Abstract
Background
Smoking cessation medications have been shown to yield higher success rates and sustained abstinence than unassisted quit attempts. In Japan, the treatments available include nicotine replacement therapy (NRT) and varenicline; however, unassisted attempts to quit smoking remain common.
Objective
The objective of this study was to compare the health and economic consequences in Japan of using pharmacotherapy to support smoking cessation with unassisted attempts and the current mix of strategies used.
Methods
A discrete-event simulation that models lifetime quitting behaviour and includes multiple quit attempts (MQAs) and relapses was adapted for these analyses. The risk of developing smoking-related diseases is estimated based on the duration of abstinence. Data collected from a survey conducted in Japan were used to determine the interventions selected by smokers initiating a quit attempt and the time between MQAs. Direct and indirect costs are assessed (expressed in 2014 Japanese Yen).
Results
Using pharmacotherapy (NRT or varenicline) to support quit attempts proved to be dominant when compared with unassisted attempts or the current mix of strategies (most are unassisted). The results of stratified analyses by age imply that smoking cessation improves health outcomes across all generations. Indirect costs due to premature death leading to lost wages are an important component of the total costs, exceeding the direct medical cost estimates.
Conclusions
Increased utilisation of smoking cessation pharmacotherapy to support quit attempts is predicted to lead to an increase in the number of smokers achieving abstinence, and provide improvements in health outcomes over a lifetime with no additional costs.
Electronic supplementary material
The online version of this article (doi:10.1007/s40258-015-0204-3) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| The model described in this article makes predictions for Japanese smokers making multiple quit attempts, based on individual smoker profiles, sequences of smoking cessation strategies, time intervals between quit attempts and relapse. |
| Increased utilisation of smoking cessation pharmacotherapy to support quit attempts is predicted to lead to an increase in the number of smokers achieving abstinence and provide improvements in health outcomes over a lifetime with no additional costs. |
| The Japanese public health insurance system covers counselling and prescription medications to support a quit attempt; however, the current eligibility criteria for these services limit access to these services. Expanding public funding for smoking cessation therapy to allow greater access to smoking cessation pharmacotherapy may be worthwhile. |
Introduction
Cigarette smoking is a modifiable risk factor contributing to premature mortality in Japan, accounting for approximately 130,000 deaths annually from smoking-related diseases [1]. The benefits of quitting smoking permanently have been well-studied, and include the reduction of the risk of developing smoking-related chronic diseases, as well as a lower risk of premature mortality [2]. Whilst smoking prevalence has declined in Japan over the past decade, 19.3 % (24 million people) of the population were current smokers in 2013 (32.2 % of men and 8.2 % of women) [3, 4]. Many current smokers are motivated to attempt to quit; for example, 24.6 % expressed the desire to quit smoking in a 2013 National Health and Nutrition Examination Survey [4].
Current practice guidelines recommend providing counselling and pharmacotherapy to support quit attempts, such as high-dose nicotine replacement therapy (NRT) patches and varenicline [5]. Nicotine replacement patches (low dose) and nicotine gum are available without prescription, but bupropion is not licensed for smoking cessation in Japan. Attempting to quit unassisted yields lower overall rates of success and abstinence than when assisted by smoking cessation medications [6–8]. Whilst the Japanese public health insurance system does cover physicians’ counselling and prescription medications to support a quit attempt, the eligibility criteria limit access to these services. Each smoker seeking to quit using the public health insurance system must meet criteria for nicotine dependence (assessed by the Tobacco Dependence Screener [9]) and nicotine use (assessed by the Brinkman Index [10]). As a result, overall only about 1 % (240,000 smokers) obtain smoking cessation therapy in Japan each year under the public health insurance system [11]. For the majority of smokers in Japan, unassisted quit attempts remain the most often used smoking cessation strategy [12].
The aim of this study is to assess the potential health and economic consequences in Japan of utilisation of pharmacotherapy to support smoking cessation with varenicline or NRT. The analyses were conducted from a healthcare payers’ perspective, with supplemental analyses in which costs associated with productivity losses due to smoking-related diseases were estimated. Previous economic models evaluating the impact of smoking cessation interventions in Japan have evaluated the impact of a single quit attempt (SQA) on long-term health and economic outcomes [13–15]. This study was conducted using a previously published model developed for the USA with appropriate modifications to reflect the smoking-related co-morbidities, interventions used, population characteristics, quit attempt behaviour and mortality for Japan [16, 17]. This is the first model developed for Japan that allows smokers to have multiple quit attempts (MQAs). It makes predictions for treatment success and smoking outcomes based on individual smoker profiles, sequences of smoking cessation strategies, and the time intervals between quit attempts and relapses. Japanese adults (N = 1261), who were current or former smokers at the time of the survey (June 2013), completed a web-based questionnaire. The sequence of smoking cessation strategies used in the model was derived from the data collected from current smokers participating in the online survey. The equations used for predicting the time between quit attempts and choice of next modality were also derived using this very recent Japanese data in order to align the simulated patterns with real-world behaviour in Japan.
Methods
Our model makes predictions for treatment success and the development of smoking-related diseases for a cohort of individual smokers. Each individual can experience MQAs, switching between the four smoking cessation strategies available in Japan (varenicline, NRT, behavioural modification therapy [BMT] and unassisted attempts). When a smoker successfully quits, they may relapse and start smoking, and then after a time interval initiate another attempt to quit.
The model framework and the derivation of the risk functions to estimate the treatment efficacy and to predict relapse have been previously published [16]. This discrete-event simulation (DES) was programmed in ARENA® version 12 (Rockwell Software, Inc., Warrendale, PA, USA). Details of all of the other parameters that have been replaced with functions developed specifically for Japanese smokers are provided in the Electronic Supplementary Material (ESM). These changes include developing functions to predict the interventions selected by smokers initiating a quit attempt and the time intervals between MQAs using the data collected from a web-based survey of smokers in Japan [12]. Furthermore, two additional diseases associated with smoking (stomach cancer and hepatic cancer) are now considered in the model. Published sources were used to derive mortality estimates for smokers and former smokers in Japan.
In this model, a cohort of individual smokers is created by sampling from a file of smoker profiles. Each simulated individual carries a risk profile that includes age, sex, race, medical history (history of chronic obstructive pulmonary disease [COPD] or cardiovascular disease), smoking history (years smoked, age started, reported average number of cigarettes per day in the past month, reported average number of cigarettes per day since start of smoking), quit attempt history (longest period of abstinence in past year, prior unassisted/medical/non-medical assisted quit attempt, number of prior quit attempts), Fagerström Test for Nicotine Dependence (FTND) score and whether there is frequent contact with a smoker.
The simulation starts with each smoker initiating an attempt to quit smoking. If the attempt is successful (i.e. abstinence is achieved), a time to relapse is assigned. When the attempt is a failure (i.e. fails to achieve abstinence), a time interval until the next attempt to quit and the next cessation strategy are assigned.
An individual’s smoking status (i.e. current vs. former smoker) impacts the risk of developing smoking-related diseases. Relative to a current smoker, the risk is lower for a former smoker, and the level of risk reduction is also dependent on the duration of abstinence. Six smoking-related diseases are considered in this simulation: myocardial infarction (MI), stroke, COPD, lung cancer, stomach cancer and hepatic cancer.
When one of these six smoking-related diseases occurs, utilities and life expectancy are updated, and the associated management costs are accrued. Life-years (LYs), quality-adjusted life-years (QALYs), incidence of smoking-related diseases, abstinence time and costs were estimated. Lost wages due to premature mortality caused by smoking-related diseases were also determined.
Data Sources
The simulated cohort key baseline characteristics, cost inputs and utilities are provided in Table 1. For one scenario, referred to as “market mix”, each smoker initiates a quit attempt with one of four cessation strategies used by current smokers: varenicline (4.6 %), BMT (1.5 %), NRT (21.4 %) or unassisted (72.5 %). This distribution was based on the web-based survey results for current smokers conducted in Japan [12]. Unassisted quit attempts were the most common cessation strategy.
Table 1.
Model inputs
| Baseline characteristics | Simulated cohort |
|---|---|
| Male, % [12] | 73.8 |
| Age [years], % [12] | |
| 18–34 | 11.0 |
| 35–54 | 65.5 |
| 55–75 | 23.5 |
| CVD, % [39] | 1.1 |
| COPD, % [29] | 2.2 |
| FTND score, mean (SD) [12] | 3.9 (2.3) |
| Years smoked, mean (SD) [12] | 23.4 (7.2) |
| Number of prior quit attempts, mean (SD) [12] | 1.5 (2.3) |
| Intervention | Quit attempt cost (¥; 2014 values) [5] |
|---|---|
| Varenicline | 65,510 |
| NRT | 43,620 |
| BMT | 9620 |
| Unassisted | 0 |
| Smoking-related diseases | Annual costs (¥; 2014 values) [44, 45] | Utilities |
|---|---|---|
| MI | 1,036,000 | 0.88 [38] |
| Stroke | 1,780,000 | 0.56 [47] |
| COPD | 678,000 | 0.79 [27] |
| Lung cancer | 3,381,000 | 0.87 [38] |
| Stomach cancer | 1,610,000 | 0.87 [38] |
| Hepatic cancer | 2,499,000 | 0.87 [38] |
¥ Japanese yen, BMT behavioural modification therapy, COPD chronic obstructive pulmonary disease, CVD cardiovascular disease, FTND Fagerström test for nicotine dependence, MI myocardial infarction, NRT nicotine replacement therapy, SD standard deviation
For the other scenarios, all smokers in the cohort initiate the first quit attempt with the same smoking cessation strategy. The cessation strategy for subsequent quit attempts is estimated, conditional on each individual’s quit attempt history profile (number of prior attempts and previous cessation strategies), by applying a function derived from the same web-based survey of current and former smokers (parameter estimates are provided in ESM Table S1). The time intervals between quit attempts are estimated based on this survey, and are conditional on sex and the number of attempts (parameter estimates are provided in ESM Table S2).
The functions developed to estimate the outcome of a quit attempt with varenicline or NRT and also a relapse in the first year are based on five 52-week varenicline clinical trials and have been previously published [16] (details of the logistic regression to estimate quit attempt outcome are in ESM Table S3). Each of these clinical trials included a 12-week treatment period and a 40-week follow-up period. A quit attempt was considered to be successful if the subject was abstinent during weeks 9–12 of these studies [18–22]. This criteria was commonly used for the primary endpoint of varenicline in clinical studies. As in the previous model, BMT and unassisted quit attempt probabilities have an additional odds ratio term (0.552 [23] and 0.171 [24] vs. varenicline, respectively); however, these values were obtained from Japanese studies to better reflect their outcomes and applied to modify the prediction of the quit attempt outcome from the logistic regression.
Each of the varenicline clinical trials included a 40-week follow-up period to check whether the patients who successfully quit had a relapse. The relapse risk in the first year following a successful quit attempt was derived from these follow-up periods (ESM Table S4). The predictors of relapse in the first year include age, measures of smoking history (e.g. number of years smoked), frequent contact with smokers and a history of COPD [16]. Older former smokers are at lower risk of relapse, but starting smoking at an older age increases the risk to relapse, as do smoking for a longer time and at a higher frequency or COPD. After the first year, annual probabilities of experiencing a relapse were applied: 14.5 % in year 1; 2.8 % in years 2–5; 2.1 % in years >5 [25].
The impact of smoking cessation on the incidence of each smoking-related disease is estimated taking into consideration the individual’s age and duration of abstinence. The relative risk of each disease for a former and current smoker is derived using a published model [26]. The risk of developing each smoking-related disease is lower for former smokers than for current smokers, and continues to decrease the longer abstinence is sustained. The hazards for developing the smoking-related diseases for current and former smokers (λcurrent and λformer, respectively) are determined as follows (Eqs. 1 and 2):
| 1 |
| 2 |
where λnever is the hazard among never smokers, RRcurrent is the relative risk among current smokers and RRformer is the relative risk among former smokers.
RRformer during the simulation is determined for each of the smoking-related diseases as a function of the individual’s age and duration of abstinence using the model published by Hoogenveen et al. [26] (disease-specific coefficient estimates were provided in the publication and are reproduced in ESM Table S5). The coefficient published by Hoogenveen et al. [26] for stomach cancer was assumed to also apply to hepatic cancer, as there are no Japanese studies on how this risk ratio will change over the years of abstinence. In order to implement these functions, the relative risks for current versus non-smokers for each of the smoking-related diseases and also the hazards for Japanese non-smokers (never smokers) were derived from published sources (MI [27], stroke [27], COPD [29], lung cancer [30–32], stomach cancer [30, 32] and hepatic cancer [30]). Details are provided in ESM Tables S6 and S7.
Estimates for survival following smoking-related diseases were derived from published sources of data collected in Japan (MI [33, 34], stroke [33], COPD [35], and lung, stomach and hepatic cancer [36]). (Parameter estimates are provided in ESM Table S8.) Parameters used to assign a time of death to individuals in the absence of developing smoking-related diseases (ESM Table S9) are based on the life tables developed by the Ministry of Health, Labour and Welfare for Japan [37].
The utility values assigned take into consideration age and any smoking-related diseases the individual develops (Table 1). The utility assigned is the minimum of the individual’s age-dependent utility [16] (see ESM for more details) and the utility associated with any of the smoking-related diseases that the individual has experienced up to that point in time. The values for stroke and COPD were obtained from studies conducted in Japan, but other values were from a survey in Korea (2005 Korea National Health and Nutrition Examination Survey [38]) and the age-dependent decline was from a study in the USA (2000–2002 Medical Expenditure Panel Survey [39]) as there were no data identified from studies conducted in Japan.
The analyses also considered lost wages due to premature mortality caused by smoking: these were estimated up to 75 years of age. Data on labour force participation in Japan and wages by age 18–75 years were used to estimate these costs [40–42].
Analyses
Costs and QALYs are discounted at 3 % per annum. For each analysis, 500,000 smokers were run through the lifetime simulation. Several scenario analysis and probabilistic sensitivity analyses were also conducted. Direct medical costs were assessed from the health care payer’s perspective in Japan (expressed in 2014 Japanese Yen [¥]). Supplemental analyses were conducted from a restricted societal perspective, in which lost wages were included.
Two scenarios compared the outcomes when each smoker initiates a quit attempt with one of the cessation strategies (varenicline or NRT) versus an unassisted attempt. The outcomes are reported for both when smokers are limited to an SQA versus being allowed to initiate MQAs over a lifetime time horizon.
The third scenario compared a “market mix”, where the smokers use one of four cessation methods for the first quit attempt based on the survey results (varenicline 4.6 %, BMT 1.5 %, NRT 21.4 %, unassisted 72.5 %) versus 100 % using varenicline. Subgroup analyses were conducted to evaluate the change in results by baseline age categories in order to provide insight into the policy implications of promoting smoking cessation in particular age groups. Net monetary benefit is estimated assuming a willingness-to-pay threshold of ¥5,000,000 per QALY gained [43].
To account for multivariate and stochastic uncertainty in the model, a probabilistic sensitivity analysis (PSA) was performed. The PSA was run for 5000 replications. In each replication, 1000 patients were simulated for each intervention arm. A summary of the parameters and their distributions included in the PSA is provided in ESM Table S10. Only those parameters around which there is analytical or quantifiable uncertainty were included in the PSA, and only direct medical costs were considered. Distributions were selected and fitted according to the statistical properties of those parameters included.
Results
Pharmacotherapy (Nicotine Replacement Therapy or Varenicline) Versus Unassisted
Over a lifetime, both pharmacotherapy scenarios are projected to lead to a net increase in abstinence time, with 3.8 years per patient using NRT, and 5.8 years per patient using varenicline for the MQA scenario (Table 2). The increase in abstinence time translates into improved health outcomes (i.e. lower incidence of smoking-related diseases and longer life expectancy) and is associated with additional QALYs (discounted QALY gain of 0.07 per patient with NRT, 0.10 per patient with varenicline). Medication and monitoring costs are higher, but this is offset by the lower management costs associated with smoking-related diseases, leading to savings in direct medical costs. The incremental cost per QALY gained and the direct medical cost savings estimated with both NRT and varenicline versus unassisted quit attempts leads to pharmacotherapy being dominant (i.e. more effective and with lower costs) over a lifetime.
Table 2.
Comparison of outcomes when the first quit attempt is supported by nicotine replacement therapy or varenicline versus an unassisted attempt—single and multiple quit attempt scenarios per patient over a lifetime
| Outcomes | Unassisted | NRT net | Varenicline net |
|---|---|---|---|
| Abstinence time, years | |||
| SQA | 1.52 | 1.65 | 2.89 |
| MQA | 9.92 | 3.79 | 5.84 |
| Life-years | |||
| SQA | 28.91 | 0.12 | 0.17 |
| MQA | 29.26 | 0.21 | 0.32 |
| Discounted QALYs | |||
| SQA | 14.87 | 0.04 | 0.05 |
| MQA | 14.96 | 0.07 | 0.10 |
| Discounted costs (¥) | |||
| Smoking-related disease | |||
| SQA | 3,506,972 | −283,579 | −311,073 |
| MQA | 3,340,219 | −323,986 | −391,680 |
| Direct medical, total | |||
| SQA | 3,506,972 | −240,128 | −245,814 |
| MQA | 3,340,219 | −152,192 | −176,140 |
| Indirect (lost wages) | |||
| SQA | 6,414,867 | −159,640 | −122,101 |
| MQA | 6,235,857 | −198,146 | −314,343 |
| Total costs | |||
| SQA | 9,921,839 | −399,768 | −367,915 |
| MQA | 9,576,075 | −350,338 | −490,483 |
Results for unassisted at first attempt are presented in absolute terms, while results for NRT and varenicline are presented as the difference from the unassisted SQA or MQA, respectively
¥ Japanese yen, MQA multiple quit attempts, NRT nicotine replacement therapy, QALYs quality-adjusted life-years, SQA single quit attempt
The analyses also allow comparison of the predicted outcomes when single attempts are allowed versus MQAs, and this illustrated a marked difference in the mean cumulative abstinence time (Table 2). When the first quit attempt is unassisted, on average, a smoker accrued fewer years of abstinence time over their lifetime (mean of 1.5 years) when compared with MQAs (mean of 9.9 years). The impact of SQAs versus MQAs on other outcomes is smaller—there is a difference in mean total discounted QALYs of 0.09 over the course of the simulation with MQAs, and the costs associated with smoking-related diseases are approximately 5 % higher in the SQA scenario.
Varenicline Versus Market Mix (Multiple Quit Attempts [MQAs])
Increased use of varenicline to support quit attempts is projected to lead to a net increase in abstinence and life expectancy (mean of 4.6 years abstinence gained per patient, and 0.25 LYs gained) when compared with the current mix of strategies being used (Table 3). This is associated with additional QALYs (discounted QALY gained 0.08 per patient). Direct medical costs are 6 % lower due to the lower costs estimated for managing smoking-related diseases. The incremental cost per QALY gain and direct medical cost savings estimates leads to varenicline being dominant over a lifetime. Indirect costs due to lost wages are an important component of the total costs, and exceeded the direct medical cost estimates.
Table 3.
Comparison of outcomes when the first quit attempt uses 100 % varenicline versus the current market mix of interventions (multiple quit attempts per patient over a lifetime)
| Outcomes | Market mix | Varenicline net |
|---|---|---|
| Incidence of smoking-related diseases | ||
| COPD | 0.26 | −0.01 |
| Lung cancer | 0.11 | −0.01 |
| MI | 0.07 | −0.0003 |
| Stroke | 0.17 | −0.005 |
| Stomach cancer | 0.08 | −0.0006 |
| Hepatic cancer | 0.04 | 0.0001 |
| Abstinence time, years | 11.19 | 4.56 |
| Life-years | 29.30 | 0.25 |
| Discounted QALYs | 14.96 | 0.08 |
| Discounted costs (¥) | ||
| Smoking-related disease | 3,316,453 | −357,587 |
| Direct medical total | 3,379,894 | −206,095 |
| Indirect (lost wages) | 6,148,085 | −246,814 |
| Total costs | 9,527,979 | −452,909 |
Results for the market mix scenario are presented in absolute terms, while results for varenicline are presented as the difference from the market mix strategy
¥ Japanese yen, COPD chronic obstructive pulmonary disease, MI myocardial infarction, QALYs quality-adjusted life-years
Results from the subgroup analyses by age at baseline are presented in Table 4. Whilst the additional discounted QALY gains show some small differences by age (ranging from 0.06 to 0.10 per patient), much more substantial differences are observed for direct medical and indirect costs. The direct medical cost savings increase with the age at which smokers are making an attempt to quit, whereas, as expected, the indirect cost savings decrease. Net discounted lifetime direct medical costs per patient differ, with net costs for the youngest subgroup (¥43,409 per patient, age 20–30 years at baseline) and net savings that increase with age (up to ¥319,186 per patient, age ≥60 years). The abstinence time over a lifetime is higher for the smokers who were younger when making the first quit attempt, leading to a lower frequency of smoking-related diseases, and the associated costs do not offset the smoking cessation medication-related costs.
Table 4.
Comparison of outcomes by age category when the first quit attempt uses 100 % varenicline versus the current market mix of interventions (multiple quit attempts per patient over a lifetime)
| Age (years) | QALYs | Direct costs (¥) | Net monetary benefita | Indirect costs (¥) | |||
|---|---|---|---|---|---|---|---|
| Market mix | VAR net | Market mix | VAR net | Market mix | VAR net | ||
| 20 to <30 | 21.35 | 0.058 | 2,214,242 | 43,409 | 246,394 | 6,804,260 | −366,428 |
| 30 to <40 | 19.06 | 0.076 | 2,546,561 | −47,781 | 425,678 | 8,058,944 | −384,920 |
| 40 to <50 | 15.91 | 0.096 | 3,201,342 | −191,731 | 671,897 | 7,469,926 | −345,057 |
| 50 to <60 | 12.67 | 0.088 | 3,773,296 | −275,014 | 717,212 | 4,891,395 | −179,511 |
| ≥60 | 9.28 | 0.062 | 4,522,490 | −319,186 | 630,685 | 1,934,117 | −58,948 |
Results for the market mix scenario are presented in absolute terms, while results for varenicline are presented as the difference from the market mix strategy
¥ Japanese yen, MQA multiple quit attempts, QALYs quality-adjusted life-years, VAR varenicline
aNet monetary benefit = (net QALYs) × ¥5,000,000 − (net direct cost)
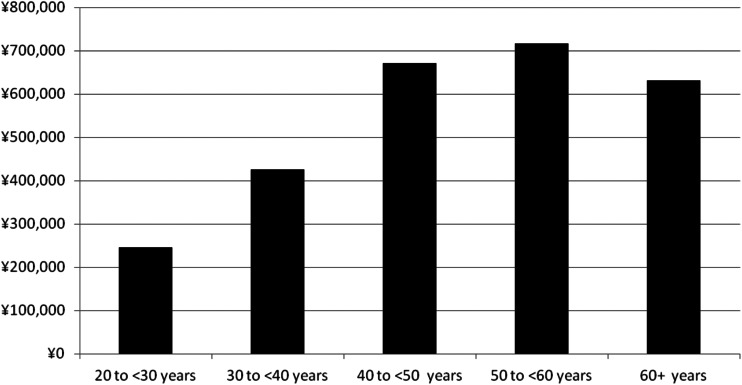
Overall, the greater net monetary benefit observed for the 50- to <60-year age category means this ranks as the most cost-effective scenario, followed by 40 to <50 years, ≥60 years, 30 to <40 years, and 20 to <30 years (Fig. 1).
Fig. 1.
Varenicline versus market mix for the first quit attempt: net monetary benefit (discounted) by age category. Net monetary benefit = (net QALYs) × ¥5,000,000 − (net direct cost). ¥ Japanese yen, QALYs quality-adjusted life-years
Sensitivity Analyses (MQAs)
Sensitivity analyses conducted around the base-case scenario (market mix vs. varenicline) did not have a major impact on model outcomes. Modifying rates of long-term relapse (±50 %) had a modest impact, with mean LYs gained per patient ranging from 0.21 to 0.31, and discounted QALYs gained from 0.06 to 0.10. Varenicline remained dominant over a lifetime, and was associated with total direct medical savings of ¥159,494 per smoker with these higher relapse rates. Variation in the probability of a successful attempt (±25 %) with varenicline had a larger impact on the health outcomes predicted, with mean LYs gained per patient ranging from 0.11 to 0.37, and discounted QALYs gained from 0.04 to 0.12. However, even with a 25 % reduction in effectiveness, varenicline remained dominant over a lifetime and was associated with total direct medical savings of ¥115,804 per smoker.
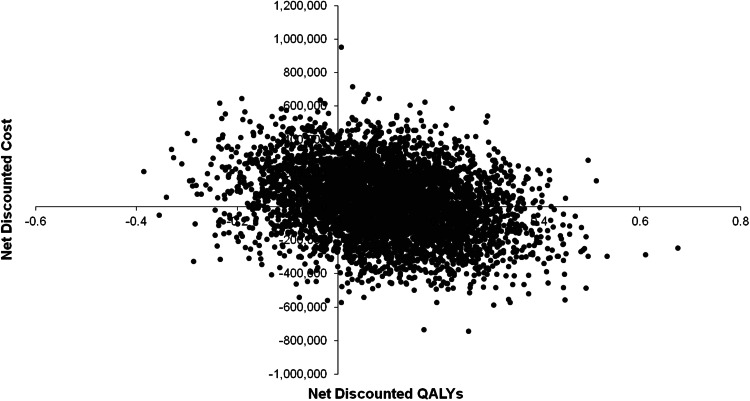
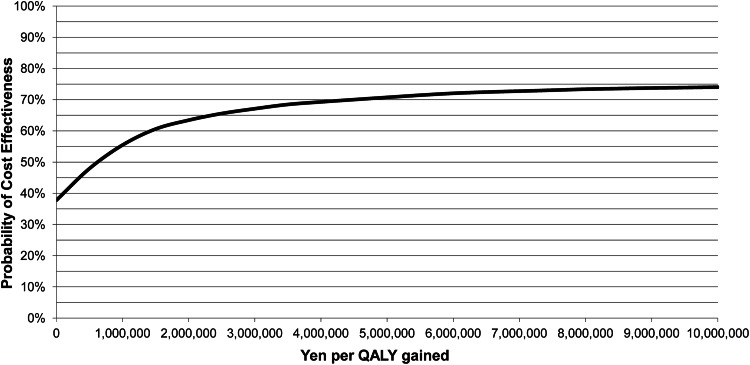
The cost-effectiveness plane for market mix (MQA) versus varenicline provided in Fig. 2 with benefits expressed in QALYs shows a clustering in the lower right quadrant, meaning that varenicline is less costly but also more effective than market mix (MQA) in terms of QALYs gained. Both direct medical costs and benefits are discounted in this graph. As can be seen, this uncertainty results in a split clustering mainly between varenicline being cost effective and dominant. On average, the probabilistic analysis shows dominance for varenicline. The probability of varenicline providing a cost-effective alternative to market mix (MQA) is 38 % at a threshold of ¥0 per QALY gained (due to dominating in 38 % of the replications), 66 % at a threshold of ¥2,500,000 or 71 % at a threshold of ¥5,000,000 (Fig. 3). Comparable PSA results were obtained for varenicline versus unassisted (MQA) and are provided in ESM Figs. S1 and S2.
Fig. 2.
Cost-effectiveness plane for varenicline (multiple quit attempts) versus market mix. QALYs quality-adjusted life-years
Fig. 3.
Cost-effectiveness acceptability curve for varenicline (multiple quit attempts) versus market mix. ¥ Japanese yen, QALY quality-adjusted life-year
Discussion
Increased utilization of smoking cessation pharmacotherapy to support quit attempts is predicted to lead to an increase in the number of smokers achieving abstinence and to improvements in health outcomes over a lifetime with no additional costs. The results from this simulation indicate that if more quit attempts were supported with varenicline, there could be an increase in abstinence and life expectancy when compared with the current mixture of strategies being used in Japan, where most are unassisted attempts. The age-stratified analyses implied smoking cessation improved health outcomes across all ages, and these benefits are achieved with cost savings from managing tobacco-related diseases. Indirect costs due to premature death leading to lost wages are an important component of the total costs, as these estimates exceeded the direct medical cost estimates. These results were consistent with a previous study conducted by Igarashi et al. [14], in which adding varenicline to smoking cessation consultation was found to be dominant, and also the study by Fukuda [44], which proved that smoking cessation consultation itself would be dominant to an unassisted quit attempt. In our model, the increase in healthcare costs other than for tobacco-related diseases, due to prolongation of the life expectancy of quitters, were not included. Such types of “unrelated medical costs” would be important, especially for smoking cessation treatment, since there are so many tobacco-related diseases, which implies that the life-expanding effects due to smoking cessation would be large. However, according to the Japanese guideline for health economic analysis (Fukuda [45]), medical costs should be limited to costs of related diseases. As was noted in the guideline, small variations of unrelated costs would mask the difference in related costs, since the number of unrelated costs may be much bigger than that of related costs (Igarashi et al. [46]). Therefore, unrelated costs were excluded from the analyses.
This model reflects smokers’ behaviour while they attempt to stop smoking entirely, as it allows them to make MQAs and also allows them, even when successful, to relapse. The predictions for treatment success and smoking outcomes consider individual smoker profiles and smoking cessation strategies, and the time intervals between quit attempts. Whilst the framework for this simulation has been previously published [16], two additional diseases associated with smoking (stomach cancer and hepatic cancer) have been added to this model. In this simulation, an individual’s age and duration of abstinence directly impact the risk of developing the six smoking-related diseases; however, the potential impact of abstinence on the progression of a complication (e.g. the effect of abstinence on the progression of COPD) is not captured. Japanese studies were identified to estimate the prevalence of these diseases among non-smokers and the relative risk for smokers. However, the reduction in the risk ratio applied for each disease over the years following stopping smoking was estimated from a study conducted in The Netherlands by Hoogenveen et al. [26] as no comparable study conducted in Japan has been identified. The model has also been modified to estimate the lost wages due to premature mortality caused by smoking, which was not previously considered.
A key strength of this specific study is how the original model framework was adapted to allow smokers to have MQAs using the new prediction equations for sequences of smoking cessation strategies, and the time intervals between quit attempts based on the patterns observed in a recent survey conducted in Japan [12]. The analyses presented are therefore based on inputs specifically derived for Japan, and ensure these analyses can be used to inform decision making related to public funding of smoking cessation therapies. The important exceptions to note are that the equations used to estimate success and relapse rates in the year following initiation of a quit attempt with varenicline or NRT were unchanged from the earlier publication (these were derived from an analysis of five clinical trials [16]) and were assumed to apply in Japan. In addition, utility estimates for some smoking-related diseases were not available from studies in Japan. Sensitivity analyses are presented, therefore, that explored the impact of varying the efficacy and relapse assumptions, as well as utilities.
The Japanese public health insurance system covers counselling and prescription medications to support a quit attempt, but the current eligibility criteria for these services limit access to these services. According to current insurance coverage criteria, the Brinkman Index is calculated by multiplying smoking years by the number of cigarettes smoked per day, and must be over 200. This criterion can be easily met by older smokers, since they started smoking many years before; however, it can be a hurdle for younger people to obtain coverage. In particular, this index makes it much more difficult for people in their early 20s to be covered by public health insurance for smoking cessation treatment. In this study, monetary benefit was positive for all ages, which implied that smoking cessation treatment would be cost effective for younger smokers who were not eligible for insurance coverage with current criteria. The Brinkman Index could be re-considered in order to enhance smoking cessation at a younger age.
Conclusions
Unassisted quit attempts remain the most common cessation strategy; however, an increased use of smoking cessation pharmacotherapy to support quit attempts is predicted to lead to an increase in the number of smokers achieving abstinence and also to be socially beneficial in terms of both a reduction in healthcare costs associated with smoking-related diseases and increased productivity. Considering an expansion of public funding to allow greater access to smoking cessation pharmacotherapy may be worthwhile.
Electronic supplementary material
Acknowledgments
This model was based on a discrete-event simulation framework developed for a US model that has been modified to include additional smoking-related diseases, and reflect cessation attempts in Japan [16]. The authors wish to acknowledge that the simulation for Japan was built on the concepts of the research published previously.
Compliance with Ethical Standards
Funding
This study was funded by Pfizer Japan Inc., Tokyo, Japan. Evidera received funding from Pfizer in connection with conducting this study and with the development of this manuscript.
Conflict of interest
Ataru Igarashi and Rei Goto have disclosed that they received a research grant from Pfizer Japan Inc. in connection with the conduct of this study, and have been on a Speaker’s Bureau for Novartis Pharma K.K. In addition, Ataru Igarashi has disclosed that he received research grant from CSL Behring Japan Inc. and Gilead Sciences K.K., received advisor/consultant fee from AbbVie GK., Novartis Pharma K.K., CRECON Research and Consulting Inc., Milliman Inc., Sony Inc., Kantar Health Inc. and Pfizer Japan Inc., has been on a Speaker’s Bureau for AbbVie GK, Astellas Pharma K.K., Boehringer Ingelheim Japan Inc., Chugai Pharmaceutical Co. Ltd, Novartis Pharma K.K. and Pfizer Japan Inc., and has been employed in a laboratory funded by TOWA Pharmaceuticals Co. Ltd.
Kiyomi Suwa and Reiko Yoshikawa have disclosed that they are employees of Pfizer Japan Inc. Alexandra J. Ward and Jörgen Möller are employees of Evidera, which provides consulting and other research services to pharmaceutical, device, government and non-government organizations. In their salaried positions, they work with a variety of companies and organisations and are precluded from receiving payment or honoraria directly from these organisations for services rendered.
Since this manuscript discusses a model informed by the published literature, the authors do not have full control of the primary data, which is in the hands of the authors of the published literature. Therefore, they could not grant Applied Health Economics and Health Policy access to review the primary data if requested.
Author’s contributions
Dr. Igarashi and Dr. Goto worked on the design of, preparation for and conduct of the study, plus data analysis, interpretation of results, and the writing, editing and final approval of the manuscript. Kiyomi Suwa and Reiko Yoshikawa, employees of Pfizer Japan Inc., were involved in the preparation for and conduct of the study, and the preparation, writing, review and final approval of the manuscript. Alexandra J. Ward and Jörgen Möller, employees of Evidera, worked on the design preparation and conduct of the study, plus data analysis, interpretation of results, and the writing, editing, review and final approval of the manuscript.
Dr. Ataru Igarashi will act as overall guarantor.
Ethical standards: approval and informed consent
The manuscript cites only published sources, and does not describe any new clinical studies or patient data collection. No approval by an Institutional Review Board was necessary, and informed consents were not required.
References
- 1.Ikeda N, Inoue M, Iso H, et al. Adult mortality attributable to preventable risk factors for non-communicable diseases and injuries in Japan: a comparative risk assessment. PLoS Med. 2012;9:e1001160. doi: 10.1371/journal.pmed.1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Printed with corrections January 2014. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf. Accessed 28 Oct 2014.
- 3.A Study Meeting on Smoking and Health Problems [Edited]. A study meeting on smoking and health problems: new version—smoking and health [in Japanese]. Tokyo: Hokendohjinsha Inc.; 2002.
- 4.Ministry of Health, Labour and Welfare. 2013 National Health and Nutrition Examination Survey [in Japanese]. Tokyo Ministry of Health, Labour, and Welfare. 2015. http://www.mhlw.go.jp/stf/houdou/0000067890.html. Accessed 3 Apr 2015.
- 5.Japanese Circulation Society. Clinical practice guidelines for smoking cessation pharmacotherapy and counseling, sixth edition [in Japanese]. 2014. https://www.jrs.or.jp/uploads/uploads/files/information/non-smoking_06.pdf. Accessed 28 Oct 2014.
- 6.Hagimoto A, Nakamura M, Morita T, et al. Smoking cessation patterns and predictors of quitting smoking among the Japanese general population: a 1-year follow-up study. Addiction. 2010;105:164–173. doi: 10.1111/j.1360-0443.2009.02735.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M, Oshima A, Ohkura M, et al. Predictors of lapse and relapse to smoking in successful quitters in a varenicline post hoc analysis in Japanese smokers. Clin Ther. 2014;1(36):918–927. doi: 10.1016/j.clinthera.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Ranney L, Melvin C, Lux L, et al. Systematic review: smoking cessation intervention strategies for adults and adults in special populations. Ann Intern Med. 2006;5(145):845–856. doi: 10.7326/0003-4819-145-11-200612050-00142. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami N, Takatsuka N, Inaba S, et al. Development of a screening questionnaire for tobacco/nicotine dependence according to ICD-10, DSM-III-R, and DSM-IV. Addict Behav. 1999;24:155–166. doi: 10.1016/S0306-4603(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 10.Brinkman GL, Coates EO., Jr The effect of bronchitis, smoking, and occupation on ventilation. Am Rev Respir Dis. 1963;87:684–693. doi: 10.1164/arrd.1963.87.5.684. [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Health Labour and Welfare. 2013 Survey of Medical Care Activities [in Japanese]. Tokyo: Ministry of Health, Labour and Welfare; 2014. http://www.mhlw.go.jp/toukei/saikin/hw/sinryo/tyosa13/. Accessed 3 Apr 2015.
- 12.Igarashi A, Negishi S, Goto R, et al. Web-based survey on smoking cessation behaviors of current and former smokers in Japan. Curr Med Res Opin. 2014;30:1911–1921. doi: 10.1185/03007995.2014.938149. [DOI] [PubMed] [Google Scholar]
- 13.Ezura M, Inagaki A, Yamauchi K, et al. Economic evaluation of ethical drugs for smoking cessation. Jpn J Clin Psychopharmacol. 2011;14:1667–1677. [Google Scholar]
- 14.Igarashi A, Takuma H, Fukuda T, et al. Cost-utility analysis of varenicline, an oral smoking-cessation drug, in Japan. Pharmacoeconomics. 2009;27:247–261. doi: 10.2165/00019053-200927030-00007. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda H, Ikeda S. Cost-effectiveness of smoking cessation in Japan. Jpn J Pharmacopidemiol. 2009;14:61–68. doi: 10.3820/jjpe.14.61. [DOI] [Google Scholar]
- 16.Getsios D, Marton JP, Revankar N, et al. Smoking cessation treatment and outcomes patterns simulation: a new framework for evaluating the potential health and economic impact of smoking cessation interventions. Pharmacoeconomics. 2013;31:767–780. doi: 10.1007/s40273-013-0070-5. [DOI] [PubMed] [Google Scholar]
- 17.Xenakis JG, Kinter ET, Ishak KJ, et al. A discrete-event simulation of smoking-cessation strategies based on varenicline pivotal trial data. Pharmacoeconomics. 2011;29:497–510. doi: 10.2165/11589230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Aubin HJ, Bobak A, Britton JR, et al. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;5(296):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;5(296):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 21.Rigotti NA, Pipe AL, Benowitz NL, et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;19(121):221–229. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tashkin DP, Rennard S, Hays JT, et al. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest. 2011;139:591–599. doi: 10.1378/chest.10-0865. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Oshima A, Fujimoto Y, et al. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;29:1040–1056. doi: 10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Kasza KA, Hyland AJ, Borland R, et al. Effectiveness of stop-smoking medications: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2013;108:193–202. doi: 10.1111/j.1360-0443.2012.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wetter DW, Cofta-Gunn L, Fouladi RT, et al. Late relapse/sustained abstinence among former smokers: a longitudinal study. Prev Med. 2004;39:1156–1163. doi: 10.1016/j.ypmed.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Hoogenveen RT, van Baal PH, Boshuizen HC, et al. Dynamic effects of smoking cessation on disease incidence, mortality and quality of life: the role of time since cessation. Cost Eff Resour Alloc. 2008;6:1. doi: 10.1186/1478-7547-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igarashi A, Oyamada M, Kubota K. The issues and provision of chronic obstructive pulmonary disease (COPD) in Japan. The validation of QOL, productivity loss and socioeconomic liability [in Japanese]. Tokyo: Health and Global Policy Institute; 2014. https://www.hgpi.org/handout/COPDReport_HGPI.pdf. Accessed 28 Oct 2014.
- 28.Ministry of Health, Labour and Welfare. Patient Survey 2011: Statistics Table 13: Total number of patients by sex, year and classification of diseases [in Japanese]. Tokyo: Ministry of Health Labour, and Welfare, Health Statistics Office, Vital, Health and Social Statistics Division; 2011. http://www.mhlw.go.jp/toukei/saikin/hw/kanja/11/. Accessed 20 Oct 2015.
- 29.Fukuchi Y, Oyamada M, Kubota K, et al. COPD in Japan: the Nippon COPD Epidemiology study. Respirology. 2004;9:458–465. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda A, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2014;44:388–396. doi: 10.1093/jjco/hyu003. [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Center. Cancer Statistics in Japan, 2008: smoking and cancer [in Japanese]. Tokyo: National Cancer Center, Foundation for Promotion of Cancer Research (FPCR); 2008. http://ganjoho.jp/professional/statistics/backnumber/2008_jp.html. Accessed 28 Oct 2014.
- 32.Sobue T. The epidemiological study of the relationship between smoking and disease via pooled analysis of large scale cohort studies, Research Grant of MHLW in Japan. General research project against life-style diseases. Research for reviewing scientific evidences around tobacco (PI: Sobue T). Saitama, Japan: National Institute of Public Health; 2006. http://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=200624022A. Accessed 21 Aug 2015.
- 33.Kubo M, Kiyohara Y, Kato I, et al. Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: the Hisayama study. Stroke. 2003;34:2349–2354. doi: 10.1161/01.STR.0000090348.52943.A2. [DOI] [PubMed] [Google Scholar]
- 34.Okura N, Ogawa H, Katoh J, et al. Long-term prognosis of patients with acute myocardial infarction in the era of acute revascularization (from the Heart Institute of Japan Acute Myocardial Infarction [HIJAMI] registry) Int J Cardiol. 2012;6(159):205–210. doi: 10.1016/j.ijcard.2011.02.072. [DOI] [PubMed] [Google Scholar]
- 35.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Cancer Center. Cancer Statistics in Japan, 2013: survival rate in the member hospitals of the Association of Clinical Cancer Centers [in Japanese]. Tokyo: National Cancer Center, Foundation for Promotion of Cancer Research (FPCR); 2013. http://ganjoho.jp/data/professional/statistics/backnumber/2013/cancer_statistics_2013.pdf. Accessed 28 Oct 2014.
- 37.Ministry of Health, Labour and Welfare. Abridged life table 2012 [in Japanese]. Tokyo: Ministry of Health, Labour and Welfare; 2013. http://www.mhlw.go.jp/toukei/saikin/hw/life/life12/dl/life12-11.xls. Accessed 9 Nov 2014.
- 38.Kang EJ, Ko SK. A catalogue of EQ-5D utility weights for chronic diseases among noninstitutionalized community residents in Korea. Value Health. 2009;12(Suppl 3):S114–S117. doi: 10.1111/j.1524-4733.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan PW, Ghushchyan V. Mapping the EQ-5D index from the SF-12: US general population preferences in a nationally representative sample. Med Decis Mak. 2006;26:401–409. doi: 10.1177/0272989X06290496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ministry of Health, Labour and Welfare. Wage and labour welfare statistics: office employment, basic survey on wage structure, 2013 [in Japanese]. Tokyo: Ministry of Health, Labour and Welfare; 2013. http://www.mhlw.go.jp/toukei/itiran/roudou/chingin/kouzou/z2013/. Accessed 28 Oct 2014.
- 41.Ministry of Internal Affairs and Communications. Total population: Japanese population: population estimates 2012 [in Japanese]. Tokyo: Ministry of Internal Affairs and Communications, Statistics Bureau; 2012. http://www.stat.go.jp/data/jinsui/2.htm#monthly. Accessed 28 Oct 2014.
- 42.Ministry of Internal Affairs and Communications. Labour Force Survey by age group, 2014 [in Japanese]. Tokyo: Ministry of Internal Affairs and Communications, Statistics Bureau; 2014. http://www.stat.go.jp/data/roudou/index2.htm#kekka. Accessed 28 Oct 2014.
- 43.Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19:422–437. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda T. Health economical evaluation of smoking cessation, Research Grant of MHLW in Japan. Study of development of effective smoking cessation method and institutionalization for dissemination (PI: Oshima A). Saitama, Japan: National Institute of Public Health; 2005. http://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=200500482A. Accessed 21 Aug 2015.
- 45.Fukuda T, Shiroiwa T, Ikeda S, et al. Guideline for economic evaluation of healthcare technologies in Japan. J Natl Inst Public Health. 2013;62:625–640. [Google Scholar]
- 46.Igarashi A, Hashimoto Y, Shiroiwa T, et al. How to handle (with) unrelated cost in health economic analyses? Jpn J Pharmacoepidemiol. 2012;17:21–26. doi: 10.3820/jjpe.17.21. [DOI] [Google Scholar]
- 47.Hattori N, Hirayama T, Katayama Y. Medical care for chronic-phase stroke in Japan. Neurol Med Chir (Tokyo). 2012;52:175–80. doi: 10.2176/nmc.52.175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.