Abstract
Purpose
We tried to evaluate the difference in the expression of carbonic anhydrase (CA) III and heat shock protein (Hsp) 70 between laryngopharyngeal reflux disease (LPRD) and non-LPRD patients.
Materials and Methods
The study involved 28 patients who underwent laryngeal microsurgery due to benign laryngeal disease from March to August 2008. Reflux symptom index (RSI) and reflux finding score (RFS) were measured for each person, and they were assigned either to the LPRD group (n=10) or non-LPRD group (n=18). Tissue samples were obtained from the mucosa of posterior commissure, and immunohistochemistry (IHC) staining of CAIII and Hsp70 was performed. The IHC scores were measured and compared with clinical features including RSI and RFS.
Results
Total 10 patients were assigned as LPRD group, and 18 patients were as control group. The mean IHC score of CAIII and Hsp70 was 1.70±1.06 and 1.90±0.88, respectively, in LPRD patients, whereas the mean IHC score of CAIII and Hsp70 was 0.78±0.73 and 0.94±0.87, respectively, in non-LPRD patients. The difference between two groups was statistically significant (p<0.05).
Conclusion
CAIII and Hsp70 expressions were higher in LPRD patients that in non-LPRD patients, suggesting the possibility as one of biomomarker in LPRD diagnosis.
Keywords: Carbonic anhydrase-III, heat shock protein 70, laryngopharyngeal reflux disease, deglutition, deglutition disorders
INTRODUCTION
Laryngopharyngeal reflux disease (LPRD) is due to retrograde flow of gastric contents or gastric acid into the laryngopharyngeal mucosa.1 It has been estimated that 15 to 20% of patients present to an otolaryngologist with symptoms that include chronic cough, globus sensation, dysphonia, and sore throat.2 LPRD has been correlated with the development of atypical laryngopharyngeal inflammatory disease and cancer development.3,4
Although LPRD is a relatively common disease, the diagnosis remains difficult. Ambulatory 24-hour double probe pH monitoring has been the most important diagnostic tool to date.5 However, this approach cannot be applied to all patients suspected of having LPRD because of its invasive nature and high false positive rate.6 Multi-channel intraluminal impedance monitoring is reported to be a better method to evaluate gastric contents, nevertheless, its potential remains speculative.7 Due to the limitation of these diagnostic procedures, diagnosis generally relies on indirect, simple, and painless approaches that include history taking of specific symptoms, grading of laryngeal endoscopic finding, change of the symptoms to behavioral modification, and empirical treatments.8 These approaches, therefore, rely on the experience and judgment of the physician and results can vary between individuals, thus limiting objective diagnosis.
The present study investigated the immunohistochemistry (IHC) staining patterns of carbonic anhydras (CA) III, a known factor related to pepsin and corrosive gastric regurgitation, and the expression of heat shock protein (Hsp) 70, a famous chaperone molecule which acts as a danger associated molecular pattern.9,10 The results were compared with reflux symptom index (RSI) and reflux finding score (RFS) to assess the value of CAIII and Hsp70 as biomarkers for the diagnosis of LPRD.
MATERIALS AND METHODS
Patients
This study was approved by Institutional Review Board (IRB) of Chung-Ang University [IRB Number: C2010018 (313)]. The study involved 28 patients (16 men and 12 women; mean age 49.6 years, range 30–69 years) who provided informed consent and underwent laryngeal microsurgery for a benign vocal cord lesion, such as vocal cord polyp or nodule. They all agreed for the biopsy from posterior commissure area. At study entry, smoking history (nonsmoker, smoker, ≥1 year ex-smoker, <1 year ex-smoker), alcohol intake (non-alcoholic, social drinker, over-drinker), and body mass index (BMI; overweight, ≥25 kg/m2; normal/underweight, <25 kg/m2) were determined.
Diagnosis of LPRD
Prior to laryngeal microsurgery, patients were categorized as LPRD group (RSI>13, RFI>7) and non-LPRD group (RSI<13, RFI<7). RSI is a 9-item self-administered outcome instrument that accurately documents symptoms of patients with LPRD (Supplementary Table 1, only online).11 The scale for each individual item ranges from 0 (no problem) to 5 (severe problem), with a maximum total score of 45. Direct rigid angulated laryngoscopy (70°) with video documentation and determination of the RFS was also performed by two otolaryngologists at study entry. The RFS is an 8-item clinical severity rating scale, based on the direct rigid laryngoscopic findings (Supplementary Table 2, only online).12 It ranges from 0 (no abnormal findings) to maximum of 26 (worst score possible).
Tissue samples
Laryngeal epithelial biopsy specimens were taken from all patients. The 2 mm-sized specimens were taken from the laryngeal posterior commissure using a KLEINSASSER™ operating laryngoscope with Nagashima™ 2.5 mm cupped, round, straight laryngeal forceps. Consistently, there was almost no bleeding at the biopsy site. Instances of minimal bleeding were controlled using Bosmin. There were no postoperative specific complications.
IHC of CAIII and Hsp70
Formalin-fixed, paraffin-embedded tissue sections (4 to 5 µm thickness) on slides were placed in an incubator at 60℃ for 30 minutes to secure the tissue sections. The sections were deparaffinized in xylene three times over a 5-minute period, dehydrated in 90%, 75%, and 50% ethanol, each for 2 minutes, and washed in distilled water for 5 minutes. The antigen retrieval technique was used to maximize the immunohistochemical signal. Sections were pretreated with 0.01 mol/L citrate buffer (pH 6.0) and microwaved for five 2-minute cycles. The slides were cooled in buffer before further treatment. Endogenous peroxidase activity was blocked with 0.5% hydrogen peroxidemethanol for 10 minutes, and sections were washed in distilled water for 10 minutes. Immunohistochemical staining was done using an AUTOSTAINER 480 (Labvision, Fremont, CA, USA) and detection kit that incorporated an Ultravision LP detection system, horseradish peroxidase polymer, and AEC chromogen (Labvision). Primary antibodies were mouse monoclonal anti-CAIII (clone 4A12-1A3; Abnova, Taipei City, Taiwan) and mouse monoclonal anti-Hsp70 (Thermo Fisher Scientific, Fremont, CA, USA). Striated muscle tissue and breast cancer tissue were used as positive controls.
Evaluation of antibodies
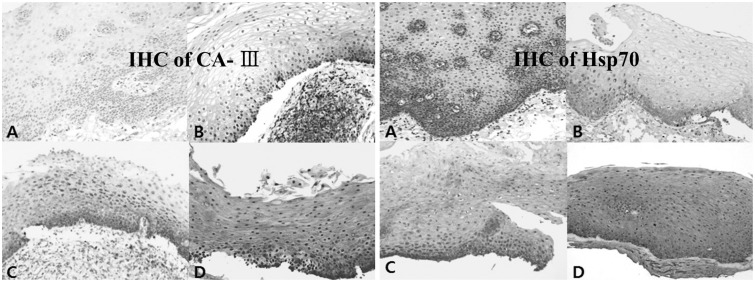
Brown staining of CAIII and Hsp70 in cytoplasm was defined as positive. Each tissue specimen was independently examined for CAIII and Hsp70. The levels of CAIII and Hsp70 expression were assessed using a semiquantitative scoring system by two experienced pathologists. Expression was coded as follows: cells absent, 0; <50% cells positive, 1; 50% to 90% cells positive, 2; and >90% cells positive, 3 (Fig. 1).
Fig. 1. Immunohistochemical (IHC) staining for CAIII and Hsp70 in the posterior commissure epithelium (×200). IHC reactivity for CAIII and Hsp70 was read by our scoring system, based on the quantity of brown pigmentation in the cytoplasm of cells. (A) Not expressed in all cells, showing IHC score 0. (B) Present less than 50% cells, showing IHC score 1. (C) Present between 50% and 90% cells, showing IHC score 2. (D) Expressed more than 90% of posterior commissure epithelial cells, showing IHC score 3. CAIII, carbonic anhydrase-III; Hsp70, heat shock protein 70.
Statistical analyses
All data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Since the data were not normally distributed, nonparametric statistics was used to detect a correlation between smoking, alcohol intake, and BMI on LPRD symptoms, and RSI, RFS, and expression of CAIII and Hsp70. Correlations between factors were tested using Spearman's correlation test. The difference in level of the expression of CAIII and Hsp70 detected between normal controls and LPR patients were tested using the Mann-Whitney test. Statistical significance was accepted as a p value <0.05.
RESULTS
Diagnosis of LPRD by means of RSI, RFS and clinical features of LPRD
There were 10 patients with LPRD (four males, six females; mean age, 51.9 years) according to RFS and RSI. The non-LPRD group consisted of 12 males and six females with a mean age of 48.6 years. There were seven non-smokers and three smokers in LPRD group, and seven non-smokers, three <1 year ex-smokers, and eight smokers in non-LPRD group. The LPRD group comprised seven non-alcoholics, one social drinker, and two over-drinkers, and non-LPRD group comprised six non-alcoholics and 12 social drinkers. The mean BMI was 25.36±2.35 kg/m2 with five normal (BMI <25 kg/m2) and five overweight (BMI ≥25 kg/m2) individuals in LPRD group. In non-LPRD group, there were 10 normal (BMI <25 kg/m2) and eight overweight (BMI ≥25 kg/m2) individuals (mean BMI 25.55±2.91 kg/m2). Among LPRD patients, 4 patients took proton pump inhibitor pills, and mean medication duration of medication was 48 days.
Distribution of immunohistochemical staining score of CAIII and Hsp70
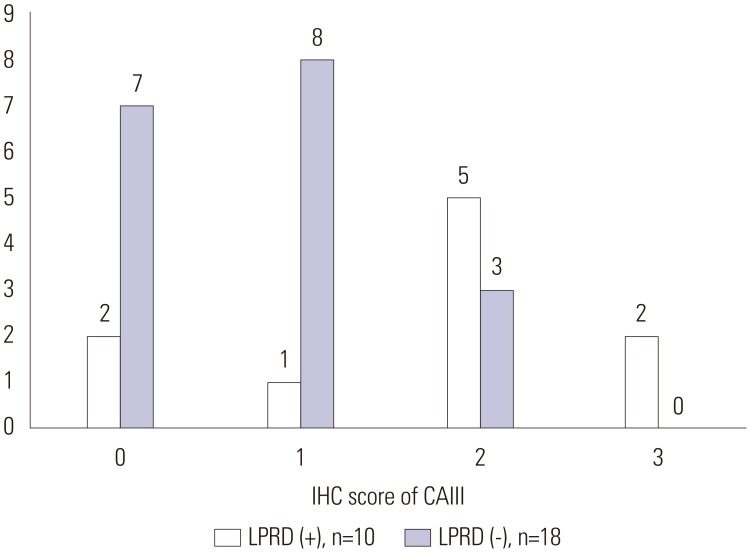
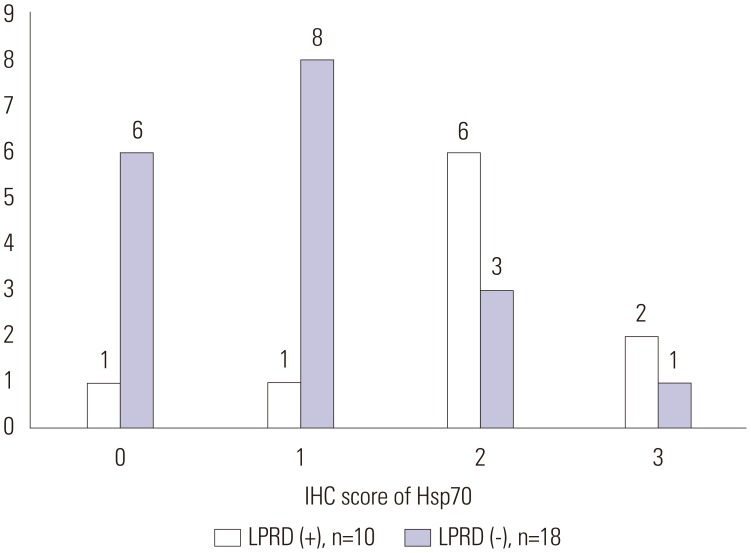
In LPRD group (n=10), the semiquantitative IHC staining score of CAIII was 0 (n=2), 1 (n=1), 2 (n=5), and 3 (n=2). In non-LPRD group (n=12), IHC staining score of CAIII was 0 (n=1), 1 (n=8), and 2 (n=3) (Fig. 2). The IHC staining scores of Hsp70 for the LPRD patients were 0 (n=1), 1 (n=1), 2 (n=6), and 3 (n=2). The IHC staining scores of Hsp70 for the 18 non-LPRD patients were 0 (n=6), 1 (n=8), 2 (n=3), and 3 (n=1) (Fig. 3).
Fig. 2. IHC score of CAIII in LPRD and non-LPRD patients. In LPRD group (n=10), the semiquantitative IHC staining score of CAIII was 0 (n=2), 1 (n=1), 2 (n=5), and 3 (n=2). In non-LPRD group (n=12), IHC staining score of CAIII was 0 (n=1), 1 (n=8), and 2 (n=3). IHC, immunohistochemical; CAIII, carbonic anhydrase-III; LPRD, laryngopharyngeal reflux disease.
Fig. 3. IHC score of Hsp70 in LPRD and non-LPRD patients. The IHC staining scores of Hsp70 for the LPRD patients were 0 (n=1), 1 (n=1), 2 (n=6), and 3 (n=2). The IHC staining scores of Hsp70 for the 18 non-LPRD patients were 0 (n=6), 1 (n=8), 2 (n=3), and 3 (n=1). IHC, immunohistochemical; Hsp70, heat shock protein 70; LPRD, laryngopharyngeal reflux disease.
Correlations between RSI, RFS and expression of CAIII, Hsp70
Significant associations between RSI, RFS, and the IHC scores of CAIII and Hsp70 in the 28 subjects were identified by statistical analysis. Smoking, alcohol intake, and BMI showed no statistically significant correlation with RSI, RFS, and expressions of CAIII and Hsp70 (all p>0.05) (Table 1).
Table 1. Comparison of RSI and RFS with CAIII and Hsp70 as LPRD-Related Factors.
| n=28 | RSI | RFS | CAIII | Hsp70 | Smoking Hx | Alcohol Hx | BMI |
|---|---|---|---|---|---|---|---|
| RSI | |||||||
| Correlation coefficient | 1 | 0.867 | 0.499 | 0.527 | -0.098 | -0.148 | 0 |
| p value | 0.000* | 0.007* | 0.004* | 0.618 | 0.452 | 0.999 | |
| RFS | |||||||
| Correlation coefficient | 0.867 | 1 | 0.622 | 0.651 | -0.158 | -0.151 | -0.258 |
| p value | 0.000* | 0.000* | 0.000* | 0.423 | 0.444 | 0.185 | |
| CAIII | |||||||
| Correlation coefficient | 0.499 | 0.622 | 1 | 0.748 | -0.081 | -0.157 | -0.158 |
| p value | 0.007* | 0.000* | 0.000* | 0.681 | 0.424 | 0.422 | |
| Hsp70 | |||||||
| Correlation coefficient | 0.527 | 0.651 | 0.748 | 1 | -0.050 | -0.166 | 0.033 |
| p value | 0.004* | 0.000* | 0.000* | 0.802 | 0.389 | 0.867 |
RSI, reflux symptom index; RFS, reflux finding score; CAIII, carbonic anhydrase-III; Hsp70, heat shock protein 70; BMI, body mass index; LPRD, laryngopharyngeal reflux disease; Hx, history.
*p value<0.05.
Correlation of expression of CAIII, Hsp70 between LPRD and normal group
In the LPRD group, semiquantitative IHC staining score of CAIII and Hsp70 was 1.70±1.06 and 1.90±0.88, respectively. In non-LPRD group, the CAIII and Hsp70 score was 0.78±0.73 and 0.94±0.87, respectively. Since the data were not normally distributed, nonparametric statistics, Mann-Whitney test was used. The expressions of CAIII and Hsp70 were significantly higher in LPRD patients (p<0.05) (Table 2).
Table 2. Correlation of LPRD (RSI≥13 and RFS≥7) with CAIII and Hsp70 Expression.
| LPRD (+) (n=10) | LPRD (-) (n=18) | p value | |
|---|---|---|---|
| CAIII | 1.70±1.06 | 0.78±0.73 | 0.024* |
| Hsp70 | 1.90±0.88 | 0.94±0.87 | 0.014* |
LPRD, laryngopharyngeal reflux disease; RSI, reflux symptom index; RFS, reflux finding score; CAIII, carbonic anhydrase-III; Hsp70, heat shock protein 70.
*p value<0.05.
DISCUSSION
Subjective diagnostic methods for LPRD include laryngopharyngeal reflux related history, grading laryngeal endoscopic findings, change of the symptoms to behavioral modification and empirical treatments. Objective diagnoses include ambulatory 24-hour double probe pH monitoring and multi-channel intraluminal impedance monitoring.5,6,13 While these methods are generally used and are helpful in the diagnosis of LPRD, their reliability and accuracy are debatable. Therefore, RSI and RFS based on the patient's medical history and laryngoscopic findings are usually used for the diagnosis of LPRD.14
In our present study, patients were divided in to LPRD and non-LPRD groups based on RSI and RFS score. The RSI is a 9-item self-administered outcome instrument. A RSI score >10 is associated with a high likelihood of pH-documented LPRD,5 and high correlation between the initial and second pretreatment surveys had demonstrated reproducible nature of this approach.11 RFS is an 8-item clinical severity scale based on findings during fiber optic laryngoscopy that was developed to standardize the laryngeal findings of LPRD. A RFS >7 is defined as LPRD status.2
Reflux-related laryngeal disease results in the breakdown of mechanisms that normally protect against these esophageal and laryngeal epithelial damages by corrosive refluxate.1 One such defense mechanism that is important in protecting the mucosa from the damaging effects of gastric reflux is CA.9,15 CA can neutralize gastric reflux directly and participates indirectly in diminishing gastric acid activity by regulating pH. Currently, 11 catalytically active CA isoenzymes have been identified, which differ in activity, susceptibility to inhibitors, and tissue distribution.15 Among them, CAIII has been found to be upregulated in gastroesophageal reflux diseases (GERD) patients, and is one of the mechanisms that compensates for the gastric acid-mediated damage.9,16 Also, a redistribution of CAIII from the basal to suprabasal cell layers have been reported in inflamed esophageal tissue in patients with GERD.16 These changes indicate that the epithelial expression of CAIII is increased to protect esophagus from gastric reflux-mediated damage in patients with GERD.
In laryngeal area, expressions of CA isoenzyme I, II, and III in normal laryngeal epithelial cells9 have been proved. Interestingly, however, the expression of CAIII has been found to differ depending on the laryngeal location in LPRD. In LPRD, the expression of CAIII was decreased in vocal fold, whereas increased in the posterior commissure.16 Comparison study of expression differences among the biopsy location might be an important concern.
The novelty of our study is that we obtained posterior commissure tissue in which the level of CAIII was higher in LPRD than non-LPRD. Previously, Western blot assays showed 73% positive detection rate.9 In the present study, however, we performed IHC assay, and the detection rate of CAIII was higher than the previous study. Our study revealed that the expression level of CAIII was significantly associated with RSI and RFS. Expression of CAIII in the LPRD group was higher than in the CAIII in non-LPRD group (p<0.05). There is an earlier study which should that the level of CAIII was associated with symptom severity.16 We proved that not only subjective symptom but also endoscopic finding represented by RFS was associated with the level of CAIII in posterior commissure mucosa. Reverse correlation of the level of CAIII with RSI and RFS suggest that CAIII in posterior commissure might be involved in the protection mechanism from laryngeal damage mediated by gastric acid and gastric contents.
Hsps are one of the most highly conserved and widely recruited cellular defense pathways known.17 Hsps are induced by cellular stresses that include heat shock, alcohol exposure, inhibitors of energy metabolism, heavy metals, amino acid analogues, fever, inflammation, and ischemia.18,19 There are various Hsp families (25, 40, 60, 70, 90, and 110) according to molecular weight, and Hsp70 is the most highly conserved of Hsps.10,20 Hsp70 serves as a molecular chaperone, and also protect cells from various stressful conditions including low pH.10 The laryngeal expression of Hsp70 in LPRD has been proved by Western blot assay, and the level of Hsp70 did not changed after exposure to low pH in vitro culture system.21 However, this study included only 4 control samples by Western blot assay.21 In our study, however, we examined relatively larger number of non-LPRD samples, and found that expression level of Hsp70 was higher in LPRD and was significantly associated with both RFS and RSI (p<0.05). Our results suggest that Hsp70 expression might be involved in the repair mechanism to maintain laryngeal epithelial homeostatis under the assault of gastric reflux, similar to CAIII. Interestingly, the level of local Hsp70 expression was not influenced by smoking, alchol or BMI of enrolled individuals, suggesting that local expression of Hsp70 might be different from the systemic level of Hsp70.
The limitation of our study is that the diagnosis of LPRD is based only on RSI and RFS. Although RSI and RFS are useful tool for diagnosis of LPRD, they have also some limitations. One question of RSI regarding voice quality also weakens the objectivity of questionnaire. Second, our study is based on the IHC only, and mucosal biopsy is an invasive procedure. Therefore, clinical application of these biomarkers for the diagnosis of LPRD is not easy. Because of the limitation of small sized samples, we could not perform other various molecular experiments regarding the expression of CAIII and Hsp70. Further study with larger number of cases might deduce confident statistical correlation of CAIII and Hsp70 with the diagnosis of LPRD.
In conclusion, as far as we are aware of this is the first study, that evaluated the association of CAIII, and/or Hsp70 with LPRD by IHC staining using posterior commissure mucosa samples. The correlations between RFS and RSI with the expressions of CAIII and Hsp70 are statistically significant. Our findings suggest that these molecules could be one of objective diagnostic biomarkers for the diagnosis of LPRD.
ACKNOWLEDGEMENTS
This research was supported by the National Research Foundation of Korea (NRF) grant funded by Korea government (MOE) (NRF-2015R1D1A1A01058771) (to Sei Young Lee) and (MSIP) (NRF-2014R1A1A1036052) (to Hyun Jin Min).
Footnotes
The authors have no financial conflicts of interest.
Supplementary Material
Reflux Symptom Index Summary
Reflux Finding Score Summary
References
- 1.Hopwood D. Oesophageal damage and defence in reflux oesophagitis: pathophysiological and cell biological mechanisms. Prog Histochem Cytochem. 1997;32:1–42. doi: 10.1016/s0079-6336(97)80005-8. [DOI] [PubMed] [Google Scholar]
- 2.Mesallam TA, Stemple JC, Sobeih TM, Elluru RG. Reflux symptom index versus reflux finding score. Ann Otol Rhinol Laryngol. 2007;116:436–440. doi: 10.1177/000348940711600608. [DOI] [PubMed] [Google Scholar]
- 3.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101(4 Pt 2 Suppl 53):1–78. doi: 10.1002/lary.1991.101.s53.1. [DOI] [PubMed] [Google Scholar]
- 4.Ward PH, Hanson DG. Reflux as an etiological factor of carcinoma of the laryngopharynx. Laryngoscope. 1988;98:1195–1199. doi: 10.1288/00005537-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Woo P, Noordzij P, Ross JA. Association of esophageal reflux and globus symptom: comparison of laryngoscopy and 24-hour pH manometry. Otolaryngol Head Neck Surg. 1996;115:502–507. doi: 10.1016/S0194-59989670003-7. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PE, Koufman JA, Nowak LJ, Belafsky PC, Postma GN. Ambulatory 24-hour double-probe pH monitoring: the importance of manometry. Laryngoscope. 2001;111(11 Pt 1):1970–1975. doi: 10.1097/00005537-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Masiak W, Wallner G, Wallner J, Pedowski T, Solecki M. Combined esophageal multichannel intraluminal impedance and pH monitoring (MII -pH) in the diagnostics and treatment of gastroesophageal reflux disease and its complications. Pol Przegl Chir. 2011;83:488–496. doi: 10.2478/v10035-011-0076-7. [DOI] [PubMed] [Google Scholar]
- 8.Belafsky PC, Rees CJ. Laryngopharyngeal reflux: the value of otolaryngology examination. Curr Gastroenterol Rep. 2008;10:278–282. doi: 10.1007/s11894-008-0056-1. [DOI] [PubMed] [Google Scholar]
- 9.Johnston N, Knight J, Dettmar PW, Lively MO, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope. 2004;114:2129–2134. doi: 10.1097/01.mlg.0000149445.07146.03. [DOI] [PubMed] [Google Scholar]
- 10.Beere HM, Green DR. Stress management - heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/s0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- 11.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI) J Voice. 2002;16:274–277. doi: 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 12.Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS) Laryngoscope. 2001;111:1313–1317. doi: 10.1097/00005537-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Postma GN, Belafsky PC, Aviv JE, Koufman JA. Laryngopharyngeal reflux testing. Ear Nose Throat J. 2002;81(9 Suppl 2):14–18. [PubMed] [Google Scholar]
- 14.Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA. 2005;294:1534–1540. doi: 10.1001/jama.294.12.1534. [DOI] [PubMed] [Google Scholar]
- 15.Gill GA, Johnston N, Buda A, Pignatelli M, Pearson J, Dettmar PW, et al. Laryngeal epithelial defenses against laryngopharyngeal reflux: investigations of E-cadherin, carbonic anhydrase isoenzyme III, and pepsin. Ann Otol Rhinol Laryngol. 2005;114:913–921. doi: 10.1177/000348940511401204. [DOI] [PubMed] [Google Scholar]
- 16.Axford SE, Sharp N, Ross PE, Pearson JP, Dettmar PW, Panetti M, et al. Cell biology of laryngeal epithelial defenses in health and disease: preliminary studies. Ann Otol Rhinol Laryngol. 2001;110:1099–1108. doi: 10.1177/000348940111001203. [DOI] [PubMed] [Google Scholar]
- 17.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 18.Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest. 1995;95:3–12. doi: 10.1172/JCI117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagui-Beltran A, Craig AL, Lawrie L, Thompson D, Pospisilova S, Johnston D, et al. The human oesophageal squamous epithelium exhibits a novel type of heat shock protein response. Eur J Biochem. 2001;268:5343–5355. doi: 10.1046/j.0014-2956.2001.02468.x. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992;267:21987–21990. [PubMed] [Google Scholar]
- 21.Johnston N, Dettmar PW, Lively MO, Postma GN, Belafsky PC, Birchall M, et al. Effect of pepsin on laryngeal stress protein (Sep70, Sep53, and Hsp70) response: role in laryngopharyngeal reflux disease. Ann Otol Rhinol Laryngol. 2006;115:47–58. doi: 10.1177/000348940611500108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reflux Symptom Index Summary
Reflux Finding Score Summary