Abstract
Summary
The aims of this study are to develop a cloud-based application of the Fracture Liaison Service for practitioners to coordinate the care of osteoporotic patients after suffering primary fractures and provide a performance feedback portal for practitioners to determine quality of care. The application provides continuity of care, improved patient outcomes, and reduced medical costs.
Introduction
The purpose of this study is to describe the content development and functionality of a cloud-based application to broadly deploy the Fracture Liaison Service (FLS) to coordinate post-fracture care for osteoporotic patients.
Methods
The Bone Health Collaborative developed the FLS application in 2013 to support practitioners’ access to information and management of patients and provide a feedback portal for practitioners to track their performance in providing quality care. A five-step protocol (identify, inform, initiate, investigate, and iterate) organized osteoporotic post-fracture care-related tasks and timelines for the application. A range of descriptive data about the patient, their medical condition, therapies and care, and current providers can be collected. Seven quality of care measures from the National Quality Forum, The Joint Commission, and the Centers for Medicare and Medicaid Services can be tracked through the application.
Results
There are five functional areas including home, tasks, measures, improvement, and data. The home, tasks, and data pages are used to enter patient information and coordinate care using the five-step protocol. Measures and improvement pages are used to enter quality measures and provide practitioners with continuous performance feedback. The application resides within a portal, running on a multitenant, private cloud-based Avedis enterprise registry platform. All data are encrypted in transit and users access the application using a password from any common web browser.
Conclusion
The application could spread the FLS model of care across the US health care system, provide continuity of care, effectively manage osteoporotic patients, improve outcomes, and reduce medical costs.
Electronic supplementary material
The online version of this article (doi:10.1007/s00198-015-3260-5) contains supplementary material, which is available to authorized users.
Keywords: Cloud-based application, FLS, Fracture Liaison Service, Osteoporosis, Post-fracture
Introduction
Osteoporosis is a debilitating, deadly, and costly bone disease [1]. It is characterized by weakened and fragile bone tissue and is the main underlying cause of fracture in an aging population. Estimates from Healthy People 2010 [2] describe about 5.3 million older women and men in the USA with osteoporosis of the femur and another 34.5 million with low bone mass. Moreover, estimates for 2010 depicts 10.2 million adults age 50 or older with osteoporosis and 43.4 million with low bone mass of either the femoral neck or lumbar spine [3]. One in two Americans over age 50 is projected to develop or be susceptible to osteoporosis of the femur by 2020, with even more at-risk of osteoporosis at other skeletal sites [4].
A powerful predictor of an osteoporotic fracture is a previous fracture [5], which has important public health consequences [1]. Two thirds of femur fracture patients never regain their former level of independence and 26 % become permanently disabled within the first year [6]. Twenty percent of older people who suffer an osteoporotic fracture die in the first year [4], and direct care costs per patient are about $30,000 for femur fracture, $11,300 for other nonvertebra fracture, and $8,380 for vertebra fracture [7].
Studies show that post-fracture osteoporotic patients are not receiving calcium, vitamin D, or a prescription medication to prevent further fracture [8]. In the 6 months following their fracture, only 21 % of osteoporotic women age 67 or older had either a bone mineral density (BMD) test or were prescribed medication to treat or prevent this disease [9, 10]. A primary impediment for post-fracture care is a lack of awareness about the severe consequences of osteoporosis among the general public, primary care physicians and other specialists, and policy makers [11].
Improving the continuity of care for osteoporotic patients has received limited attention in the USA. Fracture Liaison Service (FLS) programs that identify and manage patients after a fracture are used in several closed US health care systems and demonstrated promise for improvement. The FLS model of care connects bone health specialists with the patient’s primary care physician and employs a coordinator (nurse practitioner, physician’s assistant, registered nurse, or health care professional) to ensure patients are appropriately diagnosed, treated, and followed up. FLS programs have reduced secondary fracture rates and health care costs and improved the quality of patient care at Geisinger Health and in the UK [12, 13].
Despite their success, FLS programs are not an integral part of post-fracture care throughout the USA. Health information technology could be leveraged to facilitate widespread implementation of such programs. Current technologies, however, do not communicate across hospitals, and in some cases across care settings in the same hospital, making crucial patient data difficult to access and coordinated care challenging [14, 15]. A potential solution is a cloud-based system where practitioners across the health care sector can communicate and jointly manage osteoporotic patients. This paper describes the content development for the FLS application, deployment of the application via a cloud-based platform, and functionality of the application in a clinical setting to coordinate post-fracture care for osteoporotic patients.
Background
The FLS application (FLS App) was developed for a Fracture Liaison Service Model of Care. The objectives in building the FLS App were to develop a system that broadly deployed the FLS model of care to health care settings, to make coordination of post-fracture care and access to patient information quick and reliable, and to provide a feedback portal for practitioners to track their performance in providing quality care.
The FLS App was developed by the Bone Health Collaborative in 2013 and included a team of health care professionals with expertise in the FLS model of care, who worked closely with CECity.com, Inc®, the technology partner, the National Bone Health Alliance (NBHA), and the National Osteoporosis Foundation (NOF). The NBHA and NOF engaged leaders in the field to guide content development of the application. CECity.com® built the FLS software application on their web-based platform. They are a software as a service provider, wherein they develop, manage, and operate software applications, and end-users access these applications and submit data across the cloud. They were a logical technology partner because of these capabilities and because their focus is the health care industry and they also offer quality reporting, performance improvement, and continuing education services. Researchers from the Johns Hopkins Medicine Armstrong Institute for Patient Safety and Quality joined the collaborative once the FLS App was built to develop and manage the research project.
Content development for FLS application
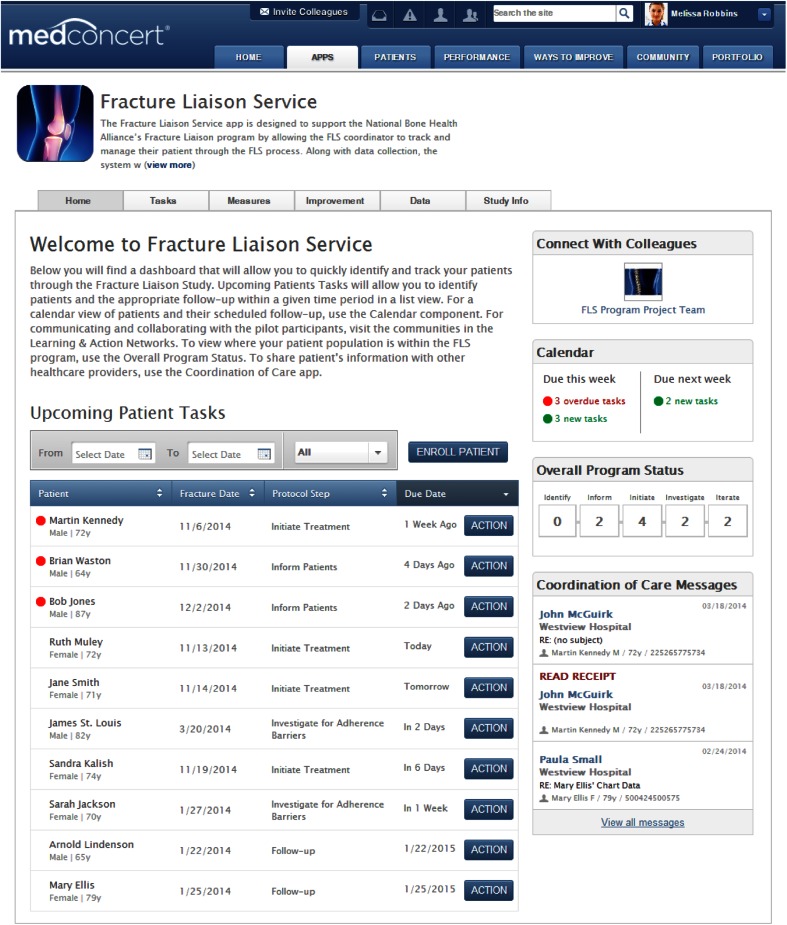
The content development team comprised experts in bone health and osteoporotic care from NBHA and NOF, and two clinicians with expertise in the fracture liaison service. They defined the optimum workflow for post-fracture care (initial patient identification, assessment, and follow-up care) and developed a five-step protocol to deliver the FLS model of care. The protocol was informed by a meta-analysis of existing post-fracture models of care for osteoporotic patients [16], and care-related tasks divided into the following five behaviors: identify, inform, initiate, investigate, and iterate. Table 1 describes each behavior and their relation to the workflow stages of post-fracture care. The protocol was built as the clinical decision-making tool for the FLS App, in which each step had to be completed by the health care practitioner coordinating the patient’s care before the next step could be initiated. Figure 1 displays the application home page and contains a 2-week window of upcoming patient tasks and how patients are progressing through the 5-step protocol.
Table 1.
Five steps and tasks of Fracture Liaison Service protocol
| Step | Description of tasks |
|---|---|
| Identify | Gather demographic information about patient, as well as information about home caregivers and other medical professionals who provide care. |
| Inform | Gather test results and communicate with patient about osteoporosis at time of discharge. Evaluate additional patient information (e.g., alcohol use, exercise level) to assess risk of future falls. |
| Initiate | Discuss osteoporosis diagnosis, treatment options, and medication with patients. Counsel patient and present educational materials about balance and muscle strengthening exercise, fall prevention, and other preventive measures. |
| Investigate | Follow up with patient to gather information about medication adherence, additional falls (if any), and other conditions. |
| Iterate | Further investigation, as described above, after 1 year. |
Fig. 1.
MedConcert platform to the Fracture Liaison Service Home page. The figure shows the MedConcert portal to the Fracture Liaison Service application (FLS App) and the Home page once a user has logged into the site. There are five functional areas to the FLS App, denoted by the tabs near the top of the screen. The current display is the Home page, which provides a comprehensive view of upcoming patient tasks over a 2-week period, each patient’s fracture date, current step in the FLS protocol, and length of time relative to the due date, which could be before or after. Users can also send and receive messages to coordinate patient care with other practitioners and connect with FLS program team members
The Collaborative also sought to provide a portal for practitioners to track and benchmark their performance on post-fracture quality of care measures for osteoporotic patients. The team identified over 35 candidate quality measures endorsed by the National Quality Forum [17], The Joint Commission [18], and the Centers for Medicare and Medicaid Services [19]. They worked with the two fracture liaison service content experts (see the Acknowledgments) and used a deliberative process, in which the group used logic and reasoning to thoughtfully reach consensus [20], to reduce candidate measures to a final set of seven (Table 2). A measure was included if it described an evidence-based therapy or process of care that directly linked to identifying osteoporotic-related fractures and managing post-fracture care for women and men.
Table 2.
Quality of care variables and epidemiological measurements
| Source | Description of measure |
|---|---|
| National Quality Forum (NQF) #0053 [17] | Osteoporosis management in women who had a fracture (primary study objective). Assesses whether women over 67 years of age who had one or more bone fractures received, within 6 months of the fracture, a bone density test to determine if osteoporosis was the underlying cause of the fracture and/or received appropriate prevention/treatment for osteoporosis. |
| Centers for Medicare and Medicaid Services (CMS) Physician Quality Reporting System (PQRS) Measure #40 [19] | Management following fracture of hip, spine, or distal radius for men and women aged 50 years and older (display measure in FLS App). Percentage of patients aged 50 years and older with fracture of the hip, spine, or distal radius who had a central dual-energy X-ray absorptiometry (DXA) measurement ordered or performed or pharmacologic therapy prescribed. |
| CMS, PQRS Measure #24 [19] | Communication with the physician managing ongoing care post-fracture of hip, spine, or distal radius for men and women aged 50 years and older (display measure in FLS App). Percentage of patients aged 50 years and older treated for a hip, spine, or distal radial fracture with documentation of communication with the physician managing the patient’s ongoing care that a fracture occurred and that the patient was or should be tested or treated for osteoporosis. |
| The Joint Commission (Osteoporosis-Associated Fracture) #OAF-01 [18] | Laboratory investigation for secondary causes of fracture (display measure in FLS App). Patients with fragility fracture who have had appropriate laboratory investigation for secondary causes of fracture ordered or performed prior to discharge from inpatient status. |
| The Joint Commission #OAF-03 [18] | Discharge instructions—Emergency Department (display measure in FLS App). Patients age 50 or over with a fracture of the vertebra, pelvis, wrist, ankle, or humerus discharged from the Emergency Department to home, or their caregivers, who have received written discharge instructions regarding the need to follow up with a primary care physician, hospital outpatient department, or specialist for possible osteoporosis to reduce the risk of future fracture, or who were contacted by a fracture liaison service. |
| NQF #0037 [17] | Osteoporosis testing in older women (exploratory objective). Examine the rate of primary osteoporosis screening in post-fracture patients by estimating the proportion of post-menopausal women over the age of 65 who had a bone density test for osteoporosis, classify these patients, and evaluate their post-diagnosis outpatient-related treatment patterns. |
| Bone Health Collaborative | FLS process adherence measure. Measure FLS Coordinator adherence to the tasks and timelines of the standardized FLS process. |
Web-based platform
The FLS App resides within CECity’s MedConcert® portal, running on the multitenant, cloud-based Avedis enterprise registry platform. The platform is a private cloud, and only authorized users can access the site and data. It is HIPAA compliant and housed across multiple enterprise class data centers. All data are encrypted in transit using a secure AES-256 protocol, and the platform automatically scales up as utilization increases. Data for the FLS App are stored within MongoDB “Big Data” databases and SQL Server 2012 Enterprise. Users can access the FLS App and portal using any common web browser, such as Chrome, Internet Explorer, Firefox, or Safari, and the portal is constantly updated as browsers evolve.
CECity followed its internal project implementation methodology (discovery, modeling, and implementation) to manage the application development. Discovery was accomplished through the content development, in which the FLS process and data elements were first defined, and CECity documented this entire process in the modeling phase. The CECity team used this modeling to configure the FLS App on its registry platform to collect the requisite data using the optimal workflow to manage osteoporotic patients and to ensure ease of data entry (implementation phase).
Functionality of FLS application
The FLS App is accessed through the MedConcert portal under the Apps page (Fig. 1). There are five functional areas for the application, including home, tasks, measures, improvement, and data. The Home page (Fig. 1) contains a comprehensive dashboard of Upcoming Patient Tasks, total number of patients being managed by protocol step, and number of due and overdue tasks for the current and upcoming week. There are also interfaces to enroll new patients, link to learning opportunities (e.g., NOF Clinician Guide to Prevention and Treatment of Osteoporosis), and communicate with practitioners. The Coordination of Care Messages interface connects practitioners from different sites, wherein messages can be sent and received to co-manage patients, and provide continuity of care.
The Tasks page shows patients currently being managed and includes their fracture date, current protocol step, and action button to view the required tasks to complete the step, and deadline for completion. This list can be filtered by protocol step, specific time period, or protocol status. Practitioners can also view patients and required protocol tasks in a monthly calendar on their scheduled follow-up date.
Practitioners enter patient information through the Data page of the application. Information on each patient encounter can be uploaded from an electronic medical record or billing system or manually entered into the system. A range of descriptive data about the patient, their medical condition, medical care, current providers, and the like are collected to inform each step of the protocol (Table S1). Details of a patient encounter includes the date and location (picked from a dropdown menu) of the visit, current step in the FLS protocol (dropdown menu), follow-up date, whether the patient remains enrolled in the program (dichotomous variable), and whether they filled or refilled their prescription (dichotomous variable).
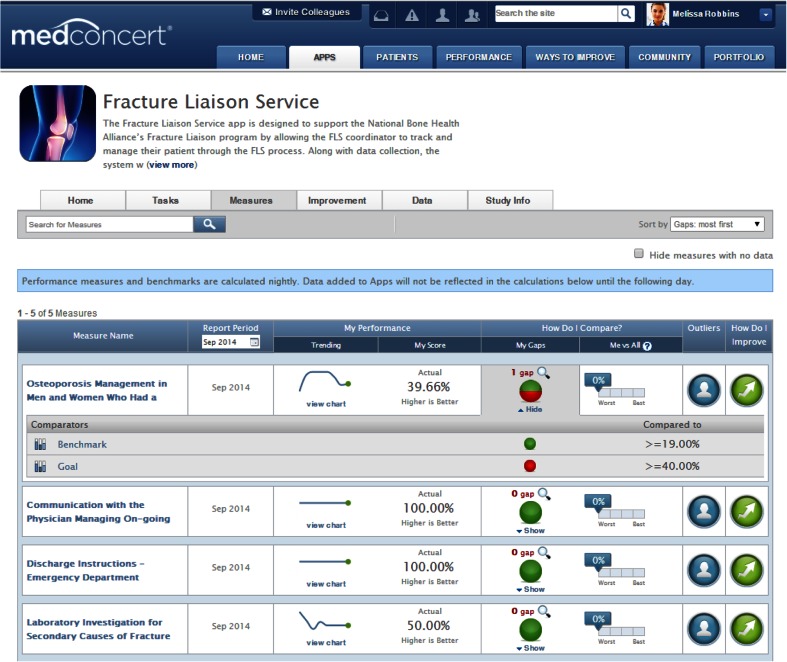
Practitioners enter data related to quality metrics (Table 2) through the Measures page (Fig. 2). Both the Measures and Improvement pages provide practitioners with continuous performance feedback and peer-to-peer benchmarks and can link to dynamically targeted learning opportunities. Performance measures and benchmarks are automatically updated every night to reflect data entered during the day. Through an intuitive interface (Fig. 2), practitioners can browse their current performance rates, drill down to identify quality of care gaps based on one or more comparators, view patient outliers, and access improvement interventions intended to close quality performance gaps related to the measures. Here is an example of how the Measures portal functions, using the first measure in Fig. 2. After data entry, a provider can go see that 39.66 % of their patients received appropriate care linked to osteoporosis management as of September 2014, and that one gap in care (laboratory testing) was causing low performance with this measure. Their performance in managing osteoporosis for their post-fracture patients was well above the national benchmark of ≥19 % of patients not receiving appropriate osteoporosis management. These data are updated daily so providers can routinely assess their fidelity to the FLS model of care. When gaps in performance are identified, providers can link directly from this page to educational and training materials that are specific to the areas identified for improvement through the “How do I improve?” icon.
Fig. 2.
Fracture Liaison Service performance feedback. The figure displays the Measures page for the FLS App. This area is where practitioners can see how they are performing on seven quality of care measures endorsed by the National Quality Forum, The Joint Commission, and the Centers for Medicare and Medicaid Services. They can also compare their performance against other benchmarks and determine the gaps in their quality of care for these measures
Preliminary testing of FLS application
The data collection tools and functionality of the FLS App were tested by CECity’s Quality Assurance team, comprising four people totaling 12 years of training and experience in quality testing on similar applications. Over a 4-week period, each person independently tested timing rules of the FLS protocol, general usability of the FLS App, and quality of data input relative to expected output. To undertake this testing, they entered simulated data on patients based on expected measure results to test and verify the input and output of the system. Prototypes of the FLS App were reviewed by members of NBHA and 12 end-users (FLS coordinators and bone specialists) to validate the content and test its usability. This was an iterative process, in which multiple demonstrations were done by CECity and updates made to the FLS App until all stakeholders reached consensus on its readiness for use.
Discussion
Secondary fracture prevention remains an underdeveloped area of osteoporotic care. This gap in care is not surprising given the limited diagnosis and treatment of osteoporosis at the time of a fragility fracture [21]. Fracture Liaison Service programs to prevent further osteoporotic fractures have a limited presence in US health care. Yet, Ganda et al’s [16] meta-analysis of existing care models to prevent secondary fractures concluded that fully coordinated models, such as the FLS program, were better for closing the quality of care gap and improving patient outcomes than education or alerts. Studies of the FLS model in other countries have increased the detection and management of osteoporosis [22] and patient adherence to long-term treatment for the disease [23]. Increased adherence is a plus because some patients fail to keep taking their medication [24, 25]. This model has also improved post-fracture treatment for osteoporosis,[26, 27] and in a predictive analysis, reduced femur fractures and saved hospital costs [28].
The FLS App is cost-effective and easily accessible. It is delivered through a secure, HIPAA compliant, cloud platform using a Software as a Service (SaaS) model. This model eliminates the need to install any software and enables the FLS App to cost-effectively scale to support large numbers of users and patient data sets, while maintaining an acceptable service level in terms of availability and response time. Care providers can remotely access the application from any device with internet access, enabling better coordination of care among providers and more reliable patient follow-up. Continuity of care is focused on two premises: one, that there is an ongoing caring relationship between provider and patient, and two, that care is informed through an integrated process where providers have access to patient information that can be shared within the system as the FLS App allows [29].
An important quality improvement feature of the FLS App is its performance feedback tool, in which practitioners can measure and track the quality of care delivered to osteoporotic patients. Quality performance is increasingly a central focus of accreditation organizations and pay for performance programs. The quality measures in the FLS App are recommended by The Joint Commission [18], and tied to the Centers for Medicare and Medicaid Services payment program [19]. Using the application to track and improve performance will meet these recommendations, benefiting practitioners and health care organizations. Also, performance measurement has been used in studies to increase use of evidence-based therapies and gained dramatic improvements in outcomes [30, 31].
Moreover, the FLS App helps integrate health information technologies within and across hospitals. Practitioners can upload patient information from electronic medical records or billing systems. Historically, these technologies have not communicated across different care settings, making crucial patient data difficult to access and coordinated care challenging [14, 15]. One of the demonstration sites found that it required up to 2 hours to collect patient information from multiple sources to complete follow up on an g an osteoporotic fracture (Thompson, Lee, et al. unpublished data 2014). The most common data sources are paper documentation, laboratory medicine radiology, and electronic medical records [32]. The FLS App stores data in a population registry and offers a single source for accessing patient information.
In conclusion, osteoporosis is a silent disease and many individuals are not aware of their fragility until they fracture a bone. The Surgeon General describes a fracture as the start of a “downward spiral in many individual’s physical and mental health” [4]. The FLS App has the potential to spread this model of care across the US health care system, provide continuity of care, effectively manage osteoporotic patients, improve outcomes, and reduce medical costs.
Electronic supplementary material
(DOCX 15 kb)
Acknowledgments
The authors wish to thank Anne F. Lake, DNP, APRN, FNPC, Fracture Liaison Service program coordinator for Wake Forest Baptist Medical Center, Winston Salem, and Paul Mitchell, founder of Synthesis Medical; both were paid consultants who provided expertise in the selection of appropriate quality measures for osteoporotic care. They also thank Brian Marchand, PMP, and Mary Franzen, MPH, both employed by CECity, for helping coordinate production of this paper.
Conflicts of interest
David A. Thompson has received a subcontract from CECity for this research and Christine G. Holzmueller has received salary support from this subcontract. Simone Karp is co-founder of CECity.com. Debbie Zeldow and David B. Lee report no conflicts of interest.
Funding for the study
Partial support for the MedConcert FLS application tool was provided to CECity.com through a contract from Merck Sharp & Dohme Corp. Merck was not involved in the development of the FLS application or the decision to submit this manuscript for consideration for publication.
References
- 1.Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68:1243–1251. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics . Healthy people 2010 final review. Atlanta: Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 3.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Office of the Surgeon General . Bone health and osteoporosis: a report of the surgeon general. Rockville: U.S. Department of Health and Human Services; 2004. [PubMed] [Google Scholar]
- 5.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 6.Magaziner J, Fredman L, Hawkes W, Hebel JR, Zimmerman S, Orwig DL, Wehren L. Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol. 2003;157:1023–1031. doi: 10.1093/aje/kwg081. [DOI] [PubMed] [Google Scholar]
- 7.Tosteson AN, Melton LJ, 3rd, Dawson-Hughes B, Baim S, Favus MJ, Khosla S, Lindsay RL, National Osteoporosis Foundation Guide Committee Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19:437–447. doi: 10.1007/s00198-007-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siris ES, Bilezikian JP, Rubin MR, Black DM, Bockman RS, Bone HG, Hochberg MC, et al. Pins and plaster aren’t enough: a call for the evaluation and treatment of patients with osteoporotic fractures. J Clin Endocrinol Metab. 2003;88:3482–3486. doi: 10.1210/jc.2003-030568. [DOI] [PubMed] [Google Scholar]
- 9.Chapurlat RD, Bauer DC, Nevitt M, Stone K, Cummings SR. Incidence and risk factors for a second hip fracture in elderly women. The study of osteoporotic fractures. Osteoporos Int. 2003;14:130–136. doi: 10.1007/s00198-002-1327-6. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay R, Burge RT, Strauss DM. One year outcomes and costs following a vertebral fracture. Osteoporos Int. 2005;16:78–85. doi: 10.1007/s00198-004-1646-x. [DOI] [PubMed] [Google Scholar]
- 11.Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE, Jr, McLellan A, Mitchell PJ, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27:2039–2046. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 12.McLellan AR, Wolowacz SE, Zimovetz EA, Beard SM, Lock S, McCrink L, Adekunle F, Roberts D. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int. 2011;22:2083–2098. doi: 10.1007/s00198-011-1534-0. [DOI] [PubMed] [Google Scholar]
- 13.Newman ED. Perspectives on pre-fracture intervention strategies: the Geisinger Health System Osteoporosis Program. Osteoporos Int. 2011;22(Suppl 3):451–455. doi: 10.1007/s00198-011-1695-x. [DOI] [PubMed] [Google Scholar]
- 14.Pronovost PJ, Bo-Linn GW, Sapirstein A. From heroism to safe design: leveraging technology. Anesthesiology. 2014;120:526–529. doi: 10.1097/ALN.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 15.Pronovost PJ, Bo-Linn GW. Preventing patient harms through systems of care. JAMA. 2012;308:769–770. doi: 10.1001/jama.2012.9537. [DOI] [PubMed] [Google Scholar]
- 16.Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR, Eisman JA, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int. 2013;24:393–406. doi: 10.1007/s00198-012-2090-y. [DOI] [PubMed] [Google Scholar]
- 17.National Quality Forum (2015) Measures, reports &tools. http://www.qualityforum.org/Measures_Reports_Tools.aspx. Accessed 29 April 2015
- 18.The Joint Commission (2013) Osteoporosis-associated fracture implementation guide. http://www.jointcommission.org/assets/1/6/Osteoporosis_Imp_Guide.pdf. Accessed 29 April 2015
- 19.Centers for Medicare and Medicaid Services (2015) Physicians quality reporting system. about PQRS. http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/index.html?redirect=/PQRS/. Accessed 29 April 2015
- 20.Abelson J, Forest PG, Eyles J, Smith P, Martin E, Gauvin FP. Deliberations about deliberative methods: issues in the design and evaluation of public participation processes. Soc Sci Med. 2003;57:239–251. doi: 10.1016/S0277-9536(02)00343-X. [DOI] [PubMed] [Google Scholar]
- 21.Edwards BJ, Bunta AD, Anderson J, Bobb A, Hahr A, O’Leary KJ, Agulnek A, et al. Development of an electronic medical record based intervention to improve medical care of osteoporosis. Osteoporos Int. 2012;23:2489–2498. doi: 10.1007/s00198-011-1866-9. [DOI] [PubMed] [Google Scholar]
- 22.Giles M, Van Der Kallen J, Parker V, Cooper K, Gill K, Ross L, McNeill S. A team approach: implementing a model of care for preventing osteoporosis related fractures. Osteoporos Int. 2011;22:2321–2328. doi: 10.1007/s00198-010-1466-0. [DOI] [PubMed] [Google Scholar]
- 23.Boudou L, Gerbay B, Chopin F, Ollagnier E, Collet P, Thomas T. Management of osteoporosis in fracture liaison service associated with long-term adherence to treatment. Osteoporos Int. 2011;22:2099–2106. doi: 10.1007/s00198-011-1638-6. [DOI] [PubMed] [Google Scholar]
- 24.Guggina P, Flahive J, Hooven FH, Watts NB, Siris ES, Silverman S, Roux C, et al. Characteristics associated with anti-osteoporosis medication use: data from the global longitudinal study of osteoporosis in women (GLOW) USA cohort. Bone. 2012;51:975–980. doi: 10.1016/j.bone.2012.08.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006;119:S3–S11. doi: 10.1016/j.amjmed.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Majumdar SR, Johnson JA, Bellerose D, McAlister FA, Russell AS, Hanley DA, Garg S, et al. Nurse case-manager vs multifaceted intervention to improve quality of osteoporosis care after wrist fracture: randomized controlled pilot study. Osteoporos Int. 2011;22:223–230. doi: 10.1007/s00198-010-1212-7. [DOI] [PubMed] [Google Scholar]
- 27.Majumdar SR, Beaupre LA, Harley CH, Hanley DA, Lier DA, Juby AG, Maksymowych WP, et al. Use of a case manager to improve osteoporosis treatment after hip fracture: results of a randomized controlled trial. Arch Intern Med. 2007;167:2110–2115. doi: 10.1001/archinte.167.19.2110. [DOI] [PubMed] [Google Scholar]
- 28.Sander B, Elliot-Gibson V, Beaton DE, Bogoch ER, Maetzel A. A coordinator program in post-fracture osteoporosis management improves outcomes and saves costs. J Bone Joint Surg Am. 2008;90:1197–1205. doi: 10.2106/JBJS.G.00980. [DOI] [PubMed] [Google Scholar]
- 29.Gulliford M, Naithani S, Morgan M. What is ’continuity of care’? J Health Serv Res Policy. 2006;11:248–250. doi: 10.1258/135581906778476490. [DOI] [PubMed] [Google Scholar]
- 30.Marsteller JA, Sexton JB, Hsu YJ, Hsiao CJ, Holzmueller CG, Pronovost PJ, Thompson DA. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40:2933–2939. doi: 10.1097/CCM.0b013e31825fd4d8. [DOI] [PubMed] [Google Scholar]
- 31.Pronovost PJ, Goeschel CA, Colantuoni E, Watson S, Lubomski LH, Berenholtz SM, Thompson DA, et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ. 2010;340:c309. doi: 10.1136/bmj.c309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melton LJ., 3rd Who has osteoporosis? A conflict between clinical and public health perspectives. J Bone Miner Res. 2000;15:2309–2314. doi: 10.1359/jbmr.2000.15.12.2309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)