Abstract
MicroRNA-206, which suppresses the expression of brain-derived neurotrophic factor, is known to be elevated in the brains of Alzheimer’s disease (AD) patients. We performed intranasal biopsy of the olfactory epithelia of early dementia patients (n = 24) and cognitively healthy controls (n = 9). Patients with significant depression (n = 8) were analyzed separately, as their cognitive impairments were thought to be caused by their depression. Real-time PCR was performed on the biopsied tissues. The relative microRNA-206 level exhibited a 7.8-fold increase (P = 0.004) in the mild cognitive impairment group (CDR 0.5; n = 13) and a 41.5-fold increase (P < 0.001) in the CDR 1 group (n = 11). However, this level was not increased in the depression group, even in those with cognitive decline. Using the optimal cutoff value, the sensitivity/specificity for diagnosing CDR 0.5 and CDR 1 dementia were 87.5%/94.1% and 90.9%/93.3%, respectively. In ROC analysis, the AUCs were 0.942 and 0.976 in the CDR 0.5 and CDR 1 groups, respectively. The olfactory mucosal microRNA-206 level and cognitive assessment scores were significantly correlated in the non-depressed subjects with cognitive impairment. In conclusion, the olfactory mucosal microRNA-206 level can be easily measured, and it can be utilized as an excellent biomarker for the diagnosis of early AD, including mild cognitive impairment.
More than 35 million people worldwide are currently suffering from Alzheimer’s disease (AD), and the number of patients will increase to >100 million by 20501. Unfortunately, no single medication showing disease-modifying effects has been approved by the US Food and Drug Administration to date2,3. Therefore, the early diagnosis of patients in the incipient stage of AD is essential for patient care and also to plan clinical trials for potential AD drugs. However, most AD diagnoses are based on clinical findings4. Multiple biomarkers have been suggested, but there are limitations associated with each biomarker, and most of them have been shown to be ineffective in differentiating individuals with mild cognitive impairment (MCI) from cognitively healthy people5. Consequently, there is still an unmet need for a better biomarker capable of the very early detection of AD.
Brain-derived neurotrophic factor (BDNF) is the most widely expressed neurotrophin in the central nervous system, and it has a neurotrophic effect on neurons by binding to its specific receptor, tyrosine receptor kinase B6. Moreover, BDNF is a key molecule involved in the maintenance of synaptic plasticity and synaptogenesis in the hippocampus, which is the core locus of memory acquisition and consolidation7,8. AD is considered to be caused by synaptic failure9, and decreased BDNF levels have been reported in the brain and blood of AD patients and AD animal models6. A low serum BDNF level has been associated with a smaller hippocampal volume and poorer memory10. Additionally, the serum BDNF level has been negatively correlated with the severity of dementia11, and the Val66Met polymorphism of the BDNF gene has been proposed to be a marker for the prediction of disease progression in MCI patients12. Recently, it has been reported that BDNF may also be reduced in healthy people who are destined to develop dementia or AD13.
MicroRNAs (miRNAs) are regulators of numerous biological processes, and alterations in miRNA levels occur in various human diseases14. miRNA-206 (miR-206) is conventionally known as one of the myomiRs, which are abundant in muscle tissue and play important roles in muscle development and muscle remodeling15. However, recent studies have shown that miR-206 also has regulatory roles in other mammalian non-muscle tissues, including tumor suppressor functions in various types of cancer16. In a previous study, we have shown that miR-206 is elevated in the brains of AD patients and animal models and that it contributes to cognitive decline by suppressing BDNF expression in the brain (Fig. 1)17. However, there is no method available to evaluate the altered expression of a specific miRNA in the brain of a living subject. Recently, the olfactory epithelium (OE) has been receiving attention for its potential use in the research of neurodegenerative disease, as it reflects pathological changes in the brain18,19,20.
Figure 1. A schematic representation of the role of miRNA-206 in Alzheimer’s disease.
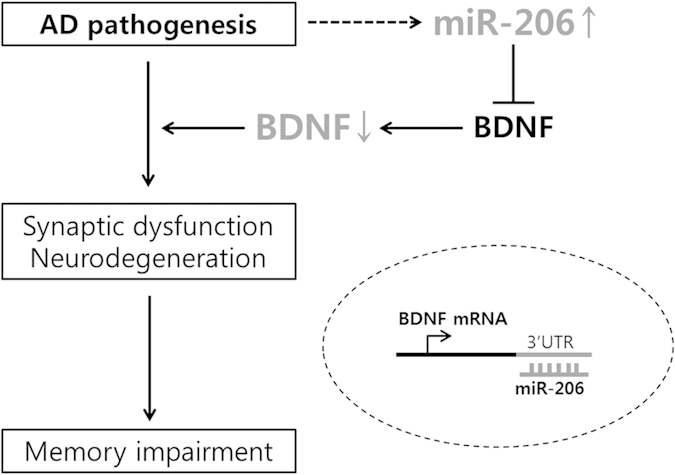
Abbreviations: AD: Alzheimer’s disease, miR: microRNA, BDNF: brain-derived neurotrophic factor, mRNA: messenger RNA, 3′ UTR: 3′ untranslated region.
In this study, we investigated whether miR-206 is elevated in the OE of early AD patients and whether the olfactory mucosal miR-206 level is an appropriate biomarker for the diagnosis of early AD.
Results
Basic characteristics of the subjects
A total of 41 subjects were enrolled in our study. The subjects were categorized based on their clinical dementia rating (CDR) score and Beck Depression Inventory-II (BDI-II) score. Nine patients who were cognitively healthy and did not exhibit depression were assigned to the CDR 0 (control) group. In addition, thirteen patients were assigned to the CDR 0.5 group, and eleven were assigned to the CDR 1 group. Eight patients with moderate to severe depression were placed in the depression group. The ages of the patients varied across the groups (P = 0.039; Table 1).
Table 1. The basic demographics and clinical assessment scores of the patients (n = 41).
| CDR 0 (n = 9) | CDR 0.5 (n = 13) | CDR 1 (n = 11) | Depression (n = 8) | P-value† | |
|---|---|---|---|---|---|
| Age, median (range)* | 63 (50–74) | 68 (56–79) | 69 (57–80) | 61.5 (56–66) | 0.039 |
| Sex (M:F) | 4:5 | 4:9 | 6:5 | 2:6 | |
| MMSE-K | 28.3 ± 0.7 | 26.8 ± 0.4 | 23.8 ± 0.6a,d | 25.1 ± 1.4 | 0.001 |
| CDR | 0 | 0.5 | 1 | 0.6 ± 0.2 | |
| ADAS-Cog-K | 8.4 ± 1.4 | 11.2 ± 1.1 | 16.0 ± 2.4c | 14.6 ± 3.2 | 0.031 |
| BDI-II | 5.2 ± 1.8 | 11.1 ± 1.4c | 9.3 ± 2.0 | 29.4 ± 2.4a,d,f | <0.001 |
| KVSS | 24.2 ± 1.1 | 23.6 ± 1.6 | 22.6 ± 0.9 | 21.9 ± 2.6 | 0.750 |
| Relative miR-206 level‡ | 1 | 7.8 ± 1.9b | 41.5 ± 6.4a,d | 1.2 ± 0.5e,f | <0.001 |
*Data presented as the median (range).
†The Kruskal-Wallis test was performed.
‡The relative miR-206 levels are divided by the mean value for the CDR 0 group.
aP < 0.001 vs. control.
bP < 0.01 vs. control.
cP < 0.05 vs. control.
dP < 0.001 vs. CDR 0.5.
eP < 0.01 vs. CDR 0.5.
fP < 0.001 vs. CDR 1.
Values are presented as the mean ± standard error of the mean (SEM). Abbreviations: CDR: clinical dementia rating, M: male, F: female, MMSE-K: Korean version of the Mini-Mental State Examination, ADAS-Cog-K: Korean version of the Alzheimer’s Disease Assessment Scale-cognitive subscale, BDI-II: Beck Depression Inventory-II.
Increased miR-206 levels in the olfactory epithelia of patients with early dementia.
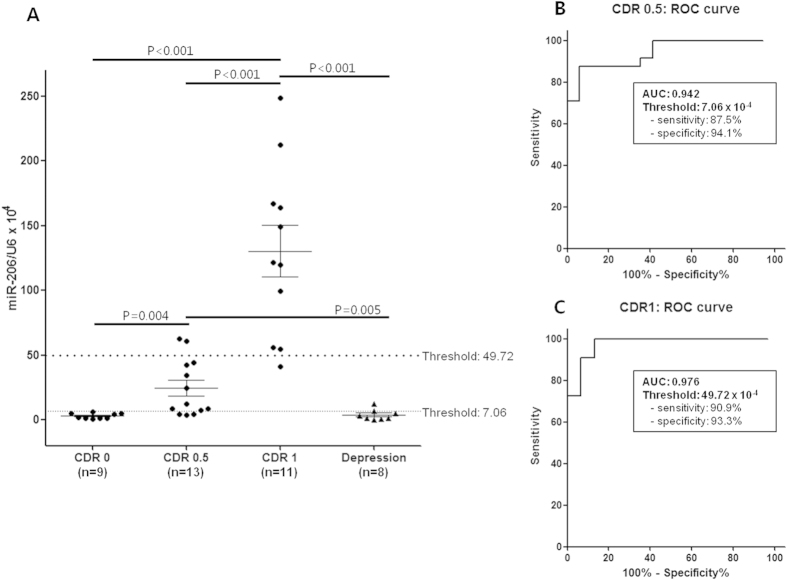
The miR-206 level was increased in the early dementia group compared with that in the control group (CDR 0.5: 7.8-fold increase, P = 0.004; CDR 1: 41.5-fold increase, P < 0.001; Fig. 2A), and it showed a remarkable tendency to increase with the progression of dementia. The patients in the depression group did not exhibit a significant alteration in the miR-206 level (1.2-fold increase, P = 0.671; Fig. 2A), although 6 of the 8 patients complained of cognitive dysfunction (Supplementary Table S1).
Figure 2. miRNA-206 real-time PCR results for olfactory epithelial samples.
Panel A demonstrates the relative miRNA-206 level (normalized to the U6 level) in each patient. The two cutoff values were determined by receiver operating curve (ROC) analysis, which revealed the highest sum of sensitivity and specificity for the diagnosis of CDR 0.5 or CDR 1 dementia from the entire population. The diagnosis of CDR 0.5 in ROC analysis signifies the detection of patients with a CDR ≥0.5. Panels B and C depict the results of ROC analyses for the diagnosis of CDR 0.5 and CDR 1 dementia, respectively. The areas under the curve (AUCs) are 0.942 and 0.976 for CDR 0.5 and CDR 1 dementia, respectively.
Receiver operating characteristic (ROC) analysis was performed, and the effectiveness of the olfactory mucosal miR-206 level for the diagnosis of CDR 0.5 or CDR 1 dementia was assessed in the entire patient population. A diagnosis of CDR 0.5 signifies the detection of patients with a CDR ≥ 0.5. The optimal cutoff values for detecting CDR 0.5 or CDR 1 patients were selected. An area under the curve (AUC) value of 0.942 indicated a diagnosis of CDR 0.5 dementia, and when the relative expression of miR-206 over U6 exceeded 7.06 × 10−4, the sensitivity was 87.5% (95% CI, 69.0–95.7) and the specificity was 94.1% (95% CI, 73.0–99.0; Fig. 2B). Only one patient in the depression group exhibited a higher miR-206 level than this cutoff value; however, this patient also complained of cognitive decline, with a CDR of 0.5 (Supplementary Table S1). None of the cognitively healthy patients had a miR-206 level of higher than this value (Fig. 2A). An AUC value of 0.976 indicated a diagnosis of CDR 1 dementia, and if the relative expression of miR-206 over U6 exceeded 49.72 × 10−4, the sensitivity was 90.9% (95% CI, 62.3–98.4) and the specificity was 93.3% (95% CI, 78.7–98.2; Fig. 2C). Two of the 13 (15.4%) patients with CDR 0.5 had a higher miR-206 level than this cutoff value, and none of the individuals with CDR 0 or depression exhibited a miR-206 level that exceeded this value (Fig. 2A). Additionally, ROC analysis was performed specifically on the non-depressed cognitive impairment patients. The olfactory mucosal miR-206 level was also useful for identifying the progression of dementia, with an AUC value of 0.944 for the diagnosis of CDR 1 dementia (Supplementary Fig. S1).
Considering early-onset AD is defined as onset before the age of 65, we assessed the ability of the olfactory mucosal miR-206 level to differentiate the presence of early dementia among patients younger than 65 years old versus those older than 65 years. Regardless of the age population, the miR-206 levels were significantly different among the groups. The diagnostic value of the olfactory mucosal miR-206 level was found to be greater among the patients who were younger than 65 years old. The patients with CDR 0.5 and CDR 1 demonstrated 11.9-fold and 57.1-fold increases in the miR-206 levels, respectively, compared with the control group (Supplementary Table S2). The AUC values for diagnosing CDR 0.5 and CDR 1 dementia were 0.952 and 1.000, respectively (Supplementary Fig. S2A,.B,C). The olfactory mucosal miR-206 level also demonstrated a fairly good ability to detect early dementia in the patients older than 65 years of age, although with less power than that observed for the patients younger than 65 years of age (Supplementary Table S2; Supplementary Fig. S2D,E,F).
Correlation of olfactory mucosal miR-206 level with clinical scores
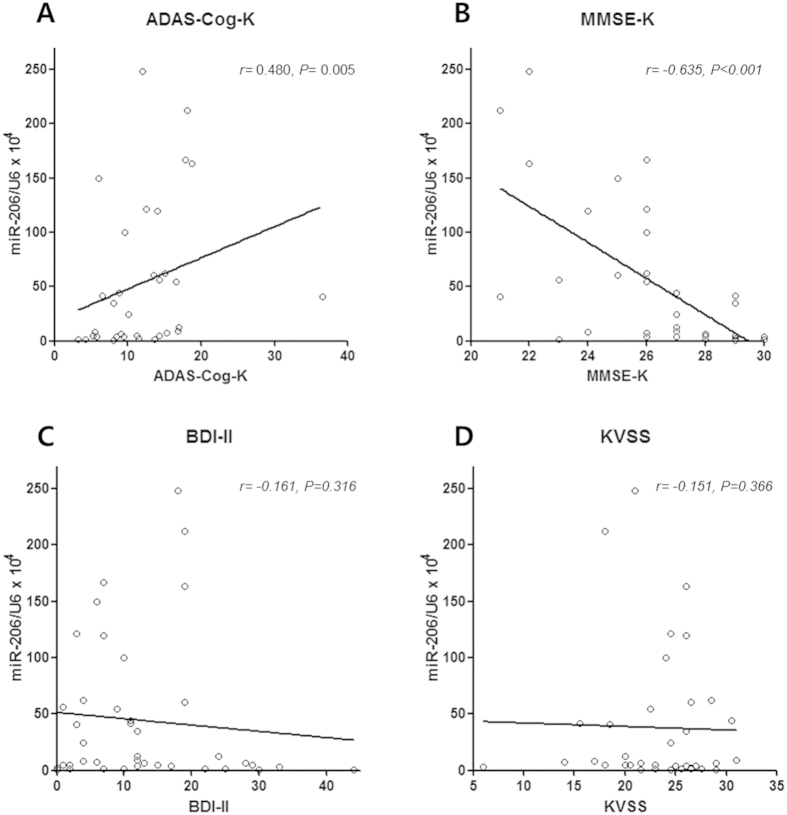
As depression is known to affect cognitive function, we assessed the correlation between the relative miR-206 level and the cognitive function scores, exclusively in the patients without depression (n = 33). A significant correlation was detected between the olfactory mucosal miR-206 level and these scores. The relative miR-206 level was positively correlated with the Korean version of Alzheimer’s Disease Assessment Scale-cognitive subscale score (ADAS-Cog-K score; r = 0.480, P = 0.005; Fig. 3A) and negatively correlated with the Korean version of the Mini-Mental State Examination score (MMSE-K; r = −0.635, P < 0.001; Fig. 3B). The correlations among the BDI-II score, the Korean version of the Sniffin’ Stick Test (KVSS) score and the relative miR-206 level were assessed for all patients. The relative miR-206 level was not found to be correlated with the BDI-II score (Fig. 3C) or KVSS score (Fig. 3D).
Figure 3. Correlation analyses between olfactory mucosal miR-206 level and clinical measures.
The correlations between the olfactory mucosal miR-206 level and cognitive assessment measures were analyzed in the non-depressed subjects. As shown in panels A and B, the olfactory mucosal miR-206 level is well correlated with the ADAS-Cog-K (P = 0.005) and MMSE-K scores (P < 0.001). As shown in panels C and D, the olfactory mucosal miR-206 level in all patients is not correlated with the BDI-II (P = 0.316) or KVSS score (P = 0.366). Abbreviations: ADAS-Cog-K: Korean version of the Alzheimer’s Disease Assessment Scale-cognitive subscale, MMSE-K: Korean version of the Mini-Mental State Examination, BDI-II: Beck Depression Inventory-II, KVSS: Korean version of the Sniffin’ Stick Test.
Safety of olfactory epithelial biopsy
Most episodes of nasal bleeding were controlled within 30 minutes after biopsy. Only one participant was taking antiplatelet agents at the time of biopsy. All of the participants returned home without any acute complications, including the one who was taking low-dose aspirin. We called each participant the next day to check for any complaints and informed them to contact us whenever they experienced any discomfort. Four of the 41 individuals (9.8%) experienced minor complications. Two of them complained of sustained nasal bleeding and had to return to the clinic. However, after additional hemostasis, they did not experience any further epistaxis. One patient complained of a mild headache, and another experienced watery rhinorrhea after biopsy, which naturally subsided within a few days. The remaining patients did not encounter any discomfort after the procedure.
Discussion
This is the first study to demonstrate the overexpression of miR-206 in the OE of patients with early dementia. The olfactory mucosal miR-206 level exhibited a sharp increasing tendency as dementia progressed, and it was significantly elevated, even in the patients with MCI (CDR 0.5 group; >7-fold increase), and was immensely increased in the patients in the CDR 1 group (>41-fold increase). However, the expression of olfactory mucosal miR-206 was not altered in the patients with depression, even in those with combined cognitive impairment. The relative miR-206 level in the OE could be a promising new biomarker for the early detection of AD, even at the MCI stage, and for the identification of subjects who might benefit from a miRNA-based treatment for AD.
Early diagnosis of AD is essential for patient care and for the success of AD drug trials. However, the diagnosis of AD still relies upon clinical decision making. Despite rapid advances in the identification of CSF markers and multiple types of neuroimaging, most current strategies are insufficient for differentiating MCI patients from cognitively healthy people5,21. The biomarkers for AD can be classified into two broad categories: (1) markers of amyloid-β (Aβ) accumulation; and (2) markers of neuronal injury or neurodegeneration21,22,23. The CSF Aβ42 assay and amyloid positron emission tomography (PET) imaging belong to the first category24; however, amyloid positivity does not reliably indicate clinical diagnosis. Although amyloid positivity on amyloid PET is reported in 90% of patients who are clinically diagnosed with AD dementia, 60% of patients with MCI and 30–40% of cognitively intact older people also exhibit amyloid deposits in the brain25. CSF phosphorylated tau (p-tau) 181 and total tau levels and 18F-fluorodeoxyglucose (FDG)-PET are markers of neuronal injury or neurodegeneration26,27. These markers help to predict progression to dementia in subjects with MCI28, but alterations are already present in many clinically normal elderly patients, making it impossible to distinguish normal aging from MCI or AD5,25.
The brain miR-206 level is elevated in patients with AD, and this miRNA may participate in the core pathogenesis of cognitive decline17. It has been reported that miR-206 post-transcriptionally suppresses BDNF expression29,30. BDNF is among the key regulators of synaptic plasticity and memory, and AD brains exhibit a decreased level of BDNF, which might be responsible for the cognitive impairment7. Several experiments have been performed with the aim of increasing the brain BDNF level in AD animal models31,32, but further study is necessary. Our group has reported for the first time that miR-206 is overexpressed in AD brains, both in animal models and in humans. We demonstrated that the brain BDNF level can be increased by antagonizing miR-206 activity, which eventually leads to the improvement of cognitive function17. The role of miR-206 in the regulation of brain BDNF was subsequently reproduced in later studies33,34. Therefore, the detection of patients with elevated brain miR-206 is important not only for making an early diagnosis of AD but also for identifying proper candidates for future AD drug trials based on the modulation of miR-206.
The OE, which is pseudostratified columnar epithelium located on the roof of the superior nasal cavity, is known to reflect pathological changes and gene expression profiles of the brain in association with many neurological diseases, including Parkinson’s disease20, schizophrenia35, and AD19. The OE of schizophrenia patients has been reported to demonstrate pathological evidence of abnormal neurodevelopment36,37. AD pathology, amyloid-β and abnormal tau protein have been reported to be present in the OE in the majority of patients with advanced AD38. The OE can be safely and easily biopsied in live human subjects via an intranasal approach39; thus, it has gained attention for its potential utility as a surrogate tissue in the research of brain disorders40.
In addition to its easy accessibility, the OE can reflect dynamic changes in the brain and can be used as a dynamic marker. Sattler et al. have demonstrated pharmacodynamic changes in the OE after drug administration41. Altered miRNA expression is known to be involved in the pathogenesis of many neurodegenerative diseases42. When it becomes possible to detect temporal changes in the levels of pathogenic miRNAs in the brain, this approach will be an excellent means to identify patients in the incipient stage of disease. Recently, Mor et al. have demonstrated that miR-382 expression is increased in the OE of schizophrenia patients, and this miRNA might be utilized as a novel biomarker of schizophrenia in living patients35.
The olfactory mucosal miR-206 level may be an excellent biomarker for the diagnosis of early AD for several reasons. First, it is significantly increased from the MCI stage; thus, it is effective for diagnosing patients with incipient AD. It is remarkable that the patients in the CDR 0 group had uniformly low olfactory mucosal miR-206 levels, while those in the CDR 0.5 group exhibited >7-fold increased levels. At the optimal cutoff value, the relative miR-206 level demonstrated fairly good sensitivity (87.5%) and specificity (94.1%) for the diagnosis of CDR 0.5 dementia. It is noteworthy that the cutoff value demonstrated greater specificity than sensitivity. This excellent specificity will help in the selection of patients in the very early stage of AD with minimal false positives. False positive results have been the major limitations of previous AD biomarkers being considered for clinical use5. The inclusion of many patients in the very early stage of AD with the minimization of false positive cases will improve the success rates of clinical trials. Notably, among the individuals who were under age 65, who are the subjects of early-onset AD by definition, the CDR 0.5 group demonstrated a larger increase (11.9-fold increase) in the miR-206 level and greater specificity (100%) at the optimal cutoff value. Accordingly, the olfactory mucosal miR-206 level will be a valuable screening tool for the selection of incipient AD patients who might be enrolled in AD drug trials.
Second, the olfactory mucosal miR-206 level showed excellent sensitivity and specificity for diagnosing CDR 1 dementia. At the optimal cutoff value, the sensitivity was 90.9% and the specificity was 93.3%. In ROC analysis, the AUC value was 0.976, which is much higher than those of previous biomarkers of AD. When the subjects were restricted to non-depressive cognitive impairment patients, the AUC value was 0.944, which suggests that the level of this miRNA can effectively indicate the progression of MCI. Many previous ROC analyses using single or combined biomarkers have yielded AUC values that were lower than our results (CSF Aβ42: 0.810–0.928, CSF t-tau: 0.80–0.911, CSF p-tau: 0.753–0.880, CSF t-tau/Aβ42: 0.85–0.917, p-tau/Aβ42: 0.84, FDG-PET: 0.88, multiple biomarkers: –0.942)23,43,44,45. Achievement of an excellent AUC value using a single biomarker is an important finding.
Third, the olfactory mucosal miR-206 level can be measured to differentiate AD from cognitive impairment related to depression. In the patients without significant depression, the level of this miRNA was strongly correlated with the ADAS-Cog-K and MMSE-K scores. However, the patients with moderate to severe depression did not exhibit any meaningful alterations in the olfactory mucosal miR-206 level, regardless of their clinical cognitive impairments. Neuropsychiatric symptoms are common in dementia46, and cognitive function can be impaired in severely depressed patients (termed pseudodementia)47. Differentiating between these two conditions will be very helpful in clinical practice because it will prevent patients with depression from being prescribed dementia drugs.
Fourth, the olfactory mucosal miR-206 level can be assessed easily without complications and at a low cost. No serious side effects occurred as a result of the 41 OE biopsies performed in our study, and <10% of the participants complained of minor side effects. One commonly used imaging biomarker for AD is the Pittsburgh compound B-PET or FDG-PET, which is quite costly. OE biopsy and real-time PCR can be performed at a much lower cost than PET. Excluding exposure to radioactive isotopes, PET studies require many prior steps before imaging is conducted, which can result in a total study time of several hours48. Conversely, olfactory biopsy takes less than 30 minutes and can be performed in an outpatient department by an otolaryngologist alone.
Lastly, measurement of the olfactory mucosal miR-206 level may aid in the selection of proper candidates for precision medicine in AD treatment. Currently, many anticancer treatments are tailored according to the specific mutation carried by the patient49,50. Precision medicine has recently been attracting increasing attention for its potential to overcome the biological complexity of neurodegenerative diseases, such as AD and Parkinson’s disease51,52. miRNA-based therapeutics are an excellent example of individualized interventions. If we can identify an overexpressed miRNA that plays a central role in the pathogenesis of a disease, then we can administer an antagomir, which is a complementary sequence to a certain miRNA, to neutralize the effect of the elevated miRNA level53. Miravirsen (Santaris Pharma), a drug used in hepatitis C treatment, is the most advanced miRNA-based drug. This agent inhibits miR-122 in the liver, and its evaluation in a phase 2 trial has been successfully completed54. We have previously demonstrated the therapeutic effect of antagomir-206 in an AD mouse model17. As we can now identify patients with an elevated miR-206 level in the brain by OE biopsy, we can consider antagomir-206 treatment for these patients. The therapeutic potential of antagomir-206 in ‘miR-206-elevated’ dementia patients is very promising, and this tailored approach would represent a breakthrough in AD drug development.
To avoid misinterpretation of the olfactory mucosal miR-206 level, it must be kept in mind that several other conditions are known to be associated with a dysregulated miR-206 level in the brain. Usually, miR-206 appears to be present at an undetectable or very low level in normal brains, except in the cerebellum55. However, upregulation of miR-206 has been reported in animal models of cerebral ischemia56 and alcohol dependence34. Patients with amyotrophic lateral sclerosis have elevated miR-206 expression in the frontal cortex57. Therefore, when these conditions are combined with cognitive impairment, it may affect the diagnostic efficacy of olfactory mucosal miR-206, which should be interpreted with caution.
It needs to be investigated in the future whether other miRNAs, which have been reported to be upregulated in the brains of AD patients (i.e. miR-146a, miR-125b, and miR-9)58,59, are also elevated in the OE of AD patients. However, considering that OE is a known source of BDNF production60 and the BDNF expression in OE is dynamically regulated61, we assume that miR-206 plays the essential role among the miRNAs and therefore it would be an excellent biomarker of early AD.
Although the current study includes a relatively small number of patients and the classifications of MCI and AD were purely based on the use of clinical scales and not current biomarkers (i.e., PET imaging, atrophy on MRI, or CSF markers), we suggest that the olfactory mucosal miR-206 level is a promising biomarker of early AD. It carries greater sensitivity and specificity than previous AD biomarkers and can detect patients in the MCI stage. Moreover, it enables clinicians to differentiate cognitive decline caused by severe depression from early AD. Large-scale studies are required to validate the efficacy and safety of the procedure. These studies will validate the cutoff values for MCI and early AD. Tailored drug trials targeting ‘miR-206-elevated’ dementia patients are promising and should be conducted in the near future.
Methods
Patient recruitment and clinical evaluation
The institutional review board of Seoul National University Hospital approved the study protocol, and informed consent was obtained from all participants. The study was carried out in accordance with the approved guidelines. Subjects over 50 years of age were included in the study. Patients with cognitive impairment were recruited from the neurology outpatient clinic of Seoul National University Hospital, and participants with normal cognitive function were recruited by advertisement. To assess cognitive function, each patient was assessed using the CDR scale, MMSE-K, and ADAS-cog-K. BDI-II was also used to examine each participant to identify the cognitive impairment caused by depression. All clinical assessments were performed by an experienced researcher (J.Y. Shim) on the same day as intranasal biopsy. Patients with moderate to severe depression (BDI-II ≥20) were assigned to the depression group regardless of their cognitive function scores. Patients who met the criteria for probable Alzheimer’s disease of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association4 were assigned to the dementia group (CDR 1), and those with cognitive impairment without a clinical diagnosis of dementia were assigned to the MCI group (CDR 0.5). People with normal cognitive function (CDR 0) were placed in the control group. The baseline olfactory function was assessed using the KVSS.
Intranasal biopsy of the olfactory epithelium
Intranasal biopsy was performed at Seoul National University Hospital Otolaryngology clinic as previously described, with some modifications35,39. A cotton pledget soaked in a mixture of 2% lidocaine and 0.1% epinephrine was inserted into the olfactory cleft for vasoconstriction and local anesthesia. The biopsy procedure was performed by an experienced otolaryngologist (I.G. Kong) under endoscopic guidance. Tissue samples were obtained from the anterior nasal septum, from a location as high as possible, just medial to the insertion of the middle turbinate. Using a small curette or small nasal biting forceps, two or three 2-mm tissue blocks were obtained from one nostril. Nasal packing was performed for 10 minutes after biopsy to control bleeding. The subjects were observed in the clinic for 15 to 30 minutes and then discharged. Tissue donors were informed not to blow their nose and to avoid heavy straining for 24 h. All but 3 patients underwent KVSS before biopsy to evaluate baseline olfactory function.
Quantitative real-time PCR of miR-206
Total RNA was isolated from the individual nasal mucosal tissue samples with Trizol (Invitrogen, USA), and miRNA was isolated using an Ambion mirVana Isolation Kit. Real-time PCR was performed using a mirVana qRT-PCR miRNA Detection Kit with primer sets for miR-206 and U6 snRNA (Applied Biosystems, USA). All real-time PCR reactions were conducted in triplicate with an ABI PRISM 7000 sequence detection system (Applied Biosystems, USA), and 40 cycles of amplification were performed. Relative expression was calculated using the comparative threshold cycle method, and the miRNA levels were normalized to the U6 levels.
Statistical analysis
Comparisons between groups were performed using Student’s t-test or the Kruskal-Wallis test. Correlations between variables were calculated using the Spearman rank correlation test, and linear logistic regression analysis was performed. ROC curves were generated to determine the best cutoff value of the relative miR-206 level to detect patients with CDR 0.5 or CDR 1 dementia. The optimal cutoff value was defined as the highest sum of the sensitivity and specificity, and the AUC was analyzed. The 95% CIs for sensitivity and specificity were derived from the Wilson score method62. All statistical analyses were performed using SPSS 21.0 for Windows (SPSS, Inc., USA), and the results were considered significant at a P < 0.05.
Additional Information
How to cite this article: Moon, J. et al. Early diagnosis of Alzheimer’s disease from elevated olfactory mucosal miR-206 level. Sci. Rep. 6, 20364; doi: 10.1038/srep20364 (2016).
Supplementary Material
Footnotes
Author Contributions J. M., S.-T.L. and I.G.K. wrote the main manuscript. J.M., S.-T.L., D.-Y.K., M.K. and K.C. designed the study. I.G.K., J.-I.B., J.-S.S., J.-Y.S. and J.-H.P. acquired the data. J.M., S.-T.L. and K.-H.J. prepared the figures and analyzed the data. J.-W.S., D.J., K.-Y.J. and S.K.L. made critical revisions of the manuscript for important intellectual content. M.K. and K.C. supervised the whole study process. All authors reviewed the manuscript.
References
- Querfurth H. W. & LaFerla F. M. Alzheimer’s disease. New England Journal of Medicine 362, 329–344, doi: 10.1056/NEJMra0909142 (2010). [DOI] [PubMed] [Google Scholar]
- Mangialasche F., Solomon A., Winblad B., Mecocci P. & Kivipelto M. Alzheimer’s disease: clinical trials and drug development. The Lancet Neurology 9, 702–716 (2010). [DOI] [PubMed] [Google Scholar]
- Lindner M. D., McArthur R. A., Deadwyler S. A., Hampson R. E. & Tariot P. N. Development, Optimization and Use of Preclinical Behavioral Models to Maximize the Productivity of Drug Discovery for Alzheimer’s. Animal and Translational Models for CNS Drug Discovery: Neurological Disorders: Neurological Disorders 2, 93 (2008). [Google Scholar]
- McKhann G. et al. Clinical diagnosis of Alzheimer’s disease Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–939 (1984). [DOI] [PubMed] [Google Scholar]
- Mattsson N. et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393 (2009). [DOI] [PubMed] [Google Scholar]
- Song J. H., Yu J. T. & Tan L. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease: Risk, Mechanisms, and Therapy. Mol Neurobiol, doi: 10.1007/s12035-014-8958-4 (2014). [DOI] [PubMed] [Google Scholar]
- Benarroch E. E. Brain-derived neurotrophic factor Regulation, effects, and potential clinical relevance. Neurology 84, 1693–1704 (2015). [DOI] [PubMed] [Google Scholar]
- Lu B., Nagappan G., Guan X., Nathan P. J. & Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nature Reviews Neuroscience 14, 401–416 (2013). [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Alzheimer’s disease is a synaptic failure. Science 298, 789–791 (2002). [DOI] [PubMed] [Google Scholar]
- Erickson K. I. et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. The Journal of Neuroscience 30, 5368–5375 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laske C. et al. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. Journal of neural transmission 113, 1217–1224 (2006). [DOI] [PubMed] [Google Scholar]
- Forlenza O. V. et al. Effect of brain-derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment. The World Journal of Biological Psychiatry 11, 774–780 (2010). [DOI] [PubMed] [Google Scholar]
- Weinstein G. et al. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol 71, 55–61, doi: 10.1001/jamaneurol.2013.4781 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim V. N., Han J. & Siomi M. C. Biogenesis of small RNAs in animals. Nature reviews Molecular cell biology 10, 126–139 (2009). [DOI] [PubMed] [Google Scholar]
- McCarthy J. J. MicroRNA-206: the skeletal muscle-specific myomiR. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms 1779, 682–691 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchelson K. R. & Qin W. Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World journal of biological chemistry 6, 162–208, doi: 10.4331/wjbc.v6.i3.162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. T. et al. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann Neurol 72, 269–277, doi: 10.1002/ana.23588 (2012). [DOI] [PubMed] [Google Scholar]
- Orru C. D. et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med 371, 519–529, doi: 10.1056/NEJMoa1315200 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S. E. et al. Olfactory epithelium amyloid-β and paired helical filament-tau pathology in Alzheimer disease. Annals of neurology 67, 462–469 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt M. et al. Biopsies of olfactory epithelium in patients with Parkinson’s disease. Movement Disorders 24, 906–914 (2009). [DOI] [PubMed] [Google Scholar]
- Jack C. R. Jr et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology 9, 119–128 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K., Hampel H., Weiner M. & Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature Reviews Neurology 6, 131–144 (2010). [DOI] [PubMed] [Google Scholar]
- Shaw L. M. et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65, 403–413, doi: 10.1002/ana.21610 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan A. M. et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Annals of neurology 59, 512–519 (2006). [DOI] [PubMed] [Google Scholar]
- Fagan A. M. et al. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64, 343–349, doi: 10.1001/archneur.64.3.noc60123 (2007). [DOI] [PubMed] [Google Scholar]
- Tapiola T. et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66, 382–389 (2009). [DOI] [PubMed] [Google Scholar]
- Herholz K. et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 17, 302–316 (2002). [DOI] [PubMed] [Google Scholar]
- Hansson O. et al. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. The Lancet Neurology 5, 228–234, doi: 10.1016/s1474-4422(06)70355-6 (2006). [DOI] [PubMed] [Google Scholar]
- Miura P., Amirouche A., Clow C., Belanger G. & Jasmin B. J. Brain-derived neurotrophic factor expression is repressed during myogenic differentiation by miR-206. J Neurochem 120, 230–238, doi: 10.1111/j.1471-4159.2011.07583.x (2012). [DOI] [PubMed] [Google Scholar]
- Radzikinas K. et al. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci 31, 15407–15415, doi: 10.1523/jneurosci.2745-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara A. H. et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nature medicine 15, 331–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M. et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proceedings of the National Academy of Sciences 106, 13594–13599 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N., Cao Z. & Zhang Y. MiR-206 decreases brain-derived neurotrophic factor levels in a transgenic mouse model of Alzheimer’s disease. Neurosci Bull 30, 191–197, doi: 10.1007/s12264-013-1419-7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik J. D. et al. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci 34, 4581–4588, doi: 10.1523/jneurosci.0445-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor E. et al. MicroRNA-382 expression is elevated in the olfactory neuroepithelium of schizophrenia patients. Neurobiology of disease 55, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella N. G., Takaki M., Lin S. & Sawa A. Neurodevelopmental involvement in schizophrenia: the olfactory epithelium as an alternative model for research. Journal of neurochemistry 102, 587–594 (2007). [DOI] [PubMed] [Google Scholar]
- Arnold S. E., Smutzer G. S., Trojanowski J. Q. & Moberg P. J. Cellular and Molecular Neuropathology of the Olfactory Epithelium and Central Olfactory Pathways in Alzheimer’s Disease and Schizophreniaa. Annals of the New York Academy of Sciences 855, 762–775 (1998). [DOI] [PubMed] [Google Scholar]
- Attems J. & Jellinger K. Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clinical neuropathology 25, 265–271 (2005). [PubMed] [Google Scholar]
- Jafek B., Murrow B., Michaels R., Restrepo D. & Linschoten M. Biopsies of human olfactory epithelium. Chemical senses 27, 623–628 (2002). [DOI] [PubMed] [Google Scholar]
- Horiuchi Y. et al. Olfactory cells via nasal biopsy reflect the developing brain in gene expression profiles: Utility and limitation of the surrogate tissues in research for brain disorders. Neuroscience research 77, 247–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R. et al. Human nasal olfactory epithelium as a dynamic marker for CNS therapy development. Experimental neurology 232, 203–211 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelamanchili S. V. & Fox H. S. Defining larger roles for “tiny” RNA molecules: role of miRNAs in neurodegeneration research. Journal of Neuroimmune Pharmacology 5, 63–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C. et al. Amyloid-β (1–42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clinical chemistry 56, 248–253 (2010). [DOI] [PubMed] [Google Scholar]
- Landau S. et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75, 230–238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe C. et al. Improving CSF biomarker accuracy in predicting prevalent and incident Alzheimer disease. Neurology 76, 501–510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. L., Miller B., Hill M. A. & Neshkes R. Neuropsychiatric aspects of multi-infarct dementia and dementia of the Alzheimer type. Arch Neurol 44, 389–393 (1987). [DOI] [PubMed] [Google Scholar]
- Caine E. D. Pseudodementia: current concepts and future directions. Archives of General Psychiatry 38, 1359–1364 (1981). [DOI] [PubMed] [Google Scholar]
- Edison P. et al. Amyloid, hypometabolism, and cognition in Alzheimer disease An [11C] PIB and [18F] FDG PET study. Neurology 68, 501–508 (2007). [DOI] [PubMed] [Google Scholar]
- Rosell R. et al. Customized treatment in non-small-cell lung cancer based on EGFR mutations and BRCA1 mRNA expression. PloS one 4, e5133 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone P. et al. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. British journal of cancer 88, 1285–1291 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine T. J. & Montine K. S. Precision medicine: Clarity for the clinical and biological complexity of Alzheimer’s and Parkinson’s diseases. The Journal of experimental medicine 212, 601–605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S. & Varmus H. A new initiative on precision medicine. New England Journal of Medicine 372, 793–795 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krützfeldt J. et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438, 685–689 (2005). [DOI] [PubMed] [Google Scholar]
- Janssen H. L. et al. Treatment of HCV infection by targeting microRNA. New England Journal of Medicine 368, 1685–1694 (2013). [DOI] [PubMed] [Google Scholar]
- Olsen L., Klausen M., Helboe L., Nielsen F. C. & Werge T. MicroRNAs show mutually exclusive expression patterns in the brain of adult male rats. PL o S One 4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaseelan K., Lim K. Y. & Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke (2008). [DOI] [PubMed] [Google Scholar]
- Shioya M. et al. Aberrant microRNA expression in the brains of neurodegenerative diseases: miR‐29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathology and applied neurobiology 36, 320–330 (2010). [DOI] [PubMed] [Google Scholar]
- Sethi P. & Lukiw W. J. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neuroscience letters 459, 100–104 (2009). [DOI] [PubMed] [Google Scholar]
- Müller M., Kuiperij H. B., Claassen J. A., Küsters B. & Verbeek M. M. MicroRNAs in Alzheimer’s disease: differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiology of aging 35, 152–158 (2014). [DOI] [PubMed] [Google Scholar]
- Pastrana E. et al. BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons. Neurochemistry international 50, 491–498 (2007). [DOI] [PubMed] [Google Scholar]
- Uranagase A., Katsunuma S., Doi K. & Nibu K.-I. BDNF expression in olfactory bulb and epithelium during regeneration of olfactory epithelium. Neuroscience letters 516, 45–49 (2012). [DOI] [PubMed] [Google Scholar]
- Newcombe R. G. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in medicine 17, 857–872 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.