Abstract
Coxiella burnetii, a Gram-negative intracellular bacterium, can give rise to Q fever in humans and is transmitted mainly by inhalation of infected aerosols from animal reservoirs. Serology is commonly used to diagnose Q fever, but the early cellular immune response –i.e. C. burnetii-specific interferon(IFN)-γ production in response to antigen challenge– might be an additional diagnostic. Detection of IFN-γ responses has been used to identify past and chronic Q fever infections, but the IFN-γ response in acute Q fever has not been described. By challenging immunocompetent BALB/c mice with aerosols containing phase I C. burnetii, the timing and extent of IFN-γ recall responses was evaluated in an acute C. burnetii infection. Other cytokines were also measured in an effort to identify other potential diagnostic markers. The data show that after initial expansion of bacteria first in lungs and then in other tissues, the infection was cleared from day 10 onwards as reflected by the decreasing number of bacteria. The antigen-induced IFN-γ production by splenocytes coincided with emergence of IgM phase II-antibodies at day 10 post-infection, and preceded appearance of IgG-antibodies. This was accompanied by the production of pro-inflammatory cytokines including IL-6, KC and IP-10, followed by MCP-1, but not by IL-1β and TNF-α, and only very low production of the anti-inflammatory cytokine IL-10. These data suggest that analysis of antigen-specific IFN-γ responses could be a useful tool for diagnosis of acute Q-fever. Moreover, the current model of C.burnetii infection could be used to give new insights into immunological factors that predispose to development of persistent infection.
Keywords: Coxiella burnetii, Q fever, cellular immunity, serology, interferon-gamma, cytokines
1. Introduction
Infection with Coxiella burnetii, a Gram-negative intracellular bacterium, causes Q fever in humans. The common route of infection is through inhalation of C. burnetii-infected aerosols spread from animals, usually sheep or goats (1). Acute Q fever presents as a flu-like illness, but can be asymptomatic in over 50% of infections. A minority of cases presents as pneumonia or hepatitis (2). Generally, acute Q fever is self-limiting, yet early recognition and antibiotic treatment may shorten duration (3). In some cases, however, C. burnetii infection leads to a chronic infection (chronic Q fever), mostly Q fever endocarditis or vascular infection (4). These conditions are life-threatening if left untreated. Prevention of evolution from acute to chronic Q fever, by prolonged antibiotic treatment following initial infection, is suggested for risk groups, but the value of this intervention is debated (5, 6).
In the initial phase of the infection, cytokines and chemokines produced by monocytes and macrophages are central to recruit and activate other immune cells, promote pathogen disposal and develop adaptive immunity. Cell-mediated adaptive immune responses are essential for control of acute C. burnetii infection, probably even more important than B-cell responses (7–9). C. burnetii-specific T-cells produce interferon-γ (IFN-γ) and activate monocytes/macrophages to produce inflammatory cytokines and control intracellular C. burnetii growth (10, 11).
Currently, detection of acute Q fever infection in humans mainly relies on measurement C. burnetii-specific serum antibodies. Measurements of T-cell immune responses might be of additional value in acute Q fever, but so far have not been investigated in this context. To obtain data on early adaptive immune responses, human studies are of limited value, since patients are identified fairly late in the course of overt clinical disease. Animal models that mimic human acute Q fever can be used instead.
Animal models for Q fever usually include guinea pigs or mice (12). In mice, as in humans, C. burnetii infection can cause disease, with different mouse strains showing divergent vulnerability for infection, with mortality only in the most sensitive strains (13). The incubation time till development of symptoms depends on the inoculation dose (3), the route of infection and the phase of C. burnetii. The virulent form is the so-called phase I C. burnetii that possesses a full-length lipopolysaccharide (LPS) and is isolated from infected humans or animals (14). Phase II, obtained after several passages of phase I organisms in vitro, displays a truncated LPS molecule lacking the terminal O-antigen sugars (15), and does not lead to disease even when administered in high inocula in experimental animals (9, 16, 17). The route of infection is of importance with a shorter incubation time in animals infected intraperitoneally as compared to the natural route of respiratory infection (18). Clearly, aerosol infection resembles most closely the natural route of infection in humans and should be preferred for studying the disease (19).
The main purpose of this study was to investigate the development of cellular immunity – i.e. C. burnetii-specific IFN-γ production in response to antigen challenge – and to compare this with the timing of IgM and IgG antibody responses against phase I and II bacteria. In addition, we investigated the specific production of early inflammatory mediators – a panel of monocyte/macrophage-derived cytokines and chemokines – in an effort to identify other potential diagnostic markers. To facilitate analysis and mimic the mode of transmission for human acute Q fever most closely, we used a mouse model of aerosol infection with phase I C. burnetii in immunocompetent BALB/c mice.
2. Materials and methods
2.1 Animals
A total of 50 male BALB/c mice, 9 weeks of age, were purchased from The Jackson Laboratory (Bar Harbor, ME). This mouse strain is known to be intermediately sensitive to infection with C. burnetii (13, 19). Mice were housed in a Tecniplast Isocage system (Tecniplast, Exton, PA) in an ABSL3 facility, and given food and water ad libitum. The animal experiments were performed according to an animal protocol approved by the CDC Institutional Animal Care and Use Committee.
2.2 Bacteria
The strain used for this study was C. burnetii Nine Mile (NM) phase I (RSA493). This reference strain, isolated from a tick in 1935 (12), can cause Q fever in humans (3) and grows well in mouse models (14). It was grown in chicken eggs and purified by sucrose gradient centrifugation (20). Stocks were kept frozen at −80°C in sucrose phosphate glutamate buffer until use.
2.3 Mouse infections
On day 0, 40 mice were inoculated using the Biaera aerosol management platform (AeroMP, Biaera Technologies, Hagerstown, Maryland, USA). Ten milliliters of phosphate-buffered saline (PBS) containing NM phase I bacteria (at 10^8 organisms/mL) was placed in a nebulizer, and the aerosolized bacteria were introduced into the chamber containing the 40 mice for a 10 minute exposure period. Sixty liters of air from the chamber were sampled in an impinger containing 10 mL PBS. Quantitative PCR detected 1.68 × 10^7 C. burnetii organisms in the impinger, suggesting that the air in the chamber contained 280 organisms per ml of air. Based on a tidal volume of 0.15 mL and a respiratory rate of 163/min for mice, it is estimated that each mouse inhaled 6.8 × 10^4 C. burnetii organisms. Ten mice served as a negative control group and were left uninfected. The infected and uninfected mice were maintained in separate HEPA-filtered isolator cages.
On day 1, 3, 7, 10 and 14, groups of 8 infected and 2 uninfected mice were euthanized by exsanguination under isoflurane anesthesia, after which the euthanasia was verified by cervical dislocation. Blood was harvested by cardiac puncture and collected in heparinized tubes and blood from pairs of mice was pooled. Lungs, spleen, and liver were aseptically removed. Spleens were weighed before further processing.
2.4 Quantitative PCR
For analysis of the quantity of C. burnetii DNA in blood and tissue, blood and spleens from the 8 infected and 2 uninfected mice at each time point were pooled into 5 pairs. Spleens were homogenized into single cell suspensions by grinding the tissues between frosted ends of ground glass slides before pooling. For liver and lung, the organs from each mouse were tested independently. To quantify the C. burnetii, total genomic DNA was extracted from 100 µL blood, lung/liver tissue, or spleen cell suspensions using the QIAamp DNA mini kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. On all these samples, quantitative PCR for IS1111a was performed as described (21).
2.5 Serology
Serum titers of IgM and IgG antibodies against phase I and II C. burnetii were determined by indirect immunofluorescence antibody test (IFA). Plasma was obtained from heparinized blood through centrifugation at 1,200 × g. Slides coated with either Nine Mile phase I (RSA 493) or Nine Mile phase II (RSA 439) strains were incubated with titrations of plasma samples. After washing, they were treated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody, and binding was visualized using a fluorescence microscope. The greatest dilution of plasma that resulted in unambiguous antibody binding is reported as titer.
2.6 Splenocyte stimulation
Splenocytes were isolated by homogenizing the spleens by grinding the tissue between the frosted ends of a pair of ground glass slides, creating single-cell suspensions in sterile PBS. Splenocytes from pairs of mice were pooled. After centrifugation at 300 × g for 10 minutes at 20°C, red blood cells were lysed by osmotic shock followed by resuspension in PBS. After passage through a 100 micron cell strainer to filter debris, cells were centrifuged again at 300 × g for 10 minutes at 20°C and resuspended in RPMI culture media supplemented as described. Cells were plated at 3×10^6 cells/well in a 24-well plate, in a final volume of 1 mL per well.
Splenocytes were stimulated with either medium alone (negative control), the mitogen concanavalin A (conA) [2.5 µg/mL], or heat-killed (60 min, 80°C)(22) phase I NM at either [1×106/mL] or [1×107/mL]. After 48 hours incubation at 37°C and 5% CO2, 400 µl of splenocyte supernatant was collected from each well. Supernatants were stored at −80°C until cytokines were measured.
2.7 Cytokine analysis
Supernatant samples were gamma-irradiated (2 × 10^6 rads) before handling. Cytokine concentrations – including mouse interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, IL-10, keratinocyte-derived cytokine (KC), monocyte chemotactic protein (MCP)-1, interferon-γ induced protein (IP)-10, and interferon (IFN)-γ – were measured using a Luminex bead-based multiplex assay (R and D Systems, Minneapolis, MN, USA), in accordance with the manufacturers’ instructions. Samples were analyzed using a Bio-Plex Luminex 100 (Bio-Rad, Hercules, CA).
2.8 Statistical analysis
Data are expressed as mean ± SD (for weight and genome copies), or median ±IQR (for cytokine data). Differences between uninfected and infected mice at different time points after infection were tested using ANOVA test or Kruskal-Wallis as appropriate. GraphPad Prism 5.0 software (GraphPad) was used. A difference was considered significant if the P value was ≤ 0.05.
3. Results
3.1 C. burnetii infection in mice
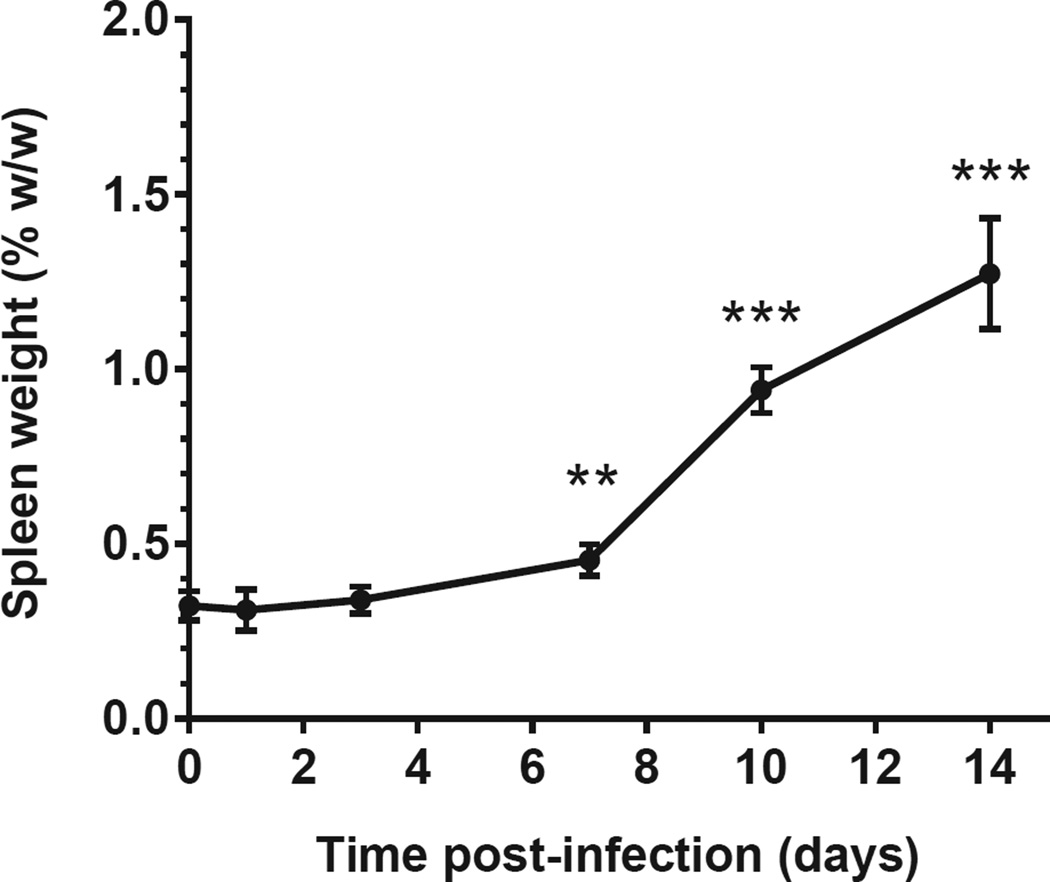
All infected mice showed some signs of lethargy and ruffled fur for 2–7 days, with onset between day 7 and day 14 post infection. Infected mice developed splenomegaly from day 3 onwards (Figure 1). None of the mice died before being sacrificed.
Figure 1. Spleen weight after aerosol infection with C. burnetii in immunocompetent BALB/c mice.
(A) Spleen-to-body weight (mean ± SD) is shown for 10 uninfected mice (t=0) and 8 infected mice per time point post-infection. ANOVA test followed by Dunn’s multiple comparison test was used to compare infected mice at different time points with uninfected mice. **, P<0.01; ***, P<0.001.
3.2 Detection of C. burnetii DNA
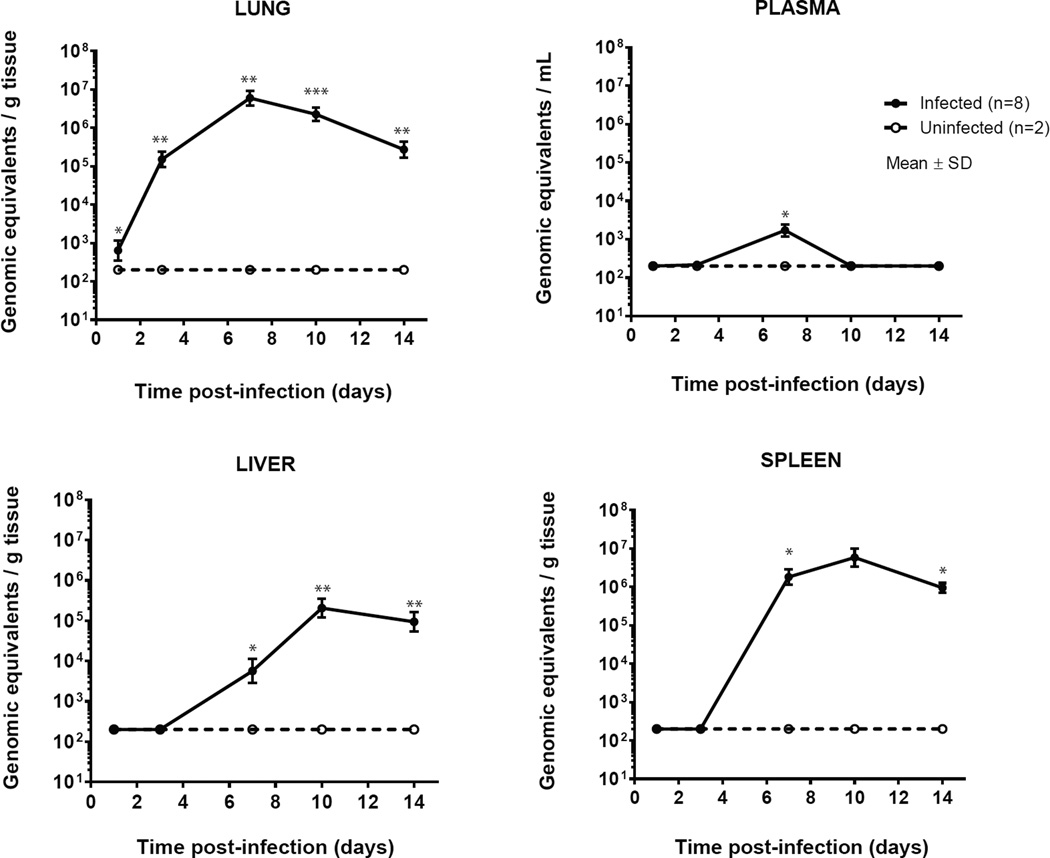
The bacterial DNA copy numbers in the lungs of infected mice increased from day 1 to day 7, and declined thereafter. In plasma, amplification products were obtained at day 3 (only in one of four pairs of mice) and reached maximum at day 7 (all pairs of mice). In liver and spleen, C. burnetii DNA was detected at day 7 and reached maximum at day 10 after which it declined (Figure 2). At day 14, of the tissues examined, spleens contained the highest load of C. burnetii DNA. No bacterial DNA was detected in plasma and tissue of uninfected mice.
Figure 2. Number of C. burnetii DNA copies in lung, plasma, liver and spleen after aerosol infection with C. burnetii in immunocompetent BALB/c mice.
The mean ± SD numbers of genomic equivalents per gram of tissue or ml of plasma are shown of 8 infected mice per time point. Uninfected mice were negative at every time point in all tissues. Samples that were negative were assigned a value of 200 genomic equivalents per g (or ml). This is the limit of detection of the assay. P values were calculated by one-sample t-test with a hypothetical value of 0. * P<0.05, ** P<0.01, *** P<0.001.
3.3 Serological response
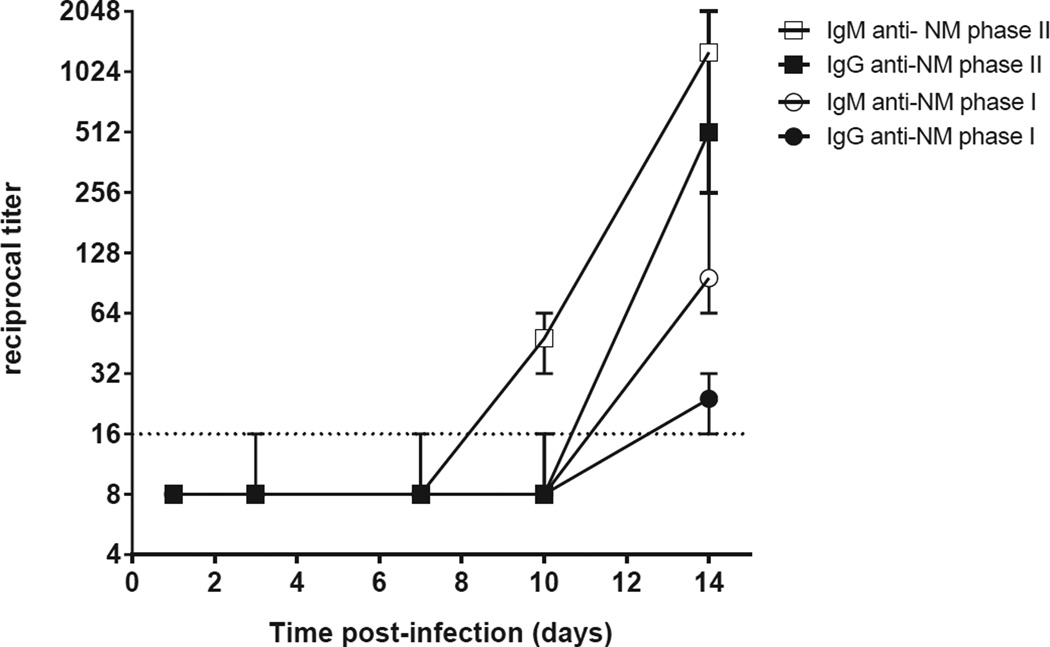
Serological responses, as measured by IFA, are shown in Figure 3. A positive response in infected mice was first detectable at day 10, with low titers (ranging from 1:32 to 1:64) for IgM phase II. These titers increased to 1:256 to 1:2048 at day 14. IgG phase II and IgM phase I were also positive in all infected mice (range 1:256 to 1:512 and range 1:64 to 1:256, respectively). At day 14, all infected mice had developed antibodies against Nine Mile phase I and II C. burnetii, and all control mice remained seronegative. IgG against phase I was only low-positive in two of four mouse pairs (maximum 1:32) at day 14.
Figure 3. Antibody responses to C. burnetii after aerosol infection with C. burnetii in immunocompetent BALB/c mice.
IgM and IgG titers to Nine Mile phase I and phase II were measured in plasma by indirect immunofluorescence assay (IFA). The median ± range reciprocal titers are shown of four pairs of infected mice per time point. The control mice were seronegative at every time point (not shown). Negative results in the IFA were assigned a value 1:8.
3.4 Cytokine production
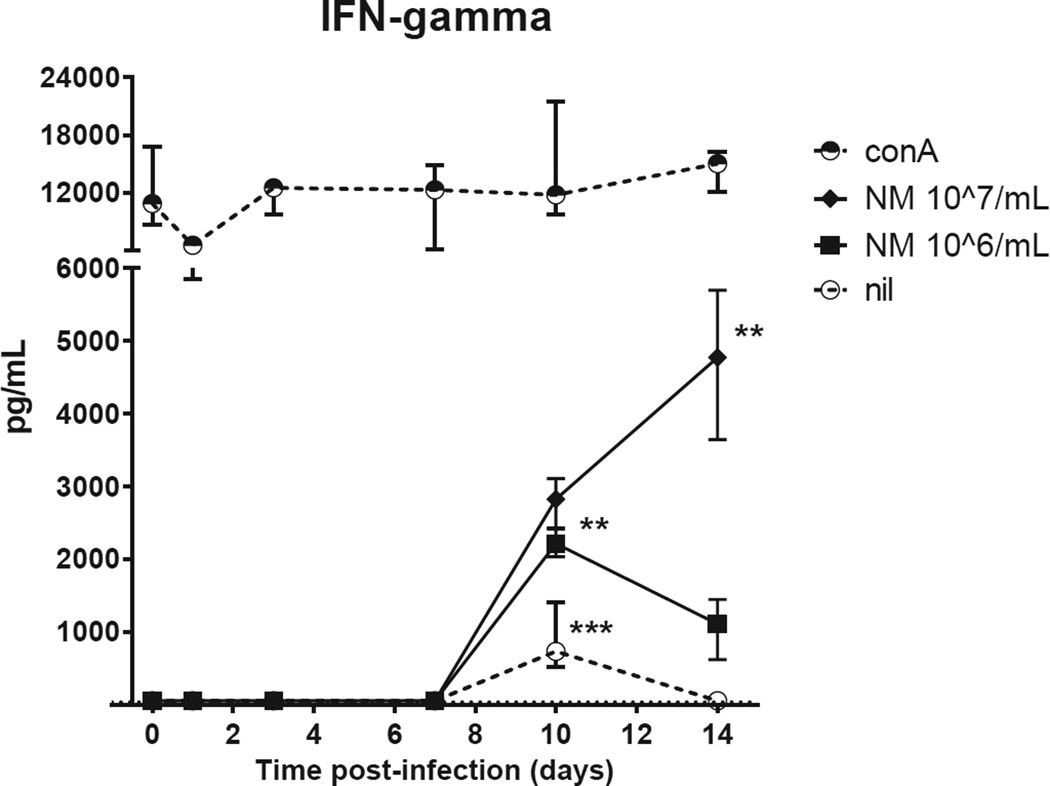
Cytokines were measured in supernatants of splenocytes stimulated for 48 hours in vitro. Splenocytes of infected mice produced substantial amounts of cytokines, with different stimulus-dependent, time post-infection patterns. The pattern of antigen-induced IFN-γ production, reflecting a specific cell-mediated immune response, was of special interest (Figure 4). ConA-induced IFN-γ production was similarly high in uninfected and infected mice. Unstimulated splenocytes produced some IFN-γ at day 10. NM-induced IFN-γ production, absent in all uninfected mice and in infected mice at day 1,3 and 7, was significantly increased at day 10 and 14 post-infection.
Figure 4. Early IFN-γ production by stimulated splenocytes of C. burnetii-infected immunocompetent BALB/c mice.
Splenocytes were stimulated for 48h with either conA [2.5 µg/mL], NM phase I [10^7/mL], NM phase I [10^6/mL], or left unstimulated (nil). The median ± IQR cytokine production is shown per time point of four pairs of infected mice. T=0 shows the median ± IQR of five pairs of uninfected mice. P values were calculated by Kruskal-Wallis test followed by Dunn’s multiple comparison test comparing cytokine concentrations of infected mice at different time points with uninfected mice. * P<0.05, ** P<0.01, *** P<0.001.
Abbreviations: conA, concanavalin A; NMI, C. burnetii Nine Mile phase I.
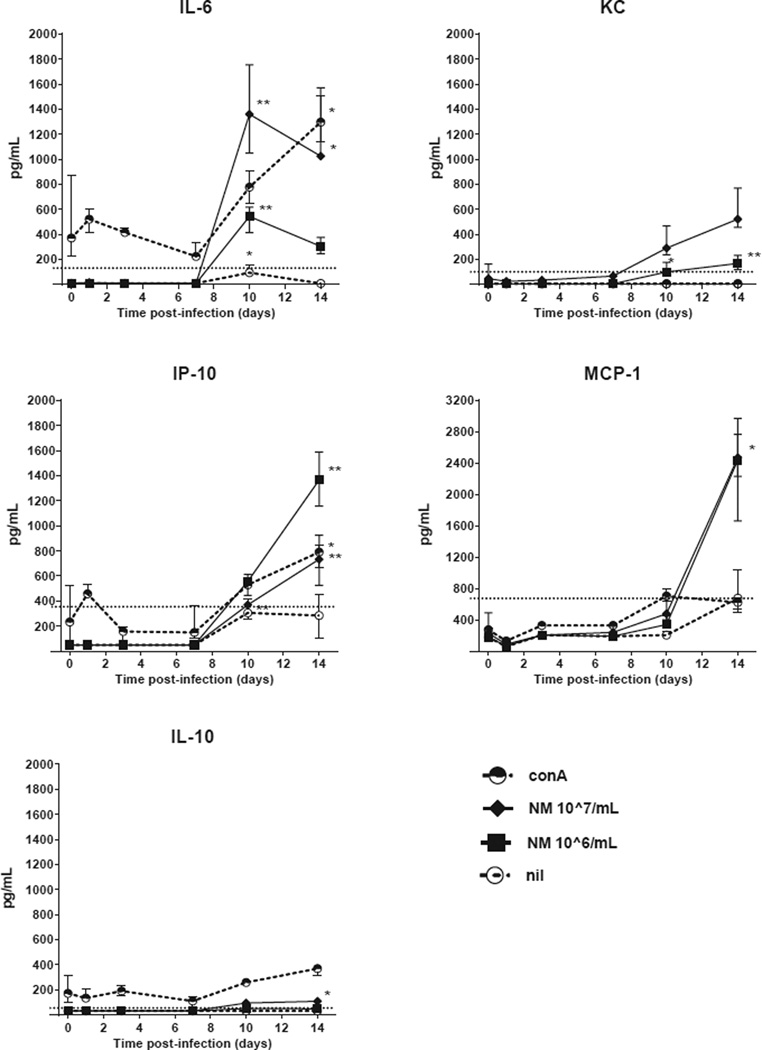
Regarding the other cytokines, NM stimulation induced significant IL-6, KC and IP-10 from day 10 onwards, while MCP-1 production and low levels of IL-10 were observed on day 14 (Figure 5). In addition, conA stimulated splenocytes showed significantly increased IL-6 and IP-10 production at day 14. Unstimulated splenocytes produced IL-6 at day 10, but otherwise no substantial amounts of any other cytokines at any timepoint. IL-1β and TNF-α production were below detection limits at all time points with all stimuli. Supplementary table 1 contains all data on all the cytokines measured.
Figure 5. Early IL-6, KC, MCP-1, IP-10 and IL-10 production by stimulated splenocytes of C. burnetii-infected immunocompetent BALB/c mice.
Splenocytes were stimulated for 48h with either conA [2.5 µg/mL], NM phase I [10^7/mL] or [10^6/mL], or left unstimulated (nil). The median ± IQR cytokine production is shown per time point of four pairs of infected mice. T=0 shows the median ± IQR of five pairs of uninfected mice. IL-1β and TNF-α production were below the lowest detection limit at all time points for all stimulations (not shown).The dashed horizontal line represents the lowest standard in the Luminex assay, values below are extrapolated. P values were calculated by Kruskal-Wallis test followed by Dunn’s multiple comparison test comparing cytokine concentrations of infected mice at different time points with uninfected mice. * P<0.05, ** P<0.01.
Abbreviations: conA, concanavalin A; NMI, C. burnetii Nine Mile phase I.
4. Discussion
In the present study, we observed effective early immune responses in immunocompetent BALB/c mice infected with C. burnetii via the aerosol route. After initial expansion of bacteria in lungs and spread to other tissues, the infection was cleared from day 10 onwards as reflected by the decreasing number of bacterial DNA copies. Antigen-induced IFN-γ production by splenocytes, indicating a cell-mediated immune response, coincided with emergence of IgM phase II antibodies at day 10 post-infection. This was accompanied by the production of pro-inflammatory cytokines including IL-6, KC and IP-10, followed by MCP-1, but not by IL-1β and TNF-α, and only very low production of the anti-inflammatory cytokine IL-10.
Previous studies in humans have looked at the in vitro IFN-γ response in people vaccinated against Q fever, people that have had a previous Q fever infection, and chronic Q fever patients (23–28). However, the testing of in vitro IFN-γ responses in acute human Q fever has not been reported. Acute cytokine responses in mice have been studied previously. This has been done by infecting mice by either an intraperitoneal or intratracheal route and then measuring circulating cytokines in serum at single time points (14). These studies have detected IFN-γ and other cytokines in mouse serum, but have not looked at in vitro antigen specific recall responses at different time points. Another study looked at some early time points and detected modest increases in IFN-γ recall responses after infection of mice by intraperitoneal or intravenous routes (29). The study reported here describes in vitro antigen specific recall cytokine responses at multiple time points shortly after C. burnetii aerosol infection. The data show a very robust antigen specific IFN-γ response that is detected at about the same time as antibody responses relative to infection and perhaps just prior to detection of C. burnetii-specific IgG. Based these observations, specific IFN-γ production assays are worthwhile to investigate for detection of human acute Q fever.
The aerosol infection route that was used in this study closely reflects the typical acquisition of human infection by inhalation. After an inoculum of 6.8 × 10^4 bacteria, the number of C. burnetii DNA copies first increased in the lungs, after which the bacteria became detectable in plasma, with subsequent spread to the spleen and liver.
After the initial expansion, bacterial numbers declined. Since we aimed to observe the very early immune response, later time points were not studied. A previous study of intratracheal infection with C. burnetii in BALB/c mice that continued to 24 days post-infection, also showed clearance of infection (8). In that study, the genome copy numbers in the lungs sharply decreased between day 9 and 16, similarly to our observations. Moreover, the spleen weight peaked at day 16 post-infection, but had decreased by day 24, another indication that infection was controlled.
The time-response curves of the humoral immune response suggest that IgG antibodies are redundant for early clearance of C. burnetii. Due to the absence of cellular IgM-receptors, IgM by itself – in contrast to IgG – is unable to influence cellular responses. In our model, IgG, against either phase I or phase II bacteria, became detectable only at day 14, whereas the clearance of bacteria from the lungs started between day 7 and 10. The decrease in bacterial DNA occurred, however, simultaneously with the increase of specific IFN-γ production by splenocytes.
A limitation of present study is that the results of cytokine production upon stimulation with recall antigens were obtained in splenocytes instead of peripheral blood cells, which would likely be used for testing in humans. Although there is not a specific reason to believe that IFN-γ production by peripheral blood cells would be completely different from splenocytes, there could be kinetic differences. In present study, stimulation of mouse whole blood in vitro was performed, but cytokine responses were difficult to detect due to technical limitations of whole blood stimulations in mice. IFN-γ could not be detected, while IP-10 was detectable only in low levels showing maximum levels at day 10 (not shown). Previous studies looking at past infection or vaccination against Q fever have found that human peripheral blood is a good source of cytokine producing cells in response to C. burnetii antigen stimulation (25, 26).
In addition to IFN-γ, we observed the production of IL-6, KC, MCP-1 and IP-10 in C. burnetii stimulated splenocytes at day 10 and 14 after infection. These cytokines probably play an important role in the cell infiltration in C. burnetii-infected tissues of immunocompetent BALB/c mice, which was observed by Read et al (8). The increased production of IL-6 has been described in the course of human acute Q fever, in which unstimulated peripheral blood cells showed increased production of pro-inflammatory cytokines including IL-6, TNF-α, IL-12 and the anti-inflammatory cytokine IL-10 (30). However, IL-6 production by C. burnetii-stimulated blood cells was not increased in acute Q fever patients. Likewise, MCP-1 was found to be increased in unstimulated blood cells of acute Q fever patients, but not in C. burnetii-stimulated cells (31). These human patients were, however, in a later stage of the infection than the mice in the current study.
To our knowledge, IP-10 has not been studied in the context of C. burnetii infections before. IP-10 is a chemokine produced by monocytes/macrophages, mainly in response to IFN-γ but also other cytokines including type I interferons, IL-2, IL-23 and IL-17. IP-10 has shown a promising role as an additional marker of M. tuberculosis infection (32), being specifically induced by TB-specific antigens in confirmed TB-cases and not in healthy controls. These data from human patients infected with an obligate intracellular pathogen are in line with our observations in mice infected with C. burnetii. However, Ruhwald et al. (28) showed that IP-10 levels after antigen stimulation are higher compared with IFN-γ levels in human peripheral blood. In our study, we found that IP-10 was induced in antigen-stimulated splenocytes, but levels of IP-10 (1400 pg/mL) were lower than levels of IFN-γ (5000 pg/mL).
Previous studies have shown absent IL-1β production, but substantial TNF-α production by C. burnetii-stimulated peritoneal macrophages of uninfected BALB/c mice (33). Other studies showed increased TNF-α production by peritoneal macrophages of C. burnetii-infected BALB/c mice (29). In our model, using the respiratory infection route, we were unable to induce substantial IL-1β and TNF-α production in stimulated spleen cells of infected mice. The lack of TNF-α response in our system could be due to the route of infection used, the cell types present in our spleen preparations, or to the antigen used.
Of note, only minimal levels of IL-10 were produced by splenocytes after C. burnetii infection. IL-10 has been of special interest in previous studies, since it was linked to persistent infection in humans (30, 34) and chronic Q fever in mice overexpressing IL-10 (35). Our findings show that clearance of C. burnetii in the early stage is accompanied by only a very low antigen-specific IL-10 production by splenocytes. Earlier studies in intraperitoneally C. burnetii-infected BALB/c mice, showed high levels of IL-10 production by C. burnetii-stimulated peritoneal macrophages at day 7 post-infection, before bacterial load decreased (29). Similar to TNFα, macrophages may be an important source of IL-10 in response to C. burnetii, although it is likely that regulatory T-cells and Th2 lymphocytes also play a role.
In conclusion, the model of C. burnetii infection used in this study demonstrates that detection of antigen-induced IFN-γ could be used to detect acute C. burnetii infection in mice, and this is likely to be the case in humans as well. Antigen-specific production of IFN-γ and IP-10 were both detectable prior to elevation of specific IgG antibodies. This study also showed antigen-specific induction of IL-6, KC and MCP-1 from splenocyte cultures. If applied to immune-deficient mice, or mice with anatomical risk factors for endocarditis or vascular infections, the model may offer wide opportunities to study the pathophysiological and immune derangements that occur during progression from acute to chronic Q fever.
Acknowledgments
We thank Rachael Priestley for her assistance in performing the IFA.
Funding
TS was financially supported by a grant of The Netherlands Organisation for Health Research and Development (grant number 205520002). MGN was supported by an ERC Consolidator Grant (310371). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC or the Department of Health and Human Services.
Footnotes
Conflict of interest
A patent application has been submitted by MGN, MVD and LABJ for a C. burnetii–specific IFN-γ production assay to diagnose Q fever and is registered by the number PCT/NL 2011/050564.
References
- 1.McQuiston JH, Childs JE. Q fever in humans and animals in the United States. Vector Borne Zoonotic Dis. 2002;2(3):179–191. doi: 10.1089/15303660260613747. [DOI] [PubMed] [Google Scholar]
- 2.Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006;367(9511):679–688. doi: 10.1016/S0140-6736(06)68266-4. [DOI] [PubMed] [Google Scholar]
- 3.Benenson AS, Tigertt WD. Studies on Q fever in man. Trans Assoc Am Physicians. 1956;69:98–104. [PubMed] [Google Scholar]
- 4.Million M, Thuny F, Richet H, Raoult D. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis. 2010;10(8):527–535. doi: 10.1016/S1473-3099(10)70135-3. [DOI] [PubMed] [Google Scholar]
- 5.Million M, Walter G, Thuny F, Habib G, Raoult D. Evolution from acute Q fever to endocarditis is associated with underlying valvulopathy and age and can be prevented by prolonged antibiotic treatment. Clin Infect Dis. 2013;57(6):836–844. doi: 10.1093/cid/cit419. [DOI] [PubMed] [Google Scholar]
- 6.Limonard GJ, Nabuurs-Franssen MH, Dekhuijzen PN, Groot CA. Prevention of Q fever endocarditis. Lancet Infect Dis. 2011;11(2):82–83. doi: 10.1016/S1473-3099(11)70016-0. [DOI] [PubMed] [Google Scholar]
- 7.Sidwell RW, Thorpe BD, Gebhardt LP. Studies of latent Q fever infections. II. Effects of multiple cortisone injections. Am J Hyg. 1964;79:320–327. doi: 10.1093/oxfordjournals.aje.a120386. [DOI] [PubMed] [Google Scholar]
- 8.Read AJ, Erickson S, Harmsen AG. Role of CD4+ and CD8+ T cells in clearance of primary pulmonary infection with Coxiella burnetii. Infect Immun. 2010;78(7):3019–3026. doi: 10.1128/IAI.00101-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andoh M, Zhang G, Russell-Lodrigue KE, Shive HR, Weeks BR, Samuel JE. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect Immun. 2007;75(7):3245–3255. doi: 10.1128/IAI.01767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghigo E, Capo C, Tung CH, Raoult D, Gorvel JP, Mege JL. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-gamma mediates its restoration and bacterial killing. J Immunol. 2002;169(8):4488–4495. doi: 10.4049/jimmunol.169.8.4488. [DOI] [PubMed] [Google Scholar]
- 11.Brennan RE, Russell K, Zhang G, Samuel JE. Both inducible nitric oxide synthase and NADPH oxidase contribute to the control of virulent phase I Coxiella burnetii infections. Infect Immun. 2004;72(11):6666–6675. doi: 10.1128/IAI.72.11.6666-6675.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12(4):518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott GH, Williams JC, Stephenson EH. Animal models in Q fever: pathological responses of inbred mice to phase I Coxiella burnetii. J Gen Microbiol. 1987;133(3):691–700. doi: 10.1099/00221287-133-3-691. [DOI] [PubMed] [Google Scholar]
- 14.Russell-Lodrigue KE, Andoh M, Poels MW, Shive HR, Weeks BR, Zhang GQ, et al. Coxiella burnetii isolates cause genogroup-specific virulence in mouse and guinea pig models of acute Q fever. Infect Immun. 2009;77(12):5640–5650. doi: 10.1128/IAI.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amano K, Williams JC, Missler SR, Reinhold VN. Structure and biological relationships of Coxiella burnetii lipopolysaccharides. J Biol Chem. 1987;262(10):4740–4747. [PubMed] [Google Scholar]
- 16.Moos A, Hackstadt T. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun. 1987;55(5):1144–1150. doi: 10.1128/iai.55.5.1144-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andoh M, Russell-Lodrigue KE, Zhang G, Samuel JE. Comparative virulence of phase I and II Coxiella burnetii in immunodeficient mice. Ann N Y Acad Sci. 2005;1063:167–170. doi: 10.1196/annals.1355.026. [DOI] [PubMed] [Google Scholar]
- 18.Tigertt WD, Benenson AS, Gochenour WS. Airborne Q fever. Bacteriol Rev. 1961;25:285–293. doi: 10.1128/br.25.3.285-293.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein A, Louveau C, Lepidi H, Ricci F, Baylac P, Davoust B, et al. Q fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infect Immun. 2005;73(4):2469–2477. doi: 10.1128/IAI.73.4.2469-2477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JD, Thompson HA. Permeability of Coxiella burnetii to ribonucleosides. Microbiology. 2002;148(Pt 8):2393–2403. doi: 10.1099/00221287-148-8-2393. [DOI] [PubMed] [Google Scholar]
- 21.Kersh GJ, Lambourn DM, Self JS, Akmajian AM, Stanton JB, Baszler TV, et al. Coxiella burnetii infection of a Steller sea lion (Eumetopias jubatus) found in Washington State. J Clin Microbiol. 2010;48(9):3428–3431. doi: 10.1128/JCM.00758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enright JB, Sadler WW, Thomas RC. Thermal inactivation of Coxiella burnetii and its relation to pasteurization of milk. Public Health Monogr. 1957;47:1–30. [PubMed] [Google Scholar]
- 23.Izzo AA, Marmion BP. Variation in interferon-gamma responses to Coxiella burnetii antigens with lymphocytes from vaccinated or naturally infected subjects. Clin Exp Immunol. 1993;94(3):507–515. doi: 10.1111/j.1365-2249.1993.tb08226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kersh GJ, Fitzpatrick KA, Self JS, Biggerstaff BJ, Massung RF. Long-Term immune responses to Coxiella burnetii after vaccination. Clin Vaccine Immunol. 2013;20(2):129–133. doi: 10.1128/CVI.00613-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoffelen T, Herremans T, Sprong T, Nabuurs-Franssen M, Wever PC, Joosten LA, et al. Limited humoral and cellular responses to Q fever vaccination in older adults with risk factors for chronic Q fever. J Infect. 2013;67(6):565–573. doi: 10.1016/j.jinf.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Schoffelen T, Joosten LA, Herremans T, de Haan AF, Ammerdorffer A, Rümke HC, et al. Specific interferon γ detection for the diagnosis of previous Q fever. Clin Infect Dis. 2013;56(12):1742–1751. doi: 10.1093/cid/cit129. [DOI] [PubMed] [Google Scholar]
- 27.Limonard GJ, Thijsen SF, Bossink AW, Asscheman A, Bouwman JJ. Developing a new clinical tool for diagnosing chronic Q fever: the Coxiella ELISPOT. FEMS Immunol Med Microbiol. 2012;64(1):57–60. doi: 10.1111/j.1574-695X.2011.00890.x. [DOI] [PubMed] [Google Scholar]
- 28.Schoffelen T, Sprong T, Bleeker-Rovers CP, Wegdam-Blans MC, Ammerdorffer A, Pronk MJ, et al. A combination of interferon-gamma and interleukin-2 production by Coxiella burnetii-stimulated circulating cells discriminates between chronic Q fever and past Q fever. Clin Microbiol Infect. 2014;20(7):642–650. doi: 10.1111/1469-0691.12423. [DOI] [PubMed] [Google Scholar]
- 29.Honstettre A, Meghari S, Nunès JA, Lepidi H, Raoult D, Olive D, et al. Role for the CD28 molecule in the control of Coxiella burnetii infection. Infect Immun. 2006;74(3):1800–1808. doi: 10.1128/IAI.74.3.1800-1808.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honstettre A, Imbert G, Ghigo E, Gouriet F, Capo C, Raoult D, et al. Dysregulation of cytokines in acute Q fever: role of interleukin-10 and tumor necrosis factor in chronic evolution of Q fever. J Infect Dis. 2003;187(6):956–962. doi: 10.1086/368129. [DOI] [PubMed] [Google Scholar]
- 31.Meghari S, Desnues B, Capo C, Grau GE, Raoult D, Mege JL. Coxiella burnetii stimulates production of RANTES and MCP-1 by mononuclear cells: modulation by adhesion to endothelial cells and its implication in Q fever. Eur Cytokine Netw. 2006;17(4):253–259. [PubMed] [Google Scholar]
- 32.Ruhwald M, Aabye MG, Ravn P. IP-10 release assays in the diagnosis of tuberculosis infection: current status and future directions. Expert Rev Mol Diagn. 2012;12(2):175–187. doi: 10.1586/erm.11.97. [DOI] [PubMed] [Google Scholar]
- 33.Ochoa-Repáraz J, Sentissi J, Trunkle T, Riccardi C, Pascual DW. Attenuated Coxiella burnetii phase II causes a febrile response in gamma interferon knockout and Toll-like receptor 2 knockout mice and protects against reinfection. Infect Immun. 2007;75(12):5845–5858. doi: 10.1128/IAI.00901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capo C, Zaffran Y, Zugun F, Houpikian P, Raoult D, Mege JL. Production of interleukin-10 and transforming growth factor beta by peripheral blood mononuclear cells in Q fever endocarditis. Infect Immun. 1996;64(10):4143–4147. doi: 10.1128/iai.64.10.4143-4147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meghari S, Bechah Y, Capo C, Lepidi H, Raoult D, Murray PJ, et al. Persistent Coxiella burnetii infection in mice overexpressing IL-10: an efficient model for chronic Q fever pathogenesis. PLoS Pathog. 2008;4(2):e23. doi: 10.1371/journal.ppat.0040023. [DOI] [PMC free article] [PubMed] [Google Scholar]