Abstract
Research on the health effects of fine particulate matter (PM2.5) frequently disregards the differences in particle composition between that measured on an ambient filter versus that measured in the corresponding extraction solution used for toxicological testing. This study presents a novel method for characterizing the differences, in metallic and organic species, between the ambient samples and the corresponding extracted solutions through characterization of extracted PM2.5 suspended on filters. Removal efficiency was found to be 98.0 ± 1.4% when measured using pre- and post-removal filter weights, however, this efficiency was significantly reduced to 80.2 ± 0.8% when measured based on particle mass in the extraction solution. Furthermore, only 47.2 ± 22.3% of metals and 24.8 ± 14.5% of organics measured on the ambient filter were found in the extraction solution. Individual metallic and organic components were extracted with varying efficiency, with many organics being lost entirely during extraction. Finally, extraction efficiencies of specific PM2.5 components were inversely correlated with total mass. This study details a method to assess compositional alterations resulting from extraction of PM2.5 from filters, emphasizing the need for standardized procedures that maintain compositional integrity of ambient samples for use in toxicology studies of PM2.5.
Keywords: PM2.5 filter extractions, PM2.5 filter sampling, PM2.5 toxicity
Introduction
Ambient fine particulate matter (PM2.5) has long been associated with respiratory and cardiovascular morbidity and mortality (Dockery et al., 1993; Franklin et al., 2007; Pope et al., 1995). Recently, PM2.5 and related health effects have been shown to vary across the USA (Bell et al., 2009; Sampson et al., 2013), highlighting the importance of researching the impact of compositional differences in ambient PM2.5. It is particularly important to understand differences in components that are relevant to human health, such as metallic and organic species (Melaku et al., 2008; Ravindra et al., 2001; Schaumann et al., 2004). As epidemiological evidence of PM2.5-associated health effects continues to be strengthened, toxicology studies to understand the mechanisms behind these outcomes and impacts of compositionally differing PM2.5 have advanced.
Toxicology studies allow for research into PM2.5 health effects while avoiding confounders present in many epidemiological studies, such as lifestyle and occupational factors (Jerrett et al., 2005). In order to capture the compositional complexity of PM2.5, ambient samples must be used; however, this requires collection of samples predominantly through concentrator systems or filter-based methods (Ghio & Huang, 2004). Concentrated ambient particles (CAPs) provide PM2.5 samples that maintain ratios of ambient mixtures while increasing the mass to allow for both in vitro and in vivo studies (Ghio & Huang, 2004). While CAPs provide a number of benefits to research, they require an expensive concentrator system that is fixed at a single sampling location, lacking the potential to study spatial differences in ambient PM2.5 concentration and composition (Matte et al., 2013).
Filter sampling allows for collection of ambient PM2.5 that may vary by location and source while utilizing a relatively low-cost method conducive to a variety of air sampling equipment systems (Kundu & Stone, 2014). An important consideration is the translation of PM2.5 on the filter, to a liquid suspension of PM2.5 that can be used in toxicology experiments. This process is integral to maximizing extraction efficiency while maintaining compositional integrity, so that the final extraction solution yields sufficient PM2.5 mass that remains representative of ambient PM2.5.
Preparation of PM2.5 is typically a multistep process involving removal from the filter into solution, recovery of dry PM2.5, and re-suspension into media appropriate for the toxicology application. A variety of extraction techniques have been implemented in toxicology research, which differ by the type of filter used for ambient collection, removal procedure and solvent, concentration method, and the media used for re-suspension. Table 1 summarizes a literature review of preparation procedures and emphasizes the variability of extraction methods that have been used.
Table 1.
Methods previously implemented for extraction of ambient PM2.5 for use in toxicology studies.
Removal method, solvent type, concentration, and authors are listed for toxicological assessments of ambient PM2.5 using filter extraction.
Variation in extraction techniques between research groups creates a potential for bias, where findings may be dependent on the extraction procedures used rather than on the characteristics of ambient material (Bein & Wexler, 2014). Well-characterized extraction solutions would avoid these biases as the exact concentration and composition of PM2.5 used would be known, enabling a more accurate interpretation of exposure studies. Thus far, a limited number of toxicology studies using ambient PM2.5 have reported chemical characterization of both metals and organics in extraction solutions (Huang et al., 2014; Lauer et al., 2009; Verma et al., 2012). Here a novel method was developed to measure compositional differences in PM2.5 between collected ambient material and the corresponding extraction solutions.
Materials and methods
PM2.5 collection
Sampling locations
In winter 2014, PM2.5 samples were collected in Pittsburgh, PA at five sampling locations throughout the downtown area including a regional background location in a park 14 km upwind of the downtown area.
Sampling methods
Portable ambient air samplers were deployed approximately 3m above ground level on metal utility poles and ran for 7 consecutive days at each sampling location. Samplers were enclosed in waterproof cases and equipped with 2.5 μm size-selective Harvard impactors (HIs) with 37mm Teflon™ (polytetrafluorethylene [PTFE]) filters (Pall Corporation, Ann Arbor, MI) or cyclone adapted HIs (Air Diagnostics and Engineering Inc., Harrison, ME) with 37mm quartz filters (Pall Corporation, Ann Arbor, MI). Vacuum pumps (model PCXR4, SKC Inc., Eighty Four, PA) were calibrated to 4 liters per minute air flow rate (Matte et al., 2013). Quartz filters were pre-baked at 900 ºC for 4 h to remove trace organic material.
Four samplers were co-located at each sampling location to provide equivalent samples for ambient characterization as well as for extraction. For the quantification and characterization of ambient material, two samplers per location collected PM2.5 on either a PTFE or a quartz filter. For extraction of ambient material into solution, two samplers per location collected PM2.5 on PTFE filters.
Ambient PM2.5 characterization
PTFE filters were used to determine PM2.5 concentrations through gravimetric analysis of filters pre- and post-sampling. Total PM2.5 mass was measured on an ultra-microbalance (model XP2U, Mettler Toledo, Columbus, OH) following a 48 h equilibration in a temperature and humidity controlled chamber (20.0 ºC and 35% humidity).
Ambient compositional analysis by X-ray fluorescence (XRF) of metals and by thermal desorption gas chromatography mass spectrometry (TD–GC–MS) of organics was performed on PTFE and quartz filters, respectively, at Desert Research Institutes, DRI (Reno, NV). Metals (n=51) and organics (n=34) analyzed are shown in Table 2. Compounds analyzed included 14 of the 16 EPA Priority polycyclic aromatic hydrocarbons (PAHs).
Table 2.
Metals and organics analyzed.
| Metals | Organics | ||||
|---|---|---|---|---|---|
| Ag | Cu | Na | Sn | 1-Methyl phenanthrene | Dibenzo[a,h]anthracene |
| Al | Eu | Nb | Sr | 2-Methyl phenanthrene | Dibenzothiophene |
| As | Fe | Ni | Ta | 9-Fluorenone | Fluoranthene |
| Au | Ga | P | Tb | Acenapthene | Fluorene |
| Ba | Hf | Pb | Ti | Acenaphthylene | Hopanes (n=10) |
| Br | Hg | Pd | Tl | Benzo[a]anthracene | Indeno[1,2,3-cd]pyrene |
| Ca | In | Rb | U | Benzo[a]pyrene | Phenanthrene |
| Cd | Ir | S | V | Benzo[b]fluoranthene | Pyrene |
| Ce | K | Sb | W | Benzo[e]pyrene | Steranes (n=4) |
| Cl | La | Sc | Y | Benzo(ghi)fluoranthene | |
| Co | Mg | Se | Zn | Benzo[ghi]perylene | |
| Cr | Mn | Si | Zr | Benzo(jk)fluoranthene | |
| Cs | Mo | Sm | Chrysene | ||
Metals (n=51) analyzed by X-ray fluorescence and organics (n=34) analyzed by thermal desorption gas chromatography mass spectrometry. All compounds were measured in both ambient and extraction solution samples.
PM2.5 extraction
Removal
Following sampling, PTFE filters collected for extraction (n=2/sampling location) underwent gravimetric analysis, described above, to determine the total PM2.5 mass collected. Filters were then placed particle side down in 100mL glass beakers containing a 9:1 solvent (methanol: sterile Milli-Q water) and sonicated for 2 min at 40 kHz in a water-bath sonicator (Branson Ultrasonics, Danbury, CT). Beakers were sufficiently wide to allow filters to lie flat, avoiding the need to cut filters into pieces. Cutting can intensify release of filter material during sonication, which creates difficulties in post-weighing of filters to determine removal mass. The extracts of the two filters collected from each location were pooled together (Baulig et al., 2004).
After sonication, filters and the beaker were rinsed with methanol to remove any residual particles and all rinses containing PM2.5 were stored in a closed 50mL conical tube at −20 ºC until concentration. PTFE filters were left to dry and equilibrate prior to gravimetric analysis for determination of the PM2.5 mass removed from each filter. Blank PTFE filters were prepared in the same manner as exposed filters to control for any loss of material throughout the removal process.
Concentration
PM2.5 suspended in the methanol solution were centrifuged (8000g, 15 min) prior to being frozen in liquid nitrogen and concentrated through lyophilization in a 4.5 L bench top freeze dryer (Labconco, Kansas City, MO). Dry concentrated PM2.5 samples were stored away from any light sources at −20 ºC until further analysis.
Re-suspension
Concentrated dry PM2.5 samples were re-suspended in a set volume of serum-free Dulbecco’s modified Eagle’s medium (DMEM) for future in vitro research. Samples were vigorously pipetted and vortexed to distribute PM2.5 throughout the media, then immediately prepared for PM2.5 characterization. Samples of PM2.5 that were removed from the filter, concentrated, and re-suspended in media (hereafter referred to as extracted samples) are the most accurate form of PM2.5 for characterization of samples used in toxicology research.
Extracted PM2.5 characterization
Aliquots of extracted PM2.5 samples in DMEM were suspended onto pre-weighed PTFE and quartz filters to allow for gravimetric and chemical analyses comparable to those performed for ambient samples. Due to the hydrophobic nature of PTFE filters, samples were mixed with methanol and then applied to the filters. PM2.5 in solution was left to dry on the filters and then filters were equilibrated for gravimetric analysis. PM2.5 mass was determined for extracted samples prior to characterization through XRF and TD–GC–MS analysis (Table 2). Expected masses of all constituents in extracted samples were calculated using the PM2.5 mass applied to the filter and ambient composition data. Filters suspended with DMEM-only were weighed and analyzed to allow for blank adjustment of samples due to mass and compositional components present in DMEM.
Statistical analysis
Statistical analysis for all data was performed with StataSE 13 (StataCorp, LP, College Station, TX) and Prism 6.0 (GraphPad Software, Inc., San Diego, CA). All data were reported as a mean±standard deviation (SD). Pearson correlation coefficients were determined between PM2.5 mass and specific constituents. Data were analyzed using a one-way analysis of variance (ANOVA) with Bonferroni’s test for multiple post-hoc comparisons where appropriate. Where ANOVA indicated significant differences and in all two-group comparisons, differences were investigated using Student’s t-test. Differences with p values <0.05 were considered significant.
Results
Sampling location differences
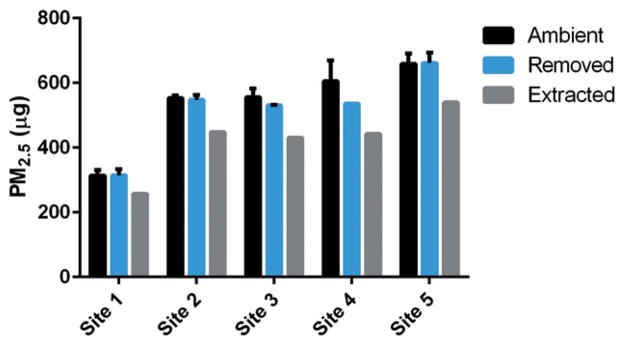
Masses for PM2.5, metals, and organics were determined for each of the five locations. PM2.5 mass was determined at three stages: (1) ambient material collected over the sampling period (“PMamb”); (2) recovered material measured based on pre- and post-removal filter weights (“PMrem”); and (3) recovered material concentrated and re-suspended into DMEM (“PMext”). Metals and organics masses were determined at stage 1 (“metalsamb, organicsamb”) and 3 (“metalsext, organicsext”). Ambient mass collected varied between sampling sites, but trends between PMamb, PMrem, and PMext were similar across locations (Figure 1).
Figure 1.
PM2.5 mass across sampling sites. Ambient mass, mass following removal from filter via sonication (“removed”), and mass following re-suspension in cell culture media (“extracted”) are displayed for each sampling location in micrograms (n=2/site for ambient and removed samples – except for site 4 (n=1) due to equipment failure during collection and n=1/site for extracted samples). Sampling sites are ordered from lowest to highest (1–5) ambient PM2.5 mass. Data are expressed as means±SD.
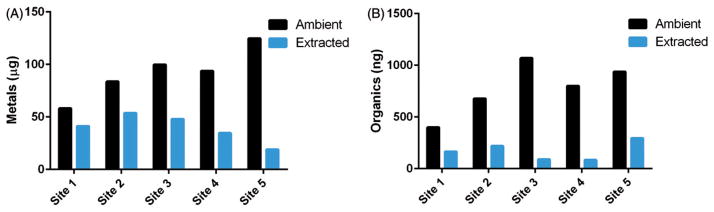
Metalsamb and metalsext (Figure 2A) were calculated by summing the masses of all species analyzed. Metalsamb did not correspond to PMamb, yet the highest PMamb sampling location also had the highest metalsamb. Metalsamb and metalsext also did not trend together indicating that extraction differences between sampling sites impacted metalsext. Interestingly, the lowest metalsext was observed at the location with the highest PMamb and metalsamb.
Figure 2.
Ambient and extracted masses of PM2.5 components. (A) Total mass of metals (μg) in ambient samples and corresponding extraction solutions at each sampling site. (B) Total mass of organics (ng) in ambient samples and corresponding extraction solutions at each sampling site. Site numbering is representative of total ambient PM2.5 mass ordering of low to high (1–5). Constituents comprising total metals and organics are shown in Table 2.
Organicsamb and organicsext were quantified by summing all species analyzed and variability was observed between all locations (Figure 2B). Similarly to ambient metals, organicsamb did not trend with PMamb. However, unlike metals, the location with the highest organicsamb was not from the sampling location with the highest PMamb. All organicsext were less than organicsamb and varied between sampling locations independent of PMamb. As with metalsext, the effect of extraction differences between sampling locations was observed in organicsext.
Extraction efficiency
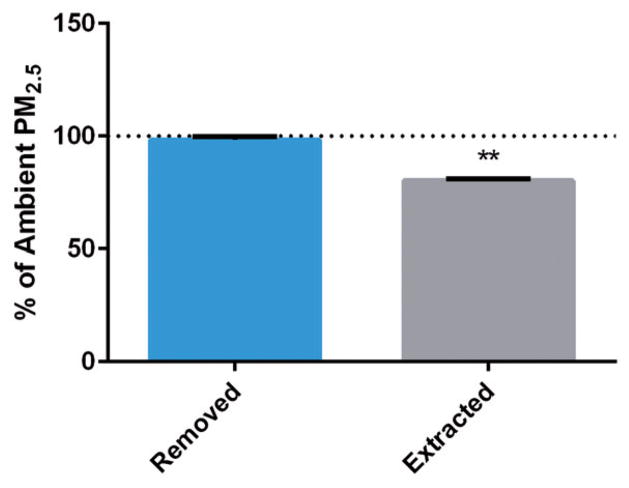
Ambient masses of PM2.5, metals, and organics were compared to extracted masses to determine the percent extracted. Removal of total PM2.5 was 98.0±1.4% following sonication; however, extraction efficiencies following concentration and re-suspension in DMEM were found to be substantially lower at 80.2±0.8% of PMamb (Figure 3).
Figure 3.
PM2.5 mass following removal and extraction. Mass following removal of PM2.5 from filter via sonication and following complete extraction (concentration and re-suspension in cell media) are displayed relative to total ambient PM2.5 mass for all sampling locations (n=5). Data are expressed as means±SD; **p value <0.001 indicating a statistically significant difference between groups.
Overall extraction efficiency for metals (Table 3) was 47.2±22.3%, with extraction efficiencies for specific metals ranging from 0.7 (for Ce) to 73.4% (for Na). High variability in extraction efficiency was also observed for specific metals between sampling locations (i.e., SD±35.8% for Ni). All averaged extraction efficiencies were less than 100% removal except for three trace metals that were present in DMEM (Ca, Mg, and P); these components were excluded from calculations of total metals. Contributions to total ambient metals for Ca, Mg, and P were 2.2, 1.1, and 0.0%, respectively.
Table 3.
Extraction efficiencies.
| Component | Percent extracted | SD |
|---|---|---|
| PM2.5 | ||
| Removed | 98.0 | 1.4 |
| Extracted | 80.2 | 0.8 |
| Metals | ||
| Al | 40.0 | 54.8 |
| Ca | 570.4 | 583.3 |
| Cd | 9.0 | 20.0 |
| Ce | 27.0 | 60.3 |
| Cl | 7.9 | 17.8 |
| Cr | 4.8 | 10.7 |
| Cs | 10.4 | 23.3 |
| Cu | 21.4 | 7.7 |
| Fe | 28.8 | 9.5 |
| Mg | 227.8 | 338.5 |
| Mn | 20.7 | 8.7 |
| Mo | 15.1 | 12.1 |
| Na | 73.4 | 49.3 |
| Ni | 26.9 | 35.8 |
| P | 17 128.8 | 8604.4 |
| Pb | 16.8 | 11.5 |
| S | 0.7 | 1.6 |
| Sn | 20.3 | 45.3 |
| Sr | 3.6 | 5.1 |
| Zn | 6.2 | 11.7 |
| Total | 47.2 | 22.3 |
| Organics | ||
| 1MP | 101.5 | 23.1 |
| Acy | 31.7 | 20.9 |
| BbFl | 20.7 | 19.3 |
| BghiPer | 98.9 | 43.9 |
| Ipyr | 17.0 | 38.0 |
| Total | 24.8 | 14.5 |
Percent extracted with SDs for total PM2.5 mass following removal and extraction as well as extracted PM2.5 components (metals and organics).
Extraction efficiencies for organics (Table 3) are displayed for the five compounds detected in both ambient and extracted samples: 1-methyl phenanthrene (1MP), acenaphthylene (Acy), benzo[b]fluoranthene (BbFl), benzo[ghi]perylene (BghiPer), and indeno[1,2,3-cd]pyrene (Ipyr). Variability of efficiency was observed between species (Ipyr to 1MP: 17.0–101.5%) as well as between sampling locations for individual species (i.e., BghiPer SD±43.9%). Extraction efficiency for total organics measured was 24.8±14.5%. All hopanes (n=10), steranes (n=4), and other organic compounds (n=15) measured were found at varying concentrations in ambient samples but were below the limit of detection in all extraction solutions, suggesting near complete loss during the extraction process.
Expected masses for constituents were calculated for extraction samples based upon PM2.5 mass and ambient composition. The expected values were compared to actual masses recorded through analysis of extraction solution (Tables 4 and 5).
Table 4.
Expected versus actual metals of extraction solution.
| Expected (μg) | Actual (μg) | |
|---|---|---|
| Al | 1.3965 | 0.3545 |
| Ca | 1.5980 | 8.7968 |
| Cd | 0.0074 | 0.0005 |
| Ce | 0.1141 | 0.0379 |
| Cl | 15.5670 | 1.3850 |
| Cr | 0.0499 | 0.0034 |
| Cs | 0.0054 | 0.0028 |
| Cu | 0.1621 | 0.0410 |
| Fe | 5.7970 | 2.0322 |
| Mg | 0.7899 | 1.1810 |
| Mn | 0.4285 | 0.1080 |
| Mo | 0.0766 | 0.0145 |
| Na | 21.7918 | 19.2882 |
| Ni | 0.0172 | 0.0059 |
| P | 0.0000 | 1.7684 |
| Pb | 0.1644 | 0.0340 |
| S | 20.3513 | 0.1847 |
| Sn | 0.0065 | 0.0048 |
| Sr | 0.0223 | 0.0008 |
| Y | 0.0066 | 0.0001 |
| Zn | 1.0115 | 0.0930 |
| Total | 69.3641 | 35.3377 |
Average expected and actual metals (μg) on filters. Expected metals were calculated using total PM2.5 mass applied to the filter and ambient composition data. Actual values were determined from XRF of extracted solutions.
Table 5.
Expected versus actual organics of extraction solution.
| Expected (ng) | Actual (ng) | |
|---|---|---|
| Acy | 132.9485 | 36.5572 |
| Ace | 0.1473 | 0.0000 |
| F | 0.1520 | 0.0000 |
| P | 2.7877 | 0.0000 |
| Flu | 2.9187 | 0.0000 |
| Pyr | 1.9161 | 0.0000 |
| 9Flo | 0.5447 | 0.0000 |
| DBT | 0.0292 | 0.0000 |
| 1MP | 0.3019 | 0.2998 |
| 2MP | 0.3923 | 0.0000 |
| Chr | 4.6438 | 0.0000 |
| BbFl | 4.0059 | 0.9423 |
| BjkFl | 6.7336 | 0.0000 |
| BaAnt | 2.3504 | 0.0000 |
| BePyr | 2.3839 | 0.0000 |
| BaPyr | 1.6650 | 0.0000 |
| Ipyr | 1.7039 | 0.2270 |
| DBahAnt | 0.2353 | 0.0000 |
| BghiPer | 7.1982 | 6.8868 |
| BghiFl | 0.9941 | 0.0000 |
| Hopanes | 1.9904 | 0.0000 |
| Steranes | 0.1141 | 0.0000 |
| Total | 176.1569 | 44.9131 |
Average expected and actual organics (ng) on filters. Expected organics were calculated using total PM2.5 mass applied to the filter and ambient composition data. Actual values were determined with TD–GC–MS of extracted samples. Organics analyzed were hopanes (n=10), steranes (n=4), and PAHs (n=20) – abbreviated: acenaphthylene (Acy), acenapthene (Ace), fluorene (F), phenanthrene (P), fluoranthene (Flu), pyrene (Pyr), 9-fluorenone (9Flo), dibenzothiophene (DBT), 1-methyl phenanthrene (1MP), 2-methyl phenanthrene (2MP), chrysene (Chr), benzo[b]fluoranthene (BbFl), benzo(jk)fluoranthene (BjkFl), benzo[a]anthracene (BaAnt), benzo[e]pyrene (BePyr), benzo[a]pyrene (BaPyr), indeno[1,2,3-cd]pyrene (Ipyr), dibenzo[a,h]anthracene (DBahAnt), benzo[ghi]perylene (BghiPer), and benzo(ghi)fluoranthene (BghiFl).
Relationship of constituents to total PM2.5
The relative contribution of measured metals and organics, as a percent of PM2.5 mass, was determined for both ambient and extracted samples (Figure 4). In ambient samples, the contribution of metals to PMamb was 120 times higher than the contribution of organics. However, in extracted samples, the contribution of metals increased to over 705 times higher than organics.
Figure 4.
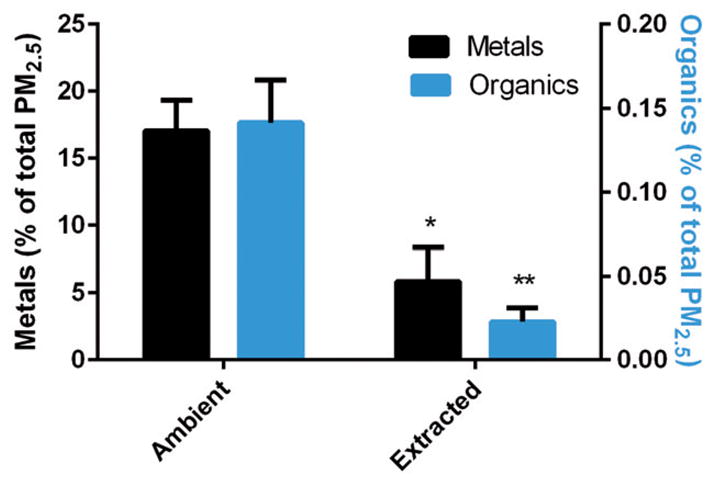
Contribution to total PM2.5 mass. Percent contribution to PM2.5 mass for total metals and organics (sum of constituents listed in Table 2) in ambient samples and extraction solutions. Left y-axis represents percent contribution of metals and the right y-axis indicates organics contribution. Data are expressed as means±SD; *p value <0.05 and **p value<0.001, indicating a statistically significant difference between ambient and extracted samples.
Correlations
Ambient to extracted
Correlations of PM2.5 to metal and organic components were calculated to compare how PM2.5 mass and constituents were related for both ambient and extracted samples (Table 6). Statistically significant positive correlations were observed between PMamb and Fe and Zn, while none of the extracted constituents exhibited a statistically significant correlation with PMext. Marked differences were observed between ambient and extracted correlations of constituents to PM2.5 mass (reported as ambient: extracted) for metals (0.911: −0.219), organics (0.805: −0.083), and a number of specific constituents including Al (0.967: −0.299), Cr (0.725: −0.350), Zn (0.975: −0.166), and Acy (0.734: −0.243).
Table 6.
Correlations of PM2.5.
| Component PM2.5 | Amb to ext 0.975* | PM2.5 mass to constituents
|
||
|---|---|---|---|---|
| Amb | Ext | % Ext −0.273 | ||
| Metals | ||||
| Al | −0.203 | 0.967 | −0.299 | −0.717 |
| Ca | 0.005 | 0.925 | −0.530 | −0.836 |
| Cd | 0.459 | 0.426 | 0.350 | 0.513 |
| Ce | 0.218 | −0.322 | −0.215 | 0.068 |
| Cl | 0.079 | 0.931 | −0.215 | 0.068 |
| Cr | 0.189 | 0.725 | −0.350 | 0.513 |
| Cs | 0.696 | 0.068 | −0.003 | 0.335 |
| Cu | 0.554 | 0.947 | 0.263 | −0.744 |
| Fe | 0.708 | 0.993* | 0.502 | −0.571 |
| Mg | 0.115 | −0.005 | −0.808 | −0.125 |
| Mn | 0.571 | 0.940 | 0.474 | −0.175 |
| Mo | −0.003 | 0.507 | −0.744 | −0.590 |
| Na | −0.639 | 0.707 | 0.051 | 0.033 |
| Ni | 0.045 | 0.951 | 0.048 | 0.184 |
| P | N/A | N/A | −0.098 | −0.928 |
| Pb | 0.504 | 0.736 | 0.213 | −0.643 |
| S | −0.189 | 0.858 | −0.215 | 0.068 |
| Sn | 0.809 | −0.081 | 0.350 | 0.513 |
| Sr | −0.427 | 0.809 | −0.555 | −0.528 |
| Tb | 1.000* | 0.079 | 0.635 | 0.079 |
| Zn | 0.312 | 0.975* | −0.166 | 0.108 |
| Total | −0.610 | 0.911 | −0.219 | −0.815 |
| Organics | ||||
| 1MP | 0.752 | 0.365 | 0.812 | −0.123 |
| Acy | −0.231 | 0.734 | −0.243 | −0.616 |
| BbFl | 0.570 | 0.953 | 0.670 | 0.467 |
| BghiPer | 0.139 | 0.630 | 0.652 | 0.279 |
| Ipyr | 0.078 | 0.579 | 0.635 | 0.079 |
| Total | −0.083 | 0.805 | −0.083 | −0.522 |
Pearson’s correlation coefficients are presented for total PM2.5 and constituents (metals and organics) between ambient (“amb”) and extraction solution (“ext”) samples as well as PM2.5 mass to: ambient values, extraction solution values, and percent extracted.
p Value<0.05, indicating a statistically significant correlation.
To determine how specific components of PM2.5 related between ambient and extracted samples, correlation coefficients were determined for each component (Table 6). Several extracted constituent values were negatively correlated with ambient measurements of the same constituent, including: total metals, total organics, and specific components such as Al, S, and Sr. Positive correlations were observed for a number of components including: Cs, Fe, Mn, Sn, 1MP, and BbFl. Statistically significant positive correlations between ambient and extracted values were present for PM2.5 mass and Tb.
Extraction percent to ambient characteristics
Correlations between calculated extraction percentages of specific components to total PM2.5 were made to investigate potential trends in extraction efficiency based upon PMamb (Table 6). The percent of total mass extracted was significantly positively correlated with PMamb. While both the percent of extracted metals and organics were negatively correlated with PMamb. These correlations suggest that as ambient PM2.5 mass increases, the efficiency of extraction for total metals and organics decreases. Similar trends were seen in individual constituents including: Al, Cu, Fe, Pb, and Acy. However, not all components measured had negative correlations between extraction efficiency and total PM2.5, these included: Cd, Cr, Sn, and BbFl.
Discussion
PM2.5 mass
The percent of mass removed via sonication was consistent across sampling locations, which is a characteristic necessary to avoid a methods bias. Less inter-filter variability was seen in removal efficiency than has previously been reported, where efficiency ranged from 59% to 95% (Imrich et al., 2000). Increased consistency in removal efficiency in this research is likely due to more deliberate selection of solvents based on anticipated chemical characteristics of PM2.5 components, as well as refinement of sonication methods. Extraction protocols using water as the sole solvent are common, and while effective for removal of water-soluble components and approximately 75% of PM2.5 mass, water is not effective for extraction of nonpolar species, including many organics compounds (Hawthorne et al., 2000; Longhin et al., 2013; Watterson et al., 2007). It should be noted that a portion of studies neglect to report removal percentages, limiting inter-method comparisons. Based solely on mass removal from the filter, the methods outlined here maintained a high PM2.5 yield and were consistent between filters and sampling locations.
The significant positive correlation between ambient and extracted PM2.5 mass suggests that ambient mass loadings do not impact the extraction of total PM2.5. Consistent extraction independent of mass makes the outlined methods translatable to many regions and sampling timescales. The methods are also effective in reducing release of filter material into the extracted solutions, as no significant loss of mass was observed with blank sonicated filters. The lack of observed fiber loss is likely due to a combination of the filter type used and decreased time and intensity of sonication. Previous methods have utilized potentially destructive probe sonication or extended sonication times, which can necessitate filtering of samples to remove fibers but also introduces a potential loss of PM2.5 (Godri et al., 2011; Huang et al., 2014; Riva et al., 2011; Van Winkle et al., 2015).
Components of PM2.5
Differences in extraction efficiency between total PM2.5 and constituents of PM2.5 demonstrate a key limitation of filter extraction methods, discussed below. Importantly, a vast majority of the PAHs (15 of the 20 analyzed), hopanes, and steranes were not extracted at any of the locations. While loss in organics was not unexpected due to the volatility of the compounds (EPA, 2014), quantifying the shift from ambient contributions is useful to establish differences from filter samples.
A number of individual components, total organics, and total metals were inversely related to corresponding ambient masses, a result of decreased extraction efficiencies as mass increased. Ideally, ambient and extracted components would be equally correlated to total mass, indicating that composition of extracted PM2.5 was similar to that of ambient PM2.5. However, positive correlations in ambient samples alone suggest that the relative composition of the ambient material is changed during the extraction process. Additionally, as ambient PM2.5 mass increases, the percentage of metals and organics extracted decreases.
Loss between removal and concentration
Translating the removal solution into dry particulate material is an imperative step before re-suspension into cell culture media to create an extraction solution for toxicology experiments. In this study, this process created a significant loss of mass and presumably loss of compounds that were volatile or soluble in the removal solution (9:1 methanol in water). However, characterization was only performed following re-suspension in cell culture media; additional characterizations of the removal and extraction solutions could identify at what point in the process the losses occurred. Better characterization of these losses is essential to accurate research, as extraction percentages are frequently reported as total mass removed from filter, without consideration of losses that occur during the subsequent preparation steps.
Impacts on toxicology applications
Studies using ambient PM2.5 extracted from filters are an integral component for assessing biological impacts of PM2.5 both in vitro and in vivo. In some cases, responses are correlated to ambient concentrations, without regard for changes that occur during the extraction process (de Kok et al., 2005). The shift in relative contributions of specific components to total PM2.5 demonstrates that the resultant extraction solution in this work is not representative of the ambient mixtures. Recently, different extraction methods were found to result in distinct biological impacts (Van Winkle et al., 2015). Identifying the specific components of PM2.5 that are not representatively extracted by protocols can suggest mechanisms responsible for the varying biological responses. In this work we identified the loss of numerous health-relevant compounds including: Cr, Fe, Ni, Pb, Zn and 10 of the 16 EPA Priority PAHs (Chen & Lippmann, 2009). There is a need to further understand what effects these losses have on subsequent toxicology analyses.
Extraction efficiencies of components of PM2.5 were shown in this research to vary between sampling locations. Similar investigations found that extraction efficiencies were influenced by the source mixture and therefore composition of PM2.5 (Bein & Wexler, 2014). These findings are particularly important for studies examining multiple sampling locations or the impacts of mixed sources. With inconsistent extraction efficiency, the variation of ambient samples will be obscured or lost, and toxicology results will not be representative of the actual exposure of interest. An additional concern is the finding that extraction efficiencies of metals and organics are inversely related to total PM2.5 loadings. High mass loadings are necessary in toxicology studies to provide adequate material for exposures, but it is ineffective to collect such loadings when they decrease the yield of metal and organic species.
In this study, extraction was performed on samples with spatially varying ambient PM2.5 concentration and composition. The impacts of temporal and seasonal variation in PM2.5 composition on extraction efficiencies were not investigated here, but future research in these areas would strengthen the correspondence to studies using temporally variant ambient samples. Furthermore, results from this study are only generalizable to the methods utilized, and efficiencies will differ based upon the extraction procedures employed (Bein & Wexler, 2015). In this study, PM2.5 was re-suspended into cell culture media; to accurately determine how composition may be impacted by re-suspension in different toxicology medium (i.e. saline, PBS, or water), further studies should be conducted. However, based on this and previous works, it is clear that complete extraction has not been achieved using any current methodology; therefore, extraction solutions will differ compositionally from ambient source material, and the issues highlighted by this extraction protocol are likewise of concern with other methods (Akhtar et al., 2014; Happo et al., 2010).
Characterization of PM2.5 was performed only on ambient material and final extraction solutions; thus, changes in composition during the intermediate stages are unknown. Identifying specific steps in extraction procedure that is most impactful on the recovery of PM2.5 components would help to establish refined procedures that maintain ambient compositions. While this work has begun to uncover compositional differences, it examined only a subset of the key components of PM2.5 other substantial contributors to mass including inorganic ions and total elemental and organic carbon would further elucidate the compositional changes following extraction.
Conclusions
This research has outlined a method for the extraction of PM2.5 from filter samples, which was effective in high mass recovery while maintaining filter integrity. Comparison of ambient and extracted samples suggests that the method was more effective in recovering metals in the extraction solution compared to organics. To the authors’ knowledge, only one study has performed a well-characterized extraction solution analysis, and this research highlighted the variance in extraction of components of PM2.5 based on the extraction procedures implemented (Bein & Wexler, 2015). However, this current work is the first to compare components measured in PM2.5 filter extract with those measured on collocated ambient filters from multiple sampling locations. The narrow understanding of alteration to PM2.5 composition as a result of extraction is a limitation that persists throughout a vast majority of toxicology research using PM2.5 collected on filters. Further awareness of the underlying mechanisms for the observed compositional shifts, in addition to the adoption of standardized extraction techniques that more efficiently extract all components of PM2.5, would allow for biological impact studies that are more readily translatable to ambient exposures, and would facilitate comparisons between studies.
Acknowledgments
The authors would like to thank Drew Michanowicz and Jessie Carr Shmool for assistance in sampling location selection.
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- Akhtar US, McWhinney RD, Rastogi N, et al. Cytotoxic and proinflammatory effects of ambient and source-related particulate matter (PM) in relation to the production of reactive oxygen species (ROS) and cytokine adsorption by particles. Inhal Toxicol. 2010;22:37–47. doi: 10.3109/08958378.2010.518377. [DOI] [PubMed] [Google Scholar]
- Akhtar US, Ratstogi N, McWhinney RD, et al. The combined effects of physicochemical properties of size-fractionated ambient particulate matter on in vitro toxicity in human A549 lung epithelial cells. Toxicol Rep. 2014;1:145–56. doi: 10.1016/j.toxrep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulig A, Poirault JJ, Ausset P, et al. Physicochemical characteristics and biological activities of seasonal atmospheric particulate matter sampling in two locations of Paris. Environ Sci Technol. 2004;38:5985–92. doi: 10.1021/es049476z. [DOI] [PubMed] [Google Scholar]
- Bein KJ, Wexler AS. A high-efficiency, low-bias method for extracting particulate matter from filter and impactor substrates. Atmos Environ. 2014;90:87–95. [Google Scholar]
- Bein KJ, Wexler AS. Compositional variance in extracted particulate matter using different filter extraction techniques. Atmos Environ. 2015;107:24–34. [Google Scholar]
- Bell M, Ebisu K, Peng R, et al. Hospital admissions and chemical composition of fine particulate matter (PM2.5) for 106 US counties. Epidemiology. 2009;20:S29. [Google Scholar]
- Cavanagh JA, Trought K, Brown L, et al. Exploratory investigation of the chemical characteristics and relative toxicity of ambient air particulates from two New Zealand cities. Sci Total Environ. 2009;407:5007–18. doi: 10.1016/j.scitotenv.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Chen LC, Lippmann M. Effects of metals within ambient air particulate matter (PM) on human health. Inhal Toxicol. 2009;21:1–31. doi: 10.1080/08958370802105405. [DOI] [PubMed] [Google Scholar]
- Choi JH, Kim JS, Kim YC, et al. Comparative study of PM2.5- and PM10- induced oxidative stress in rat lung epithelial cells. J Vet Sci. 2004;5:11–18. [PubMed] [Google Scholar]
- de Kok TM, Hogervorst JG, Briedé JJ, et al. Genotoxicity and physicochemical characteristics of traffic-related ambient particulate matter. Environ Mol Mutag. 2005;46:71–80. doi: 10.1002/em.20133. [DOI] [PubMed] [Google Scholar]
- Deng X, Zhang F, Rui W, et al. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol In Vitro. 2013;27:1762–70. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, 3rd, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Eng J Med. 1993;329:1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Dye JA, Lehmann JR, McGee JK, et al. Acute pulmonary toxicity of particulate matter filter extracts in rats: coherence with epidemiologic studies in Utah Valley residents. Environ Health Perspect. 2001;109:395–403. doi: 10.1289/ehp.01109s3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. Semi-volatile organic compounds. Mid-Atlantic Brownfields & Land Revitalization; 2014. [Last accessed: 21 Jun 2015]. Available from: http://www.epa.gov/reg3hwmd/bf-lr/regional/analytical/semi-volatile.htm. [Google Scholar]
- Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17:279–87. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- Geng H, Meng Z, Zhang Q. In vitro responses of rat alveolar macrophages to particle suspensions and water-soluble components of dust storm PM(2.5) Toxicol In Vitro. 2006;20:575–84. doi: 10.1016/j.tiv.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, Dormans JA, Bloemen HJ, et al. Toxicity of coarse and fine particulate matter from sites with contrasting traffic profiles. Inhal Toxicol. 2007;19:1055–69. doi: 10.1080/08958370701626261. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Huang YC. Exposure to concentrated ambient particles (CAPs): a review. Inhal Toxicol. 2004;16:53–9. doi: 10.1080/08958370490258390. [DOI] [PubMed] [Google Scholar]
- Godri KJ, Harrison RM, Evans T, et al. Increased oxidative burden associated with traffic component of ambient particulate matter at roadside and urban background schools sites in London. PLoS One. 2011;6:e21961. doi: 10.1371/journal.pone.0021961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri M, Longhin E, Mattioli M, et al. Gene expression profiling of A549 cells exposed to Milan PM2.5. Toxicol Lett. 2012;209:136–45. doi: 10.1016/j.toxlet.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Happo M, Markkanen A, Markkanen P, et al. Seasonal variation in the toxicological properties of size-segregated indoor and outdoor air particulate matter. Toxicol In Vitro. 2013;27:1550–61. doi: 10.1016/j.tiv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Happo MS, Hirvonen MR, Hälinen AI, et al. Seasonal variation in chemical composition of size-segregated urban air particles and the inflammatory activity in the mouse lung. Inhal Toxicol. 2010;22:17–32. doi: 10.3109/08958370902862426. [DOI] [PubMed] [Google Scholar]
- Hawthorne SB, Grabanski CB, Martin E, Miller DJ. Comparisons of soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: recovery, selectivity and effects on sample matrix. J Chromatogr A. 2000;892:421–33. doi: 10.1016/s0021-9673(00)00091-1. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhang J, Peng S, et al. Effects of water soluble PM2.5 extracts exposure on human lung epithelial cells (A549): a proteomic study. J Appl Toxicol. 2014;34:675–87. doi: 10.1002/jat.2910. [DOI] [PubMed] [Google Scholar]
- Huang SL, Hsu MK, Chan CC. Effects of submicrometer particle compositions on cytokine production and lipid peroxidation of human bronchial epithelial cells. Environ Health Perspect. 2003;111:478–82. doi: 10.1289/ehp.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imrich A, Ning Y, Kobzik L. Insoluble components of concentrated air particles mediate alveolar macrophage responses in vitro. Toxicol Appl Pharmacol. 2000;167:140–50. doi: 10.1006/taap.2000.9002. [DOI] [PubMed] [Google Scholar]
- Jalava PI, Hirvonen MR, Sillanpää M, et al. Associations of urban air particulate composition with inflammatory and cytotoxic responses in RAW 246.7 cell line. Inhal Toxicol. 2009;21:994–1006. doi: 10.1080/08958370802695710. [DOI] [PubMed] [Google Scholar]
- Jalava PI, Salonen RO, Hälinen AI, et al. In vitro inflammatory and cytotoxic effects of size-segregated particulate samples collected during long-range transport of wildfire smoke to Helsinki. Toxicol Appl Pharmacol. 2006;215:341–53. doi: 10.1016/j.taap.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Janssen NA, Yang A, Strak M, et al. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci Total Environ. 2014;472:572–81. doi: 10.1016/j.scitotenv.2013.11.099. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Ma R, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–36. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Shadie AM, Bucknall MP, et al. Differential injurious effects of ambient and traffic-derived particulate matter on airway epithelial cells. Respirology. 2015;20:73–9. doi: 10.1111/resp.12381. [DOI] [PubMed] [Google Scholar]
- Kundu S, Stone EA. Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environ Sci Process Impacts. 2014;16:1360–70. doi: 10.1039/c3em00719g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer FT, Mitchell LA, Bedrick E, et al. Temporal-spatial analysis of US-Mexico border environmental fine and coarse PM air sample extract activity in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2009;238:1–10. doi: 10.1016/j.taap.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CM, Suh HH, Kobzik L, et al. A pilot investigation of the relative toxicity of indoor and outdoor fine particles: in vitro effects of endotoxin and other particulate properties. Environ Health Perspect. 2001;109:1019–26. doi: 10.1289/ehp.011091019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhin E, Holme JA, Gutzkow KB, et al. Cell cycle alterations induced by urban PM2.5 in bronchial epithelial cells: characterization of the process and possible mechanisms involved. Part Fibre Toxicol. 2013;10:63. doi: 10.1186/1743-8977-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matte TD, Ross Z, Kheirbek I, et al. Monitoring intraurban spatial patterns of multiple combustion air pollutants in New York City: design and implementation. J Expo Sci Environ Epidemiol. 2013;23:223–31. doi: 10.1038/jes.2012.126. [DOI] [PubMed] [Google Scholar]
- Melaku S, Morris V, Raghavan D, Hosten C. Seasonal variation of heavy metals in ambient air and precipitation at a single site in Washington, DC. Environ Pollut. 2008;155:88–98. doi: 10.1016/j.envpol.2007.10.038. [DOI] [PubMed] [Google Scholar]
- Monn C, Becker S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10-2.5) in outdoor and indoor air. Toxicol Appl Pharmacol. 1999;155:245–52. doi: 10.1006/taap.1998.8591. [DOI] [PubMed] [Google Scholar]
- Mudway IS. Comparing the toxicity of particulate matter (PM) collected by different samplers. London: King’s College London; 2004. [Google Scholar]
- Ning Y, Imrich A, Goldsmith CA, et al. Alveolar macrophage cytokine production in response to air particles in vitro: role of endotoxin. J Toxicol Environ Health A. 2000;59:165–80. doi: 10.1080/009841000156952. [DOI] [PubMed] [Google Scholar]
- Pope CA, Thun MJ, Namboodiri MM, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–74. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- Ravindra, Mittal AK, Van Grieken R. Health risk assessment of urban suspended particulate matter with special reference to polycyclic aromatic hydrocarbons: a review. Rev Environ Health. 2001;16:169–89. doi: 10.1515/reveh.2001.16.3.169. [DOI] [PubMed] [Google Scholar]
- Riva DR, Magalhaes CB, Lopes AA, et al. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal Toxicol. 2011;23:257–67. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- Rivero DH, Soares SR, Lorenzi-Filho G, et al. Acute cardiopulmonary alterations induced by fine particulate matter of São Paulo, Brazil. Toxicol Sci. 2005;85:898–905. doi: 10.1093/toxsci/kfi137. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Richards M, Szpiro AA, et al. A regionalized national universal kriging model using Partial Least Squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos Environ (1994) 2013;75:383–92. doi: 10.1016/j.atmosenv.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumann F, Borm PJ, Herbrich A, et al. Metal-rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. Am J Respir Crit Care Med. 2004;170:898–903. doi: 10.1164/rccm.200403-423OC. [DOI] [PubMed] [Google Scholar]
- Schins RP, Lightbody JH, Borm PJ, et al. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol. 2004;195:1–11. doi: 10.1016/j.taap.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Skarek M, Janosek J, Cupr P, et al. Evaluation of genotoxic and non-genotoxic effects of organic air pollution using in vitro bioassays. Environ Int. 2007;33:859–66. doi: 10.1016/j.envint.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlahoyianni T, Fiotakis K. Comparative study of the formation of oxidative damage marker 8-hydroxy-2′-deoxyguanosine (8-OHdG) adduct from the nucleoside 2′-deoxyguanosine by transition metals and suspensions of particulate matter in relation to metal content and redox reactivity. Free Radic Res. 2005;39:1071–81. doi: 10.1080/10715760500188671. [DOI] [PubMed] [Google Scholar]
- Van Winkle LS, Bein K, Anderson D, et al. Biological dose response to PM2.5: effect of particle extraction method on platelet and lung responses. Toxicol Sci. 2015;143:349–59. doi: 10.1093/toxsci/kfu230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V, Rico-Martinez R, Kotra N, et al. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic subfractions to the reactive oxygen species-generating potential of fine ambient aerosols. Environ Sci Technol. 2012;46:11384–92. doi: 10.1021/es302484r. [DOI] [PubMed] [Google Scholar]
- Vincent R, Goegan P, Johnson G, et al. Regulation of promoter- CAT stress genes in HepG2 cells by suspensions of particles from ambient air. Fundam Appl Toxicol. 1997;39:18–32. doi: 10.1006/faat.1997.2336. [DOI] [PubMed] [Google Scholar]
- Watterson TL, Sorensen J, Martin R, Coulombe RA., Jr Effects of PM2.5 collected from Cache Valley Utah on genes associated with the inflammatory response in human lung cells. J Toxicol Environ Health A. 2007;70:1731–44. doi: 10.1080/15287390701457746. [DOI] [PubMed] [Google Scholar]