75%), 10 cells/mm3 for AF WBC count (sensitivity of 69% and specificity of 61%), and 2.4 ng/mL for AF IL-6 concentration (sensitivity of 62% and specificity of 78%).
Fig. 1. Receiver operating characteristic curves for each (A) non-invasive and (B) invasive parameter for predicting subsequent histologic chorioamnionitis [gestational age: area under the curve (AUC)=0.734, SE=0.042, p<0.001; serum C-reactive protein (CRP): AUC=0.617, SE=0.048, p<0.015; maternal blood white blood cell (WBC): AUC=0.640, SE=0.046, p=0.003; amniotic fluid (AF) WBC: AUC=0.702, SE=0.044, p<0.001; AF interleukin-6 (IL-6): AUC=0.743, SE=0.041, p<0.001]. SE, standard error.
In an attempt to better predict which women with PPROM would have histologic evidence of chorioamnionitis using a non-invasive technique, a multivariable analysis was conducted with a stepwise logistic regression model. All continuous variables were dichotomized using the cut-off values derived from the ROC curves, because the assumption of linearity in the logit was not verified. After the exclusion of variables (i.e., AF IL-6 level, AF WBC count, and AF culture results) for invasive amniocentesis, four variables with a significant correlation or a tendency towards an association with histologic chorioamnionitis based on the univariate analysis (p<0.2) were entered into the multivariate model (parity, gestational age at assessment, serum CRP level, and maternal blood WBC count). The final variables retained in the non-invasive model were gestational age at the time of assessment and serum CRP levels (Table 2). There was no statistically significant interaction between these two variables. The discriminatory ability of this model was 0.742 [95% confidence interval (CI), 0.661–0.823], demonstrating a reasonably good capacity to discriminate between women with and without histologic chorioamnionitis. The Hosmer-Lemeshow test showed an adequate fit of the model studied (p=0.389). A cut-off value ≥0.32 was identified as the optimal threshold for risk score, with a sensitivity of 89% (95% CI, 80–95%) and specificity of 51% (95% CI, 39–63%) to predict the occurrence of histologic chorioamnionitis. Positive and negative likelihood ratios were 1.83 (95% CI, 1.40–2.40) and 0.21 (95% CI, 0.10–0.40), respectively.
Table 2. The Final Non-Invasive Model and the Final Model That Included the Additional Invasive Test Results for Determining Risk Scores to Predict Histologic Chorioamnionitis.
| Predictor | Beta-coefficient | S.E. | Odds ratio | 95% CI | p value |
|---|---|---|---|---|---|
| Non-invasive model* | |||||
| Gestational age at assessment, ≤32.0 wks | 1.589 | 0.381 | 4.900 | 2.322–10.340 | <0.001 |
| Serum CRP, ≥5.1 mg/L | 0.960 | 0.384 | 2.612 | 1.231–5.541 | 0.012 |
| Constant | -1.289 | 0.323 | 0.276 | <0.001 | |
| Model including the additional invasive test results† | |||||
| Gestational age at assessment, ≤32.0 wks | 1.249 | 0.406 | 3.485 | 1.574–7.719 | 0.002 |
| AF IL-6, ≥2.4 ng/mL | 1.269 | 0.405 | 3.557 | 1.609–7.861 | 0.002 |
| Constant | -1.241 | 0.311 | 0.289 | <0.001 |
CRP, C-reactive protein; AF, amniotic fluid; IL-6, interleukin-6; S.E., standard error.
*Formula that was generated to predict histologic chorioamnionitis was as follows: Y=loge (Z)=-1.289+1.589 (if gestational age at assessment was ≤32.0 weeks)+0.960 (if serum CRP was ≥5.1 mg/L). Z=ey and risk (%)=[Z/(1+Z)]×100, †Formula that was generated to predict histologic chorioamnionitis was as follows: Y=loge (Z)=-1.241+1.249 (if gestational age at assessment was ≤32.0 weeks)+1.269 (if AF IL-6 was ≥2.4 ng/mL). Z=ey and risk (%)=[Z/(1+Z)]×100.
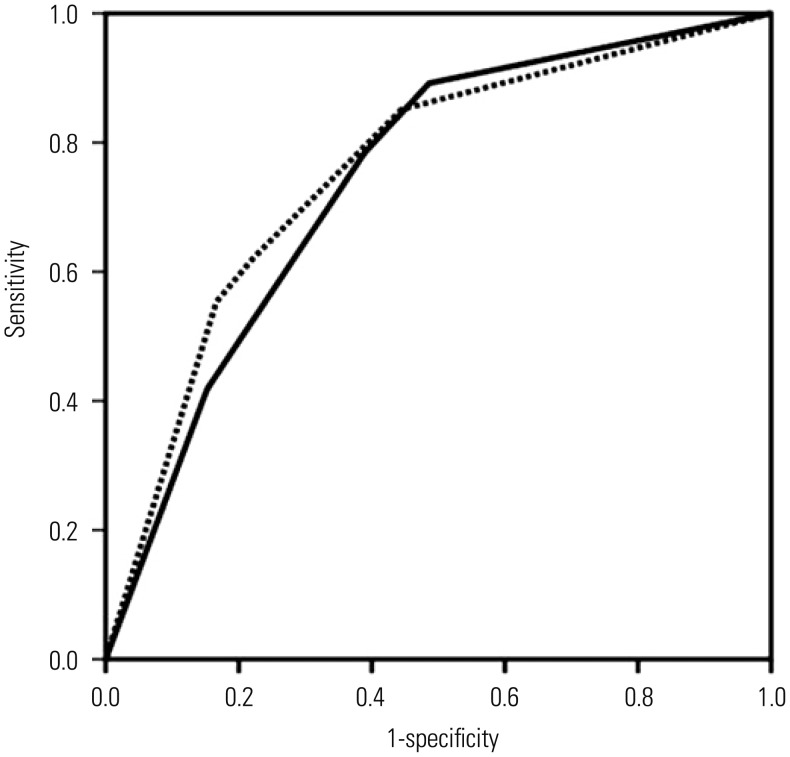
A further risk score was then identified using the four variables of the initial non-invasive model and three variables (i.e., AF IL-6 level, AF WBC count, and AF culture results) for invasive amniocentesis. Ultimately, seven variables were included in this model (model 2). When including AF IL-6 concentration, AF WBC count, and AF culture results as invasive markers in the non-invasive model (model 1), serum CRP level, parity, and maternal WBC count were insignificant and excluded from the final model (model 2), whereas AF IL-6 and gestational age were retained (Table 2). No interaction between these two predictors was demonstrated. The Hosmer-Lemeshow test showed an adequate fit of the model studied (p=0.927). AUCs for predicting histologic chorioamnionitis were 0.742 for model 1 and 0.757 for model 2 (Fig. 2). These values were not significantly different (p=0.623).
Fig. 2. ROC curves comparing the power of the non-invasive model (model 1, solid line), and the addition (model 2, dotted line) of amniotic fluid tests results as invasive markers to the non-invasive model for predicting subsequent histologic chorioamnionitis [model 1: area under the curve (AUC), 0.742; 95% confidence interval (CI), 0.661–0.823 vs. model 2: AUC, 0.757; 95% CI, 0.677–0.836; p=0.623). ROC, receiver operating characteristic.
To assess the relationship between clinical chorioamnionitis and the covariates, we repeated the same analyses described above for 146 women with PPROM. As shown in Table 3, no parameters studied, including both non-invasive and invasive factors, were found to identify women at high risk for developing clinical chorioamnionitis.
Table 3. Demographic and Clinical Characteristics of the Study Population According to the Presence or Absence of Clinical Chorioamnionitis.
| Clinical chorioamnionitis | p value | ||
|---|---|---|---|
| Presence (n=13) | Absence (n=133) | ||
| Maternal age (yrs) | 30.9±4.1 | 31.9±4.2 | 0.450 |
| Nulliparity | 46% (6/13) | 42% (56/133) | 0.778 |
| Previous spontaneous preterm birth (<37 wks) | 8% (1/13) | 8% (10/133) | 1.000 |
| Gestational age at assessment (wks) | 30.2±1.5 | 30.1±3.4 | 0.326 |
| Gestational age at delivery (wks) | 31.5±1.4 | 31.4±3.2 | 0.254 |
| Cervical length by ultrasound (mm) | 23.4±14.0 | 23.0±11.7 | 0.904 |
| Sampling-to-delivery interval [hrs, median (95% Cl)] | 405 (0–935) | 153 (60–246) | 0.315* |
| Maternal blood WBC count (cells/mm3) | 10765±3813 | 11293±3643 | 0.427 |
| Serum C-reactive protein (mg/L) | 9.0±13.6 | 8.8±13.1 | 0.680 |
| Amniotic fluid IL-6 (ng/mL) | 13.1±25.1 | 8.9±17.3 | 0.948 |
| Amniotic fluid WBC count (cells/mm3) | 915±2340 | 1063±6303 | 0.704 |
| Positive amniotic fluid cultures | 54% (7/13) | 33% (44/133) | 0.134 |
| Histologic chorioamnionitis | 77% (10/13) | 48% (64/133) | 0.078 |
| Funisitis | 62% (8/13) | 20% (27/133) | 0.001 |
| Use of antibiotics | 100% (13/13) | 95% (126/133) | 1.000 |
| Use of antenatal corticosteroids | 100% (13/13) | 82% (109/133) | 0.127 |
| Composite neonatal morbidity† | 54% (7/13) | 37% (46/126) | 0.220 |
| Early-onset neonatal sepsis | 23% (3/13) | 7% (9/126) | 0.086 |
| Respiratory distress syndrome | 31% (4/13) | 25% (31/126) | 0.738 |
| Bronchopulmonary dysplasia | 15% (2/13) | 16% (20/126) | 1.000 |
| Necrotizing enterocolitis | 0% (0/13) | 2% (3/126) | 1.000 |
| Periventricular leukomalacia | 15% (2/13) | 10% (12/126) | 0.621 |
| Neonatal mortality† | 8% (1/13) | 1% (1/126) | 0.179 |
| Birth weight (g) | 1798±337 | 1784±568 | 0.706 |
CI, confidence interval; WBC, white blood cell; IL-6, interleukin-6.
Values are presented as the mean±standard deviation or % (n) unless otherwise indicated.
*Log-rank test, †Seven infants who were not actively resuscitated at birth due to extremely low gestational age (less than 23.4 weeks) were excluded.
DISCUSSION
The principal findings of this study suggest that 1) a model based on non-invasive clinical and laboratory parameters (gestational age and maternal CRP) is effective for predicting subsequent development of histologic chorioamnionitis in women with PPROM and 2) invasive test results requiring amniocentesis, such as AF IL-6 levels, do not add predictive information to the non-invasive model. Similar observations have been reported in the setting of intra-amniotic inflammation and imminent preterm delivery in women with PPROM by our and other groups.2,10,20 Collectively, these findings are relevant given that amniocentesis (a costly and invasive procedure) may be avoided, and underscore the importance of considering multiple clinical and laboratory factors when assessing the likelihood of early intrauterine infection.
At a lower gestation, the most important characteristics of an ideal diagnostic test for infection in cases of PPROM should have very high specificity to reduce the risk of intervention in response to a false positive test, which could lead to an unnecessary elective preterm birth. Therefore, from a clinical perspective, this non-invasive model can be recommended as a screening tool for predicting intrauterine development of infection at early stage rather than a diagnostic test. While the results of our non-invasive model may not provide a definitive diagnosis, it could help identify high-risk women who require more intensive observation and treatment, as well as risk-specific intervention (i.e., amniocentesis), during antenatal periods following PPROM.
In the present study, we recorded a prevalence of histological chorioamnionitis of 50.7% (74/146), which is in agreement with findings from previous studies.7 In accordance with these previous investigations,7 we found that elevated CRP levels are associated with subsequent histologic chorioamnionitis in women with PPROM. Our data also demonstrated the superiority of measuring AF IL-6 concentrations, compared to CRP determination, in terms of predicting the occurrence of histologic chorioamnionitis. Nevertheless, the performance of CRP in this study for the prediction of histologic chorioamnionitis was worse than that observed in previous work from our institution (AUC, 0.617 vs. 0.760; p=0.039).5 The apparent discrepancy between the current finding and those from the previous study is most likely related to one of the enrolment criteria of the previous study (delivery within 72 hours after sampling). Based on the data reported by Fisk, et al.,21 showing that CRP elevation often precedes clinical infection or delivery by several days, we believe that CRP accurately serves as histologic evidence of chorioamnionitis within a few days before delivery. This might also be a contributing factor for better performance of CRP in the previous study.
Contrary to preterm labor with intact membranes,22,23 neither cervical length nor latency period (time from PPROM to delivery) was associated with the risk of subsequent histologic chorioamnionitis in women with PPROM. These observations are consistent with data from previous reports.24,25 Significant differences between PPROM and preterm labor with intact membranes in terms of cervical length and latency are probably related to the fact that systemic antibiotic therapy is routinely given to women with PPROM, while systemic antibiotic prophylaxis is not typically administered in cases of preterm labor. For patients with PPROM, administration of antibiotics, which has been shown to significantly reduce the incidence of histologic chorioamnionitis, prolong pregnancy, and improve neonatal outcomes,26,27 may potentially prevent a possible ascending infection or ameliorate local damage to the placenta already experiencing acute inflammation.
Variations in the reported incidence of clinical chorioamnionitis are wide, ranging from 7% to 29%.28 In the present study, the finding that 8.9% of women in our cohort showed clinical evidence of chorioamnionitis is similar to data from a recent report evaluating a retrospective cohort of 430 patients with PPROM that had a clinical chorioamninitis prevalence of 13%.29 In contrast to histologic chorioamnionitis, no parameters studied in our cohort could be used to predict which women were at a high risk for developing clinical chorioamnionitis. This finding is inconsistent with ones from previous reports published in the 1990s by different authors.8,28 The discrepancy between our findings and those of other groups may be related to recent changes in PPROM management regarding the recommended timing of delivery and antibiotic treatment, and different gestational ages at the time of inclusion. The routine policy of management for women with PPROM in our hospital, including the use of prophylactic broad-spectrum antibiotics and induction of labor at around 34.0 weeks, may reduce the risk of developing clinical chorioamnionitis that may occur late in the course of intrauterine infection and thus influence the identification of patients destined to develop an obvious symptomatic infection.
Our study had a number of limitations. First, the investigation was limited by its retrospective nature. Our findings should therefore be confirmed by prospective studies. Second, we lacked data for some previously reported important biomarkers in non-invasive samples of women presenting with PPROM, such as cervicovaginal fluid,20 which help clinicians discriminate between women at high risk and low risk for subsequent histologic chorioamnionitis. Third, we did not perform a full characterization of inflammatory cytokines in the AF samples, as previously reported for women with PPROM; we only measured IL-6 levels, as AF IL-6 concentrations are consistently reported to be the best predictor of histologic chorioamnionitis.1,4 Fourth, our results were not validated in a different hospital or with a different patient population. Therefore, the generalizability of our non-invasive model is limited. Fifth, the design of our study is likely to reduce the statistical power available to test our hypothesis by dichotomizing continuous independent parameters,30 although the final results are almost the same whether the variables added to the multivariate model were continuous variable or dichotomized. C-index values for model 1 and 2 with all continuous variables retained as continuous were 0.768 and 0.786, respectively. The strength of this study is that the influence of histologic chorioamnionitis on neonatal outcomes was evaluated, and the importance of predicting histologic chorioamnionitis was therefore evaluated in terms of improving the outcomes of pregnancy management for cases of PPROM.
In conclusion, a model based on non-invasive clinical and laboratory parameters (gestational age and maternal CRP) was effective for predicting the subsequent development of histologic chorioamnionitis in women with PPROM. Invasive test results requiring amniocentesis, such as AF IL-6 levels, did not add predictive information to the non-invasive model in this setting. None of the parameters studied (non-invasive or invasive parameters) could be used to identify women at high risk for the development of clinical chorioamnionitis. Additional large, prospective studies, especially those focused on serial estimations of clinical and laboratory variables, are required to confirm our findings.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Cobo T, Kacerovsky M, Palacio M, Hornychova H, Hougaard DM, Skogstrand K, et al. A prediction model of histological chorioamnionitis and funisitis in preterm prelabor rupture of membranes: analyses of multiple proteins in the amniotic fluid. J Matern Fetal Neonatal Med. 2012;25:1995–2001. doi: 10.3109/14767058.2012.666592. [DOI] [PubMed] [Google Scholar]
- 2.Park KH, Kim SN, Oh KJ, Lee SY, Jeong EH, Ryu A. Noninvasive prediction of intra-amniotic infection and/or inflammation in preterm premature rupture of membranes. Reprod Sci. 2012;19:658–665. doi: 10.1177/1933719111432869. [DOI] [PubMed] [Google Scholar]
- 3.Elimian A, Verma U, Beneck D, Cipriano R, Visintainer P, Tejani N. Histologic chorioamnionitis, antenatal steroids, and perinatal outcomes. Obstet Gynecol. 2000;96:333–336. doi: 10.1016/s0029-7844(00)00928-5. [DOI] [PubMed] [Google Scholar]
- 4.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 5.Oh KJ, Park KH, Kim SN, Jeong EH, Lee SY, Yoon HY. Predictive value of intra-amniotic and serum markers for inflammatory lesions of preterm placenta. Placenta. 2011;32:732–736. doi: 10.1016/j.placenta.2011.07.080. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsson B. Infectious and inflammatory mechanisms in preterm birth and cerebral palsy. Eur J Obstet Gynecol Reprod Biol. 2004;115:159–160. doi: 10.1016/j.ejogrb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 7.van de Laar R, van der Ham DP, Oei SG, Willekes C, Weiner CP, Mol BW. Accuracy of C-reactive protein determination in predicting chorioamnionitis and neonatal infection in pregnant women with premature rupture of membranes: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2009;147:124–129. doi: 10.1016/j.ejogrb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol. 1996;88:1034–1040. doi: 10.1016/s0029-7844(96)00339-0. [DOI] [PubMed] [Google Scholar]
- 9.Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 10.Park KH, Lee SY, Kim SN, Jeong EH, Oh KJ, Ryu A. Prediction of imminent preterm delivery in women with preterm premature rupture of membranes. J Perinat Med. 2011;40:151–157. doi: 10.1515/JPM.2011.124. [DOI] [PubMed] [Google Scholar]
- 11.Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Kim A. Intra-amniotic infection/inflammation as a risk factor for subsequent ruptured membranes after clinically indicated amniocentesis in preterm labor. J Korean Med Sci. 2013;28:1226–1232. doi: 10.3346/jkms.2013.28.8.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park KH. Transvaginal ultrasonographic cervical measurement in predicting failed labor induction and cesarean delivery for failure to progress in nulliparous women. J Korean Med Sci. 2007;22:722–727. doi: 10.3346/jkms.2007.22.4.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 15.Dulay AT, Buhimschi IA, Zhao G, Luo G, Abdel-Razeq S, Cackovic M, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. 2008;198:426.e1–426.e9. doi: 10.1016/j.ajog.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 17.Richter D, Bartmann P, Pohlandt F. Prevention of necrotizing enterocolitis in extremely low birth weight infants by IgG feeding? Eur J Pediatr. 1998;157:924–925. doi: 10.1007/s004310050968. [DOI] [PubMed] [Google Scholar]
- 18.Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 20.Jun JK, Yoon BH, Romero R, Kim M, Moon JB, Ki SH, et al. Interleukin 6 determinations in cervical fluid have diagnostic and prognostic value in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:868–873. doi: 10.1067/mob.2000.109034. [DOI] [PubMed] [Google Scholar]
- 21.Fisk NM, Fysh J, Child AG, Gatenby PA, Jeffery H, Bradfield AH. Is C-reactive protein really useful in preterm premature rupture of the membranes? Br J Obstet Gynaecol. 1987;94:1159–1164. doi: 10.1111/j.1471-0528.1987.tb02316.x. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. Ultrasonographic assessment of the uterine cervix and interleukin-8 concentrations in cervical secretions predict intrauterine infection in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 1998;12:86–92. doi: 10.1046/j.1469-0705.1998.12020086.x. [DOI] [PubMed] [Google Scholar]
- 23.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol. 1993;81:941–948. [PubMed] [Google Scholar]
- 24.Gire C, Faggianelli P, Nicaise C, Shojai R, Fiori A, Chau C, et al. Ultrasonographic evaluation of cervical length in pregnancies complicated by preterm premature rupture of membranes. Ultrasound Obstet Gynecol. 2002;19:565–569. doi: 10.1046/j.1469-0705.2002.00666.x. [DOI] [PubMed] [Google Scholar]
- 25.Ghidini A, Salafia CM, Minior VK. Lack of relationship between histologic chorioamnionitis and duration of the latency period in preterm rupture of membranes. J Matern Fetal Med. 1998;7:238–242. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<238::AID-MFM6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Ovalle A, Martínez MA, Kakarieka E, Gómez R, Rubio R, Valderrama O, et al. Antibiotic administration in patients with preterm premature rupture of membranes reduces the rate of histological chorioamnionitis: a prospective, randomized, controlled study. J Matern Fetal Neonatal Med. 2002;12:35–41. doi: 10.1080/jmf.12.1.35.41. [DOI] [PubMed] [Google Scholar]
- 27.Hutzal CE, Boyle EM, Kenyon SL, Nash JV, Winsor S, Taylor DJ, et al. Use of antibiotics for the treatment of preterm parturition and prevention of neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 2008;199:620.e1–620.e8. doi: 10.1016/j.ajog.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Ohlsson A, Wang E. An analysis of antenatal tests to detect infection in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1990;162:809–818. doi: 10.1016/0002-9378(90)91016-6. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey PS, Lieman JM, Brumfield CG, Carlo W. Chorioamnionitis increases neonatal morbidity in pregnancies complicated by preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192:1162–1166. doi: 10.1016/j.ajog.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]