Abstract
Study Objective
To examine national trends in prescription antiobesity drug use in the United States.
Design
Data analysis.
Data Source
The IMS Health Vector One National and Total Patient Tracker and Encuity Research Treatment Answers databases, the Source Healthcare Analytics Source Lx database, and IMS Life-Link database.
Measurements and Main Results
National drug use estimates from 1991–2011 were extracted from the IMS Health Vector One National database, and patient characteristics from 2008–2011 were extracted from the Vector One Total Patient Tracker and Encuity Research Treatment Answers databases. The Source Healthcare Analytics Source Lx database was used to examine duration of antiobesity drug use from 2002–2011, with a sensitivity analysis performed using the IMS LifeLink database. In 2011, approximately 2.74 million patients used antiobesity drugs, predominantly phentermine (2.43 million patients). The use of prescription orlistat and sibutramine was relatively uncommon. Eighty-five percent of antiobesity drug users were female, 62% were aged 17–44 years, and 4.5% had a body mass index of ≤ 24.9 kg/m2. Duration of use was generally short and most patients only had one episode of antiobesity drug use during the observation period. The longest episode of use was 30 days or less in 47–58% of patients. Approximately one quarter of the patients used antiobesity drugs for longer than 90 days, including phentermine and other amphetamine congeners whose labels recommend short-term use, not exceeding “a few weeks.” Only 1.3–4.2% of antiobesity drug users used them for longer than 1 year. Concomitant use of two or more prescription weight-loss drugs was generally uncommon, although phentermine was dispensed during 13–16% of benzphetamine, diethylpropion, or phendimetrazine episodes of use.
Conclusion
Phentermine dominated the prescription weight-loss market. Despite the indication of short-term use for amphetamine congeners, duration of use was similar to other antiobesity drugs. Nevertheless, the reasons for and implications of the limited duration of use observed with all prescription antiobesity drugs deserve further investigation.
Keywords: antiobesity drugs, utilization, duration, phentermine, orlistat, sibutramine
Approximately 33% of the adult population in the United States is overweight, and another 36% is obese.1 By 2030, the prevalence of obesity is projected to reach 42%.2 Patients with excess body fat are at increased risk of death, cardiovascular disease, type 2 diabetes mellitus, hypertension, dyslipidemia, certain types of cancer, osteoarthritis, depression, and reduced quality of life.3 Studies have estimated annual medical costs associated with obesity in the United States to be $147–190 billion.4, 5
Besides lifestyle interventions and surgery, pharmacologic treatment options are available to combat obesity for patients with a body mass index (BMI) of 30 kg/m2 or more, or in some instances for patients with a BMI of 27 kg/m2 or more along with additional weight-related risk factors (Table S1). After more than a decade since the last market introduction of a prescription weight-loss drug in the United States (orlistat, 1999), the United States Food and Drug Administration (FDA) has approved two new products: lorcaserin in June 2012 and a phentermine-topiramate combination product in July 2012.6 Other weight-loss options include the amphetamine congeners benzphetamine, desoxyephedrine (methamphetamine), diethylpropion, phendimetrazine, and phentermine, which were approved more than 50 years ago.7 Few patients were exposed to these drugs for more than 12 weeks in subsequent efficacy assessments, and concerns existed about the potential for abuse and addiction. Consequently, the indication for weight loss was limited to short-term use only, specified in the labels as “a few weeks,” which is often interpreted as up to 12 weeks. The labels of orlistat and sibutramine, which was withdrawn from the U.S. market in 2010, contain no such limitation on duration of use. It is unknown if the recommendations for short-term use of amphetamine congeners are reflected in actual duration of use patterns.
The objective of this study was to provide data on drug use patterns, including duration of use, for the prescription antiobesity drugs benzphetamine, diethylpropion, phendimetrazine, phentermine, orlistat, and sibutramine. We did not include desoxyephedrine because its main indication for attention-deficit–hyperactivity disorder and the potential for abuse complicate describing its use as a weight-loss drug.
Methods
Antiobesity Drug Use Analysis
To obtain national estimates of outpatient antiobesity drug use, we used the IMS Health (IMS Health, Inc., Plymouth Meeting, PA) Vector One National (VONA) and Total Patient Tracker (TPT) and Encuity Research (Encuity Research, LLC., Newtown, PA) Treatment Answers databases. IMS databases are large commercial prescription and patient databases of drugs dispensed from outpatient retail pharmacies. The IMS contracts with retail pharmacies, software providers, and pharmacy claims aggregators to obtain dispensed prescription data from two-thirds of the approximately 59,000 U.S. retail pharmacies, accounting for approximately one half of all retail prescriptions dispensed in the United States. IMS projected these data to the national level by using a proprietary projection method incorporating geography, pay type, and class of trade (e.g., retail, independent, mass merchandisers). The VONA database was used to extract the nationally projected number of dispensed prescriptions from 1991–2011 for benzphetamine, diethylpropion, phendimetrazine, phentermine, orlistat, and sibutramine, as well as fenfluramine and dexfenfluramine, which were withdrawn from the U.S. market in 1997 (Table S1). For each weight-loss drug, we calculated per capita rates of use as the annual number of dispensings/100,000 U.S. population, based on U.S. Census data.8
We further characterized the use of weight-loss drugs in the more recent period from 2008–2011. We extracted data on payer type separately for each weight-loss drug, cumulatively over these 4 years from the VONA database. For the same time period, we used TPT to calculate the number of users, patient sex, and patient age at the first dispensing of each drug.
The Treatment Answers database is a survey of over 3200 office-based physicians representing 30 specialties across the United States who report on all patient activity during one typical workday each month. We extracted BMIs associated with drug occurrences mentioned during physician office visits from Treatment Answers, rounded them to the nearest tenth, and categorized users as underweight or normal weight (≤ 24.9 kg/m2), overweight (25–26.9 and 27–29.9 kg/m2), or obese (≥ 30 kg/m2).
Duration of Use Analysis
We used the Source Lx database (Source Healthcare Analytics [SHA], Yardley, PA) to examine duration of use patterns of antiobesity drugs from 2002–2011. The database provides longitudinal patient-level data, and it captures adjudicated claims from 27,000 U.S. pharmacies including mail order pharmacies based on a mix of prescription claims from commercial plans, Medicare Part D plans, cash, and Medicaid claims. Analyses based on this patient-level database are not nationally projected.
In addition to the prescription weight-loss drugs benzphetamine, diethylpropion, orlistat, phendimetrazine, phentermine, and sibutramine, we included two comparator drugs, the antihypertensive drug captopril and the antidiabetic drug repaglinide. The purpose of using captopril and repaglinide was not to provide a head-to-head comparison with antiobesity drugs but to test if the dataset and our analytic approach can detect chronic prescription drug use, when present. Although these two drugs are not the most commonly used members of their respective therapeutic class, they are indicated for chronic use. Also, in contrast to more commonly prescribed antihypertensive or antidiabetic drugs, the number of captopril or repaglinide prescriptions was similar to the number of antiobesity drug prescriptions, thus facilitating data handling. All study drugs were on the market at the beginning and throughout the study period, with the exception of sibutramine, which was withdrawn in October 2010. An over-the-counter (OTC) version of orlistat was approved in February 2007, but prescription orlistat continues to be available. The OTC products are not part of the database, and OTC orlistat was not included in this analysis.
Among patients with at least one dispensing of the aforementioned drugs, we excluded entire patient records if any claim for any study drug included unknown sex, unknown or invalid birth year, zero or more than 100 days of supply or days of supply exceeding the number of units dispensed (quantity). Episodes of use were constructed using prescription dates and days of supply. Each episode started on the date of the first dispensing until a treatment gap of 15 days or longer or the end of study period was reached. Gaps shorter than 15 days were ignored and the surrounding dispensings were counted as one episode. For one particular analysis, duration of the longest episode, we only included episodes that were “fully observed” to ensure that episodes were not truncated by patients’ entering or leaving the database. We required that these episodes be preceded by at least one claim for any prescription drug during the period between 294 and 115 days before the start date and between 15 and 194 days after the end of the respective episode. These periods were chosen to represent 180-day windows under consideration of a maximum of 100 days of supply and the less than 15-day allowable gap period.
We considered stockpiling, which can occur when two dispensings of the same drug for the same patient have overlapping days of supply. We focused on instances where a dispensing would normally have been marked as the end of an episode but additional supply was available due to overlapping dispensings. In that case we created a new end date of that treatment episode by adding days of supply of the same drug that overlapped with the last dispensing, up to a maximum of 15 days.
We calculated the duration of each episode by subtracting the date of the first dispensing in a particular episode from the end date of that treatment episode. The total cumulative duration of use was estimated by summing the duration of all episodes of use for each patient, regardless of left or right truncation—that is, we did not require that an episode be preceded and followed by pharmacy activity. Similarly, for the analysis of the number of episodes, we included all episodes of use.
Sensitivity Analyses
We conducted two sensitivity analyses. In the first sensitivity analysis, we extended the allowable gap to less than 30 days and altered the definition of fully observed episodes accordingly to include any pharmacy activity between 309 and 130 days before the start and between 30 and 209 days after the end of an episode. Also, the maximum number of days added due to overlap was raised from 15 to 30 days.
One limitation to a pharmacy-based database is that dispensings filled in pharmacies that are not included in the database are not detected, and episodes of use could be artificially truncated if patients use in- and out-of-database pharmacies within the same episode. In a second sensitivity analysis, we used the payer-based IMS LifeLink database to address this limitation. The design was comparable to the primary analysis; however, instead of pharmacy activity before and after treatment episodes, we required that a patient be eligible for pharmacy benefits for five consecutive calendar months before and two consecutive calendar months after a fully observed episode. Because this database is based on payer data, pharmacy switching would not affect our ability to detect dispensings. However, unlike our primary analysis, this analysis would not include dispensings obtained without reimbursement, such as dispensings obtained through cash payments.
Data Analysis
Data analyses were conducted by using SAS statistical software, version 9.2 (SAS Institute Inc., Cary, NC). The study was exempted from review by the FDA Research Involving Human Subjects Committee under 45 CFR 46 101(b)(4).
Results
Trends in Antiobesity Drug Use (1991–2011)
The outpatient use of antiobesity drugs in the United States reached its peak in 1996, when 8529 antiobesity drug prescriptions were filled/100,000 U.S. population, mostly consisting of phentermine or fenfluramine (Figure 1). Prescription rates decreased dramatically starting in 1997, when fenfluramine and dexfenfluramine were voluntarily withdrawn from the market due to their association with valvular heart disorders. Sibutramine and orlistat were introduced to the U.S. market in 1998 and 1999, respectively, but never reached the popularity of phentermine. The use of phentermine started to increase in 2004 after an earlier decline, but phentermine has remained the leading prescription weight-loss drug throughout the study period. In 2011, 2554 antiobesity drug prescriptions were filled/100,000 U.S. population, 86.6% of which were for phentermine.
Figure 1.
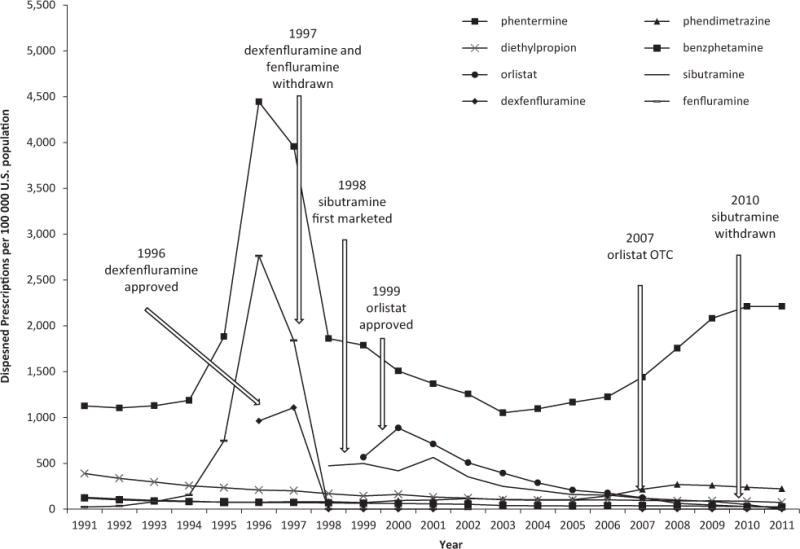
Per capita antiobesity drug prescriptions in the United States. (Data source: IMS Health Vector One National, 1991–2011.)
Characteristics of Antiobesity Drug Users (2008–2011)
Since 2008, the number of antiobesity drug users has increased from approximately 2.35 million to 2.74 million in 2011 (Table 1). In 2011, most patients used phentermine (2.43 million) or phendimetrazine (253,240). Antiobesity drugs were overwhelmingly used by women (85.0%), and most users were between 17 and 44 years old (62.3%). Phendimetrazine and phentermine tended to be used by younger patients compared with other weight-loss drugs. Overall, antiobesity drugs were mostly paid with cash (58.4%), with the exceptions of orlistat (68.5% by third-party payers) and sibutramine (57.9% by third-party payers). Medicaid only played a significant role in the reimbursement of orlistat (8.7%). Most drug occurrences were in obese patients (BMI ≥ 30 kg/m2 [65.7%]) or in patients with a BMI of 27–29.9 kg/m2 (12.7%). However, approximately 1 in 10 users of prescription weight-loss drugs (10.5%) did not meet the BMI indication: 4.5% of occurrences were in patients with a BMI of 24.9 kg/m2 or less, and 6.0% occurred in slightly overweight patients with a BMI of 25–26.9 kg/m2. The BMI was not specified for 11.1% of the sample.
Table 1.
Nationally Projected Characteristics of Antiobesity Drug Users in the United States, 2008–2011
| Benzphetamine | Diethylpropion | Orlistat | Phendimetrazine | Phentermine | Sibutramine | All Antiobesity Drugs | |
|---|---|---|---|---|---|---|---|
| No. of usersa | 69,625 | 299,374 | 133,030 | 783,614 | 6,232,462 | 233,060 | 7,276,261 |
| 2008 | 22,991 | 102,641 | 68,979 | 253,503 | 1,883,164 | 117,125 | 2,348,838 |
| 2009 | 27,003 | 105,841 | 48,133 | 250,290 | 2,301,056 | 99,579 | 2,727,723 |
| 2010 | 25,503 | 99,952 | 34,080 | 251,043 | 2,431,963 | 63,697 | 2,798,618 |
| 2011 | 22,017 | 85,542 | 23,134 | 253,240 | 2,434,061 | 106 | 2,738,084 |
| Femalea | 79.9% | 86.3% | 78.6% | 87.0% | 85.2% | 82.3% | 85.0% |
| Age (yrs) | |||||||
| 0–16 | 0.3% | 0.5% | 0.6% | 0.5% | 0.5% | 0.6% | 0.5% |
| 17–44 | 52.6% | 49.7% | 39.9% | 58.7% | 64.1% | 48.3% | 62.3% |
| 45–64 | 43.7% | 44.6% | 49.0% | 38.7% | 34.3% | 46.1% | 35.7% |
| ≥ 65 | 5.3% | 7.3% | 12.2% | 4.1% | 3.1% | 6.2% | 3.6% |
| No. of dispensingsb | 358,017 | 1,049,442 | 484,005 | 3,191,475 | 25,335,191 | 720,419 | 31,138,551 |
| Payer typeb | |||||||
| Cash | 71.1% | 66.2% | 22.8% | 70.5% | 57.5% | 41.3% | 58.4% |
| Third party | 28.9% | 33.7% | 68.5% | 29.5% | 42.3% | 57.9% | 41.3% |
| Medicaid | 0.0% | 0.1% | 8.7% | 0.1% | 0.1% | 0.8% | 0.3% |
| BMI (kg/m2)c | |||||||
| ≤ 24.9 | –d | 4.4% | 2.6% | 7.0% | 4.6% | 1.3% | 4.5% |
| 25–26.9 | – | 7.5% | 1.0% | 6.3% | 6.8% | 1.0% | 6.0% |
| 27–29.9 | – | 10.6% | 9.0% | 8.1% | 14.1% | 9.2% | 12.7% |
| ≥ 30 | – | 62.8% | 71.9% | 74.6% | 63.3% | 73.3% | 65.7% |
| Not specified | – | 14.2% | 15.3% | 3.9% | 11.3% | 14.9% | 11.1% |
BMI = Body mass index.
Source: IMS Health Vector One Total Patient Tracker.
Source: IMS Health Vector One National.
Source: Encuity Research, LLC, Treatment Answers.
Treatment Answers sample size was insufficient for reliable estimates of body mass index for benzphetamine users.
Duration of Use (2002–2011)
In the duration of use analysis, we excluded 5.1% of patients for the following reasons: unknown sex (1.7%), unknown or invalid year of birth (1.0%), dispensing with zero (0.1%) or more than 100 days of supply (0.3%) or days of supply greater than the quantity (3.1%) (see Figure S1 for exclusions specific to exposure groups). Our sample ranged from 14,329–1,369,091 patients for each study drug (Table 2) during the 10-year study period. On average, patients contributed between 4.6 years (captopril) and 5.9 years (orlistat and sibutramine) of follow-up with any prescription activity. The mean number of antiobesity drug dispensings/patient ranged from 3.1–5.3, with a median of 2. Users of comparator drugs received approximately 2–3 times the number of dispensings. Between one half and two-thirds of antiobesity drug users had only one episode of use, compared with slightly less than one half of comparator drug users. On average, patients had about two episodes of antiobesity drug use during the study period. However, this distribution was skewed, as indicated by the median of one episode. On average, users of comparator drugs had one additional episode.
Table 2.
Duration of Use Analysis, 2002–2011
| Variable | Antiobesity Drugs
|
Comparator Drugs
|
||||||
|---|---|---|---|---|---|---|---|---|
| Benzphetamine | Diethylpropion | Orlistat | Phendimetrazine | Phentermine | Sibutramine | Captopril | Repaglinide | |
| No. of patients | 14,329 | 89,207 | 232,493 | 100,052 | 1,369,091 | 238,241 | 646,455 | 293,601 |
| No. of calendar yrs with any prescription activity (mean) | 4.9 | 5.7 | 5.9 | 5.0 | 5.0 | 5.9 | 4.6 | 5.7 |
| No. of antiobesity drug dispensings/patient | ||||||||
| Mean | 5.3 | 3.6 | 3.4 | 3.6 | 3.8 | 3.1 | 10.9 | 8.5 |
| Median | 2 | 2 | 2 | 2 | 2 | 2 | 5 | 4 |
| Mode no. (%) days of supply/dispensing | 30 (66.1) | 30 (81.2) | 30 (76.9) | 30 (65.0) | 30 (87.0) | 30 (90.0) | 30 (83.3) | 30 (76.0) |
| No. of episodes of use | ||||||||
| Mean | 2.3 | 2.0 | 2.1 | 1.9 | 2.2 | 1.7 | 3.1 | 3.1 |
| Median | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| Patients with only one episode | 59.3% | 62.6% | 60.6% | 64.0% | 54.5% | 67.0% | 46.3% | 45.6% |
| Cumulative duration | ||||||||
| Mean (days) | 139 | 108 | 106 | 91 | 116 | 97 | 380 | 306 |
| Median (days) | 60 | 47 | 48 | 37 | 60 | 60 | 164 | 133 |
| 1–30 days | 43.9% | 46.4% | 45.9% | 47.9% | 37.3% | 43.9% | 21.7% | 22.0% |
| 31–90 days | 25.3% | 25.5% | 25.6% | 27.0% | 26.9% | 25.9% | 16.4% | 19.6% |
| 91–360 days | 21.6% | 22.5% | 23.0% | 21.1% | 29.9% | 26.2% | 30.2% | 31.8% |
| > 360 days | 9.2% | 5.6% | 5.5% | 4.0% | 5.8% | 4.0% | 31.7% | 26.5% |
| Duration of longest episode | ||||||||
| Mean (days) | 92 | 72 | 67 | 65 | 75 | 75 | 247 | 174 |
| Median (days) | 33 | 30 | 30 | 30 | 45 | 34 | 131 | 90 |
| 1–30 days | 49.1% | 55.0% | 58.2% | 55.2% | 47.4% | 49.4% | 22.3% | 27.7% |
| 31–90 days | 24.2% | 23.8% | 22.6% | 25.5% | 26.5% | 24.7% | 17.3% | 23.0% |
| 91–360 days | 22.6% | 19.3% | 17.5% | 18.0% | 24.8% | 24.6% | 39.0% | 36.3% |
| > 360 days | 4.2% | 1.9% | 1.7% | 1.3% | 1.3% | 1.3% | 21.5% | 13.0% |
Data source: Source Healthcare Analytics Source Lx, 2002–2011.
Average cumulative duration of antiobesity drug use was approximately 3–4 months during the 10-year study period (Table 2). The mean duration of the longest episode ranged from 67–92 days for antiobesity drugs and was 2–3 times longer for comparator drugs. Again, this distribution was skewed as indicated by the shorter median duration. Approximately one half of anti-obesity drug users had supplies for 30 days or less, a quarter of patients between 31 and 90 days, and another quarter for more than 90 days, including 1.3–4.2% with an episode that lasted longer than 1 year (Table 2 and Figure 2).
Figure 2.
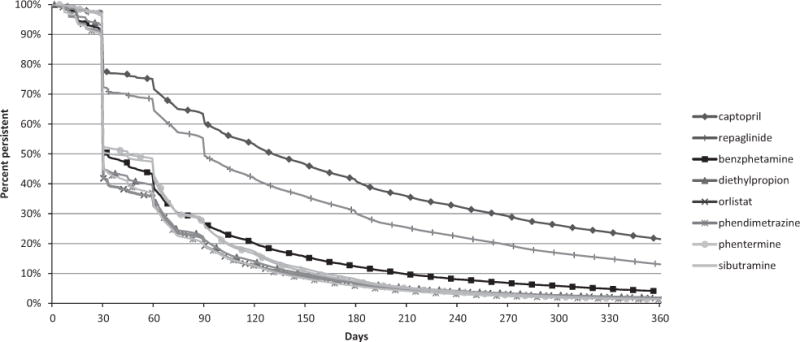
Persistence by duration of use, longest episode/patient. Captopril and repaglinide, which are both indicated for chronic use, are represented as comparator drugs to test if the dataset and analytic approach could detect chronic prescription drug use, when present. (Data source: Source Healthcare Analytics Source Lx, 2002–2011.)
As expected with the extension of gap length and overlap in the first sensitivity analysis, the average number of episodes/patient decreased and the proportion of patients with only one episode increased (Table S2). Still, only a quarter to a third of patients used antiobesity drugs for more than 90 days and only 2.3–6.9% for longer than 1 year. Results from the second sensitivity analysis in the payer-based IMS Vector One database were remarkably similar to the primary pharmacy-based analysis (Table S3).
Concomitant Use of Antiobesity Drugs (2002–2011)
Concomitant use of prescription antiobesity drugs was generally uncommon, with the exception of phentermine, which was dispensed during 3.0% of sibutramine episodes of use to 16.3% of phendimetrazine episodes of use (Table 3). Without limiting to overlapping days of supply, between 14.4% (orlistat) and 39.9% (phendimetrazine) of patients also used phentermine at any point during follow-up (Table S4), but only 8.1% of all prescription antiobesity drug users used more than one agent at any point during follow-up (data not shown).
Table 3.
Concomitant Use of Prescription Antiobesity Drugs
| Concomitant Antiobesity Drug Dispensed During Fully Observed Episodes | Benzphetamine | Diethylpropion | Orlistat | Phendimetrazine | Phentermine | Sibutramine |
|---|---|---|---|---|---|---|
| Benzphetamine | – | 1.7% | 2.5% | 2.9% | 13.9% | 0.6% |
| Diethylpropion | 0.2% | – | 2.2% | 1.8% | 13.2% | 0.8% |
| Orlistat | 0.1% | 0.8% | – | 0.9% | 6.5% | 2.9% |
| Phendimetrazine | 0.4% | 1.8% | 2.5% | – | 16.3% | 0.5% |
| Phentermine | 0.1% | 1.0% | 1.3% | 1.2% | – | 0.6% |
| Sibutramine | 0.0% | 0.3% | 3.0% | 0.2% | 3.0% | – |
Data indicate percentages of episodes of drugs listed in the first column with concomitant use of another antiobesity drug.
Data source: Source Healthcare Analytics Source Lx, 2002–2011.
Discussion
This study provided national estimates of prescription antiobesity drug use in the United States for the years 1991–2011. An earlier study followed national use patterns until 2002,9 and we extended this observation period to 2011, up to the market introduction of two new prescription weight-loss drugs, lorcaserin and phentermine-topiramate. Our analysis showed that prescription weight-loss drugs were predominantly used by women, users were mostly between 17 and 44 years old, and only a small proportion of users were not overweight or obese. Throughout the study period, phentermine dominated the market, only distantly followed by phendimetrazine in the most recent years. The branded products orlistat and sibutramine (up to its withdrawal in 2010) only represented a small portion of antiobesity drug use. The availability of OTC orlistat in 2007 may have contributed to a further decline in the use of prescription orlistat. Our data indicate that prescriptions were filled for sibutramine after its withdrawal. The reasons for these dispensings are unclear but may include pharmacies that still dispensed a prescription (even though it was withdrawn), delays or corrections in billing for previously filled prescriptions, and pharmacy data entry errors.
We found that over the 10-year study period, the duration of antiobesity drug use was generally short, with very few episodes/patient. These observations are consistent with findings of previous studies. A study based on U.S. claims data from 2002–2005 found that 75–79% of prescription weight-loss drug use was shorter than 91 days and only 2–4% was longer than 1 year.10 Conversely, a survey-based study found self-reported use of phentermine for longer than 12 weeks in 42% of respondents.11 Lastly, a study conducted in an administrative database in British Columbia, Canada, found that after 6 months, 1 year, and 2 years, 18%, 6%, and 2% of patients, respectively, were persistent with orlistat, and 26%, 8%, and 2% of patients, respectively, were persistent with sibutramine, using a more generous grace period of less than 120 days between two dispensings.12
The relatively short duration of all antiobesity drug use deserves discussion. Notably, the duration of use differed little between the various types of antiobesity drugs, despite the recommendation for short-term use in the labels of amphetamine congeners included in our study. The recommendation that use should not exceed “a few weeks” is vague. However, approximately 20–25% of patients used antiobesity drugs, including amphetamine congeners, for longer than 90 days, thus exceeding “a few weeks.” On the other hand, orlistat and sibutramine, which were not limited with regard to recommended duration of use, were used for a similar duration as amphetamine congeners. Reasons for early discontinuation in most users could include unpleasant adverse effects, as commonly reported with orlistat,13 or a perceived lack of effectiveness. In the United Kingdom, orlistat may only be continued beyond 12 weeks if a patient has lost at least 5% of body weight by that time,14 and similar provisions were included in the U.S. labels of the two recently approved weight-loss drugs,15, 16 which were not part of our analysis. However, the drop in persistence for antiobesity drugs shown at 90 days (Figure 2) is small and less pronounced than for the comparator drugs, indicating that a stopping rule at 12 weeks was not a major factor contributing to the short duration of use that we observed. Other factors potentially impacting the choice of a weight-loss drug and duration of use may be brand versus generic status and related out-of-pocket cost as well as scheduling under the Controlled Substances Act. Benzphetamine and phendimetrazine are listed under schedule III, diethylpropion, phentermine, and sibutramine are listed under schedule IV, whereas orlistat is unscheduled (Table S1).
Our study is not without limitations. First, we did not investigate appropriateness of prescribing in overweight patients—that is, the presence of comorbidities in patients with a BMI of 27–29.9 kg/m2. Also, we caution that the BMI analysis was based on a limited sample size, and estimates for BMI categories with low weight-loss drug use may not be reliable.
Second, our analyses focused on prescription drugs with a weight-loss indication. Physicians have responded to a survey that they prescribe drugs for weight loss that do not carry that indication and that they recommend certain dietary supplements to their patients.17 Our data did not allow for an assessment of off-label use or nonprescription drug or supplement use.
Third, in the duration of use analysis, the observation period was limited by the study period and patients’ presence in the database; as such, the cumulative duration of use and the lifetime number of episodes could have been underestimated.
Last, we only had information on drug dispensing and not actual drug consumption. This may be especially relevant in the duration of use analysis in the case where patients obtained only one dispensing because we would not know if the patient discontinued after one dose or finished the entire supply of the dispensing. For multiple dispensings, it may be more realistic to assume that the supply up to the last dispensing was actually consumed.
Our study has several strengths, including the use of nationally projected data based on the Vector One database, which includes 1.4 billion prescription claims every year. Our duration of use analysis was conducted in parallel in two different databases, thus overcoming limitations associated with multiple payment sources or multiple pharmacies during one patient’s treatment episode. With the use of comparator drugs, we showed that chronic use could have been detected if it were present. Finally, our conclusions remained identical, even when we tested assumptions regarding gap lengths and stockpiling in a sensitivity analysis.
Conclusion
This analysis showed that the prescription weight-loss market in the most recent years was dominated by phentermine, with orlistat and sibutramine only playing a minor role. Users of weight-loss drugs were predominantly females and between 17 and 44 years of age. Approximately 1 in 10 patients prescribed a weight-loss drug had a BMI of 26.9 kg/m2 or less. The duration of prescription antiobesity drug use was generally short, and most patients had only one episode of use during our 10-year study period. In about half of the patients, the longest treatment episode was 30 days or shorter, and only a quarter of the patients used antiobesity drugs for longer than 90 days. Despite the indication of short-term use for amphetamine congeners, duration of use was similar to other antiobesity drugs. Nevertheless, the reasons for the limited duration of use observed with all prescription antiobesity drugs deserve further investigation.
Supplementary Material
Table S1. Overview of selected prescription weight-loss drugs.
Table S2. Sensitivity analysis of duration of use extending the allowable gap period to < 30 days.
Table S3. Sensitivity analysis of duration of use with allowable gap period of < 15 days, alternative data source.
Table S4. Use of multiple prescription antiobesity drugs during follow-up.
Figure S1. Schematic of patient selection in the duration of use analysis.
Acknowledgments
We acknowledge the following individuals from the FDA Center for Drug Evaluation and Research for their helpful comments and assistance with the preparation of the manuscript: Gerald Dal Pan, M.D., M.H.S., Julie Golden, M.D., Laura Governale, Pharm. D., M.B.A., Solomon Iyasu, M.D., M.P.H., Hina Mehta, Pharm. D., Diane Wysowski, Ph.D., and Esther Zhou, M.D., Ph.D., for their review of the manuscript, and Kusum Mistry, Pharm. D., for her assistance with vendor communication.
Footnotes
This article reflects the views of the authors, and it should not be construed to represent the United States Food and Drug Administration’s views or policies.
Christian Hampp had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting Information
The following supporting information is available in the online version of this paper:
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–70. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316:104–8. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood) 2009;28:w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 5.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219–30. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Colman E, Golden J, Roberts M, Egan A, Weaver J, Rosebraugh C. The FDA’s assessment of two drugs for chronic weight management. N Engl J Med. 2012;367:1577–9. doi: 10.1056/NEJMp1211277. [DOI] [PubMed] [Google Scholar]
- 7.Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med. 2005;143:380–5. doi: 10.7326/0003-4819-143-5-200509060-00013. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Census Bureau, Population Division. Annual estimates of the resident population by sex and selected age groups for the United States: April 1, 1990 to July 1, 1999, and April 1, 2000 to July 1, 2009 and April 1, 2010 to July 1, 2011. Accessed March 15, 2013. [Google Scholar]
- 9.Stafford RS, Radley DC. National trends in antiobesity medication use. Arch Intern Med. 2003;163:1046–50. doi: 10.1001/archinte.163.9.1046. [DOI] [PubMed] [Google Scholar]
- 10.Bolen SD, Clark JM, Richards TM, Shore AD, Goodwin SM, Weiner JP. Trends in and patterns of obesity reduction medication use in an insured cohort. Obesity. 2010;18:206–9. doi: 10.1038/oby.2009.175. [DOI] [PubMed] [Google Scholar]
- 11.Blanck HM, Khan LK, Serdula MK. Prescription weight loss pill use among Americans: patterns of pill use and lessons learned from the fen-phen market withdrawal. Prev Med. 2004;39:1243–8. doi: 10.1016/j.ypmed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 12.Padwal R, Kezouh A, Levine M, Etminan M. Long-term persistence with orlistat and sibutramine in a population-based cohort. Int J Obes (Lond) 2007;31:1567–70. doi: 10.1038/sj.ijo.0803631. [DOI] [PubMed] [Google Scholar]
- 13.Xenical (orlistat) label. Available from http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020766s029lbl.pdf. Accessed September 13, 2012.
- 14.Xenical. Summary of product characteristics. Available from http://www.medicines.org.uk/emc/document.aspx?documentid=1746. Accessed February 16, 2013.
- 15.Belviq (lorcaserin) label. Available from http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf Accessed September 11, 2012.
- 16.Qsymia (phentermine/topiramate) label. Available from http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022580s000lbl.pdf. Accessed September 11, 2012.
- 17.Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 2009;17:1730–5. doi: 10.1038/oby.2009.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Overview of selected prescription weight-loss drugs.
Table S2. Sensitivity analysis of duration of use extending the allowable gap period to < 30 days.
Table S3. Sensitivity analysis of duration of use with allowable gap period of < 15 days, alternative data source.
Table S4. Use of multiple prescription antiobesity drugs during follow-up.
Figure S1. Schematic of patient selection in the duration of use analysis.


